Hepatitis E virus (HEV) is one of the identified pathogens that cause acute viral hepatitis in Mexico. It has been observed that the presence of this particular pathogen represents a great risk in pregnant women and solid organ transplant recipients. In Mexico there are no formal epidemiological reports about HEV. The objective of this study is to determine the incidence of HEV infection in Mexican patients with non-identified viral infection, and to provide a general perspective of the epidemiological characteristics of HEV.
Materials and methodsWe selected a total of 624 patient samples that where collected from January 2010 to June 2013 in 32 states of Mexico. The patients were clinically suspected of having viral hepatitis and obtained a negative result for HAV, HBV and HCV infection. The samples were then analyzed with an ELISA test.
ResultsWe determined an incidence of 1.76% in the analyzed Mexican population with clinical presentation of viral hepatitis, predominantly in the age group of 18–60 years, with no statistical difference in relation to gender.
ConclusionsThe incidence of HEV infection is probably underdiagnosed, and there is a marked lack of knowledge regarding its epidemiology in Mexico. Further studies should be performed in order to understand the epidemiology, risks factors, pathogenesis and prevention of this disease.
El Virus de Hepatitis E (VHE) es uno de los patógenos identificados que causan hepatitis viral aguda en México. Se ha observado que la presencia de este patógeno particular representa un gran riesgo en mujeres embarazadas y los receptores de trasplante de órganos sólidos. En México no hay informes epidemiológicos formales sobre información acerca del VHE. El objetivo de este estudio es determinar la incidencia de infección por VHE en pacientes Mexicanos con infección viral no identificada, así como proporcionar una perpectiva general de las características epidemiológicas del VHE.
Material y métodosSe seleccionaron un total de 624 muestras de paciente que fueron recogidas desde Enero de 2010 a Junio de 2013 en 32 Estados de la República Mexicana. Los pacientes tenían la sospecha clínica de hepatitis viral y obtuvieron un resultado negativo de infección por VHA, VHB y VHC. Posteriormente las muestras se analizaron mediante una prueba de ELISA.
ResultadosSe determinó una incidencia de 1,76% en la población mexicana analizada con presentación clínica de la hepatitis viral, predominante en el grupo de edad de 18 a 60 años, sin diferencia estadística en relación con género.
ConclusionesLa incidencia de infecciones de HEV está probablemente infradiagnosticada además de que hay una importante falta de conocimiento de la epidemiología en México. Se deben de realizar más estudios para conocer la epidemiología, factores de riesgo, patogénesis y prevención de esta patología.
The first approaches to the Hepatitis E virus (HEV) were made between 1955 and 1956 when an outbreak of viral hepatitis arose in the city of New Delhi, India.1 During the outbreak, physicians observed a similar symptomatology and transmission to that caused by the Hepatitis A virus (HAV), with differences in morphologic and epidemiologic characteristic. During this time, scientist called this virus “no-A, no-B, no-C virus”. With more and new information regarding this new virus, finally scientists called it HEV.1,2 It was classified as a member of the genus Hepevirus and Hepeviridae family, even though it was previously classified as a member of the Caliciviridae family.1,2
HEV is a small, non-enveloped virus, composed only by a single-stranded RNA genome with positive polarity of 7.2kb in length. The virus is approximately 27–34nm in diameter.1,3 The viral genome contains short non-coding regions at both the 5′ and the 3′ ends, and contains three discontinuous and partially overlapping open reading frames. The larges open reading frame (ORF) is known as ORF1 and coded for viral non-structural proteins and contains several conserved domains, which include putative methyltransferase, protease, helicase and RNA-dependent RNA polymerase. The ORF2 codes for the viral capsid protein, and the ORF3 codes for a small phosphoprotein with uncertain function.4,5
The genus Hepevirus consists so far of two species: (1) mammalian HEV, which causes disease in humans and infects other mammalian species, such as pigs; and (2) avian HEV, which is responsible for liver and spleen disease in chicken.1
HEV can be extracted from feces of persons infected with the virus, and is transmitted mainly through the fecal-oral route.1,6 Other routes have also been described. The routes of transmission of HEV infection include (1) fecal-oral transmission due to fecal contamination of drinking water, (2) food-borne transmission from ingestion of products derived from infected animals, (3) zoonotic transmission from animals to humans from exposure to infectious body fluids of infected animals, (4) transfusion of infected blood products, and (5) materno-fetal (vertical) transmission. The transmission through contaminated water is the most common form of transmission. Zoonotic transmission appears to be common in hyperendemic areas.6–8
There are four further classifications of HEV based on its genotype: genotypes 1, 2, 3 and 4. Genotype 1 is found predominantly in Africa and Asia, where the disease is hyperendemic, and is the most frequent cause of acute hepatitis. Genotype 2 has been found in Africa (especially in Nigeria and Chad) and Mexico, where it was firstly reported from an outbreak. Genotype 3 has been found in the United States, where it was firstly isolated. This genotype can also be found in Thailand, Argentina, Mexico, Japan, Australia, Korea, Greece, United Kingdom, France, Spain, Austria, Italy and Netherlands. Genotype 4 has been found in sporadic cases of acute hepatitis E in China, Japan, Taiwan and Vietnam.2,4 Genotype 1 HEV is the predominant cause of sporadic hepatitis and outbreaks of hepatitis E in hyperendemic regions.1,4,9
HEV is a virus that has an incubation period that ranges between three and eight weeks, followed by a prodromal phase, and then there is a phase that depending on the genotype are the clinical manifestations and complications. In this period the virions are excreted through the feces.5–7 HEV enters the host orally. Then, it replicates primarily in the gastrointestinal tract, and travels to the liver though the portal circulation. In the liver, the virus replicates in the cytoplasm of the hepatocytes. After replication, the virus is then secreted into the bile and the blood. From the bile, the virus travels though the gastrointestinal track in the feces.8,9
It known that each HEV genotype has different characteristics. For example, genotypes 1 and 2, also known as the epidemic genotypes, are more frequent in developing countries, and only affects humans. Also its major mode of transmission is fecal–oral and waterborne, having a high rate of icteric illness, being more common in adolescents and young adults with equal rate of infection between genders. These genotypes have a high mortality among pregnant women (between 10 and 30%), few extra hepatic features and do not cause chronic infection. Genotypes 3 and 4, also known as autochthonous, are present in both developing and developed countries, its spread pattern are sporadic, affecting wild boars, deer, pig, horses, rabbits and humans (humans are accidental host).7,8 Its major spread manner is food borne, presenting low rate of icteric illness, being more common among older adults, having higher disease rates among men, and present a spectrum of serious complications that includes liver failure and encephalopathy in patients with preexisting liver disease. Chronic infection is more common in immunosuppressed, and transplanted patients, especially recipients of solid organs.7,9–13 The overall mortality in non-pregnant patients ranges from 0.4 to 4%, as compared to the mortality caused by Hepatitis A virus, which is around 0.2%.14
It has been observed that HEV affects people ranging between 15 and 45 years old, and there is a greater incidence in pregnant women, making a more difficult and dangerous gestation and having severe problems with the course of the infection, compared with non-pregnant individuals.15,16 The clinical presentation of the HEV infection usually includes jaundice, general discomfort, abdominal pain, anorexia, nausea, fever and hepatomegaly, even though there have been reported cases of presentation without jaundice. It has been discovered that HEV infection in most cases is self-limited, having a percentage of mortality less than 0.1%. In severe cases, especially infections in pregnant women, HEV can cause fulminant hepatitis leading to death.2,11 In the case of vertical transmission, the product may have a premature course with a mortality risk of 33% or an increased risk of presenting hepatic damage at birth.13 The reasons for this remains unknown. It is hypothesized that the infection causes toxicity, hypertension, edema and kidney injury to the fetus. Fetuses usually die as a consequence of encephalopathy, hemorrhagic diathesis or kidney disease.17
It has been published that approximately 21% of the population of the United States have been exposed to HEV.11,12,18 In Mexico, between 1986 and 1987 there were two documented outbreaks in populations close to Mexico City, where genotype 2 was isolated.4,7 Nonetheless, there is lack of epidemiologic information of HEV in Mexico. The prevalence of HEV in patients younger than 30 years old in Mexico is 30%, affecting 1.1% of children and 14% of young adults.7,14 In a study performed in the state of Hidalgo, authors detected a prevalence of 6.3%, affecting primarily males older than 50 years old. Another study performed in pregnant patients in the north of Mexico detected a prevalence of 0.4–1.6%. A study detected the presence of antibodies for HEV in 10% of healthy patients and 26% in patients with cirrhosis. 33% of these patients had an unknown etiologic agent.7,14
The diagnosis of hepatitis caused by HEV is mainly by the determination of specific antibodies for HEV, usually IgM and IgG in the serum of the patient. The detection of anti-HEV IgM implies acute infection, and the detection of anti-HEV IgG implies current chronic infection or infection in the past. Another method is the use of polymerase chain reaction, which is a highly specific and sensible method and can find the virus in blood, bile, liver tissue and feces.2,19
The objective of this study is to determine the incidence of HEV infection in Mexican patients with non-identified acute viral infection, and to provide a general perspective of the epidemiological characteristics of HEV.
Materials and methodsWe performed a descriptive randomized study with a longitudinal design from January 2010 to June 2013, in 32 states of Mexico. We selected a total of 624 patient samples from across the country, with the clinical suspicion of viral hepatitis, which were divided in 7 trials. Eligible patients were those who had clinical symptoms compatible with viral hepatitis and obtained a negative result to analysis of Hepatitis A virus (HAV), Hepatitis B virus (HBV) and Hepatitis C virus (HCV). Patients were excluded from the study if the blood samples were found altered, insufficient, hemolized or lipemic.
To analyze the samples, we used the Orgenics InmunoLisa of HEV IgM assay for detection, which is a sandwich-type ELISA, using microplates covered with synthetic HEV antigens derived from Mexico and Burma strains. We used human polyclonal IgM antibodies coupled with peroxidase.
In each ELISA plate, intending to validate the test, we included a blank control, a negative control in duplicate and a positive control, which were read by a spectrophotometer (Reader 250) with a 450nm primary filter reading and a 620nm reference filter. The cutoff value for the samples reading was obtained with the average value of the duplicate of the negative controls plus 0.250. Control and blank values must be less than 0.100 for the test to be validated as a standardized trial, as indicated by the insert. According to the final readings, if the result was <1.0, the sample was considered negative for HEV; if the result was >1.2, the sample was positive for HEV and if the result was between 1.00 and 1.20 it was cataloged as indeterminate. We used the SPSS (IBM) statistics program to analyze the collected data.
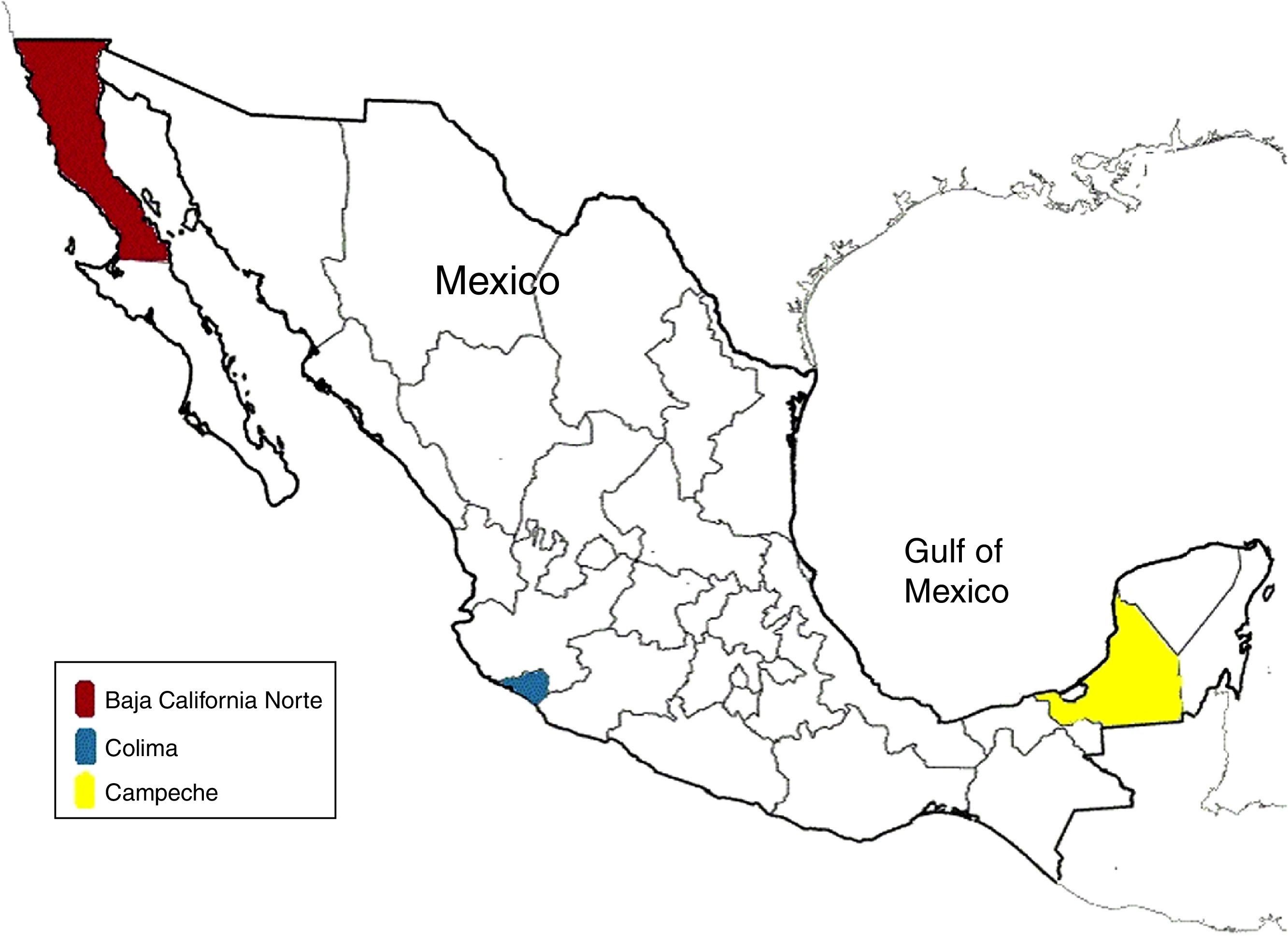
ResultsA total of 624 patients with a no-identified acute viral hepatitis infection were analyzed. Two cases where identified in Baja California Sur, one in Campeche and eight in Colima (Fig. 1). The reported incidence of viral hepatitis caused by HEV in the analyzed population was 1.76%, but only in specific regions. Of these eleven positive HEV results, we found that six of them were men (n=344), and five of them women (n=269). We found an incidence in male population of 1.74%, while we found an incidence of 1.85% in women.
In addition, we found that nine of all of the positive results according to their age were between 18 and 60 years of age, where the incidence in this age group was 2.6%. One of the positive samples was in the group of 12–18 years, which shows an incidence of 1.5% in that age group. The other positive case was in a person >60 years, with represents an incidence of 1.38% in that age group.
DiscussionAccording to our preliminary results, HEV infection is more likely to occur in adults (18–60 years old). The few reported cases are associated with a low incidence of HEV in the Mexican population in patients with a no-identified viral acute infection, although there may be an important number of cases that are asymptomatic, which were not assessed in the analyzed sample.
Based on the results, both sexes have similar rate of infection, 1.85% in women and 1.74% in males. Our incidence rates are lower than those reported in the epidemiology of viral hepatitis in Mexico, where we found a 1.76% incidence in patients in all ages whereas the other study reported a 10.5% incidence in patients who were less than 30 years old.4 Therefore, we could adjudicate the disparity of the results in the methodology and characteristics of the studied population in our study, in addition that we depend on the information collected for other health care professionals. The cases distribution shown in Fig. 1, indicate that despite just in three states the diagnosis of acute hepatitis E infection was made, this is a national spread disease, that could be associated with the coast line and distribution of potable water, in addition to poor water management and hygiene. Thus our reported incidence is low, this does not mean that HEV infection in Mexico is controlled. The results represent that in the Mexican territory, cases of HEV infection are still occurring and that we are susceptible to an outbreak that could have important repercussions, especially in groups such as pregnant woman and immunosuppressed patients.
Health care professionals should be aware of the risk factors associated with HEV infection. In addition, symptoms of acute viral infection in risk population for HEV infection, which includes pregnant woman, should be consider in the differential diagnosis due the high risk of complications therefore we encourage the private and public institutions to be able to perform rapid detect tests for HEV. For this reason, we believe that the HEV should be considered in the differential diagnosis of the susceptible population. Nevertheless, the low incidence reported in this study could be the result that mainly the infection by this pathogen undergoes without clinical manifestations, complications and without the need of medical attention, therefore we could be reporting an underestimation of HEV infection according to the general population.9,14
Currently, there is the development of a safe and effective vaccine against HEV, although the US Food and Drug Administration (FDA) and the Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS) have not approved it yet due the lack of research.15 In the future, this vaccine would be useful in the Mexican population, especially in vulnerable populations including patients with liver disease, pregnant women, children, transplanted and immunosuppressed patients.20
ConclusionsWe believe that the presented results should be taken with caution. Larger studies, especially cohort studies that perform follow-up for a period of years of bigger populations that includes children and older adults should be performed. Probably the low incidence reported in this study is an underestimation of the general population with HEV infection. However, we consider that this study will encourage other research groups to analyze and report their epidemiological experience regarding HEV infection. In addition, further studies should include viral molecular characterization to identify the different HEV genotypes present in distinctive populations, so we can learn about the behavior of the virus around the world and in Mexico.
Finally, medical professionals should pay special attention to vulnerable groups, especially pregnant patients and patients who have received a solid organ transplant, in order to decrease the morbidity and mortality associated with HEV.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interests.
Financial supportNo funding was received for this study.