Syphilis is a systemic disease caused by the spirochaete Treponema pallidum that affects the central nervous system at any time and whose clinical presentation has undergone changes in recent decades, due to the emergence of the acquired immune deficiency virus. We present the case of a 50-year-old immunocompetent woman with no significant changes in sexual behaviour, who only presented with headache and speech disturbances (mixed aphasia). MRI and CT scans initially showed left parietal injury, and later left temporal recurrence. The patient was treated for neurosyphilis for 5 weeks and showed improvement at her one-month follow-up appointment, before once again manifesting speech disturbances with sensory aphasia six months after treatment onset. Another control MRI was performed, revealing a relapse of the tumour lesion in the left temporal region. Intravenous treatment was once again initiated with benzathine penicillin and new serological and imaging tests were conducted, revealing the absence of lesions. Gummatous neurosyphilis is a rare condition, which explains why it tends to be erroneously diagnosed and treated. It is for this reason that we have presented our case study and literature review.
La sífilis es una enfermedad sistémica causada por la espiroqueta Treponema pallidum que compromete al sistema nervioso central en cualquier etapa y cuyas presentaciones clínicas se modificaron en las últimas décadas, debido a la aparición del virus de la inmunodeficiencia adquirida. Se presenta el caso de una mujer de 50 años sin alteraciones de importancia, en la conducta sexual, e inmunocompetente. Presenta solo cefalea y alteraciones del habla (afasia mixta). Cuenta con imágenes de RMN y TAC demostrando lesión parietal izquierda en primera instancia, y posteriormente recidiva en temporal izquierdo. Recibe tratamiento para neurosífilis por 5 semanas, y teniendo mejoría al mes de control, pero presentando nuevamente a los 6 meses del tratamiento, alteraciones del habla, con afasia sensitiva. Se realiza nueva RMN de control y se observa recidiva de lesión tumoral en región temporal izquierda. Se inicia nuevamente tratamiento endovenoso con penicilina benzatinica, y se realizan nuevos controles, serológicos y de imagen, encontrándose la ausencia de las lesiones. La neurosífilis gomatosa es una presentación infrecuente, por lo que la mayoría de las veces se llega a un diagnóstico y tratamiento erróneo, motivo por el cual reportamos nuestro caso y realizamos revisión de la bibliografía.
Syphilis is one of the many types of sexually-transmitted diseases. It is a systemic infectious disease caused by a spirochaete, Treponema pallidum. It can affect most organs. The most common form of transmission is through sexual relations, but in some cases vertical transmission occurs, from the mother to the foetus.1
In 2008, the World Health Organisation (WHO) reported an incidence of 10.6million and a prevalence of 36.4million, with more than 90% of cases occurring in developing countries. In America, an incidence of 2.8million and prevalence of 6.7million is estimated, and it is therefore considered to be a public health problem.2
It is a rare brain finding observed during tertiary syphilis that can sometimes simulate different neoplastic brain lesions, confusing diagnosis and treatment.
In this article we present the case of a 50-year-old patient and we provide a literature review.
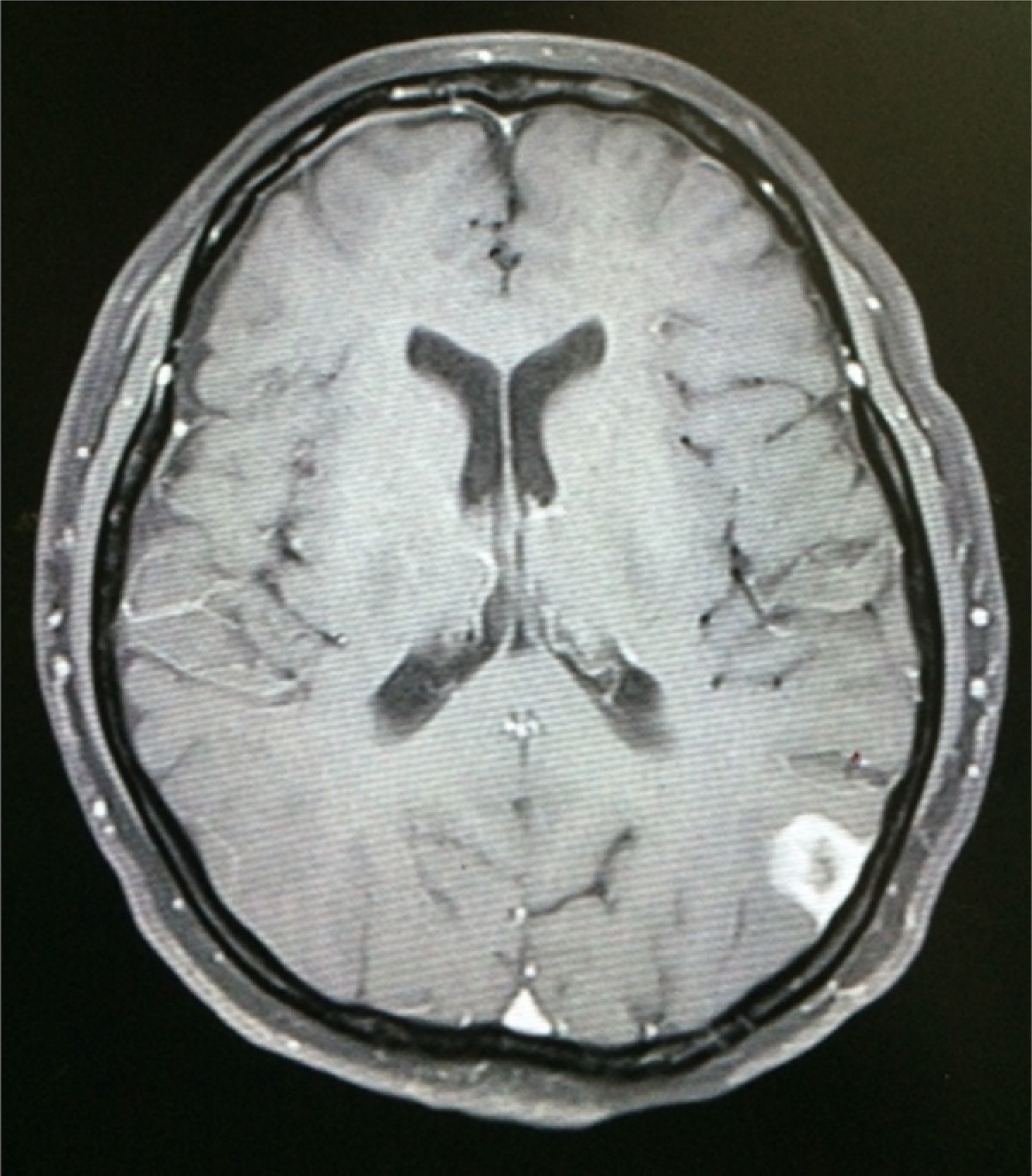
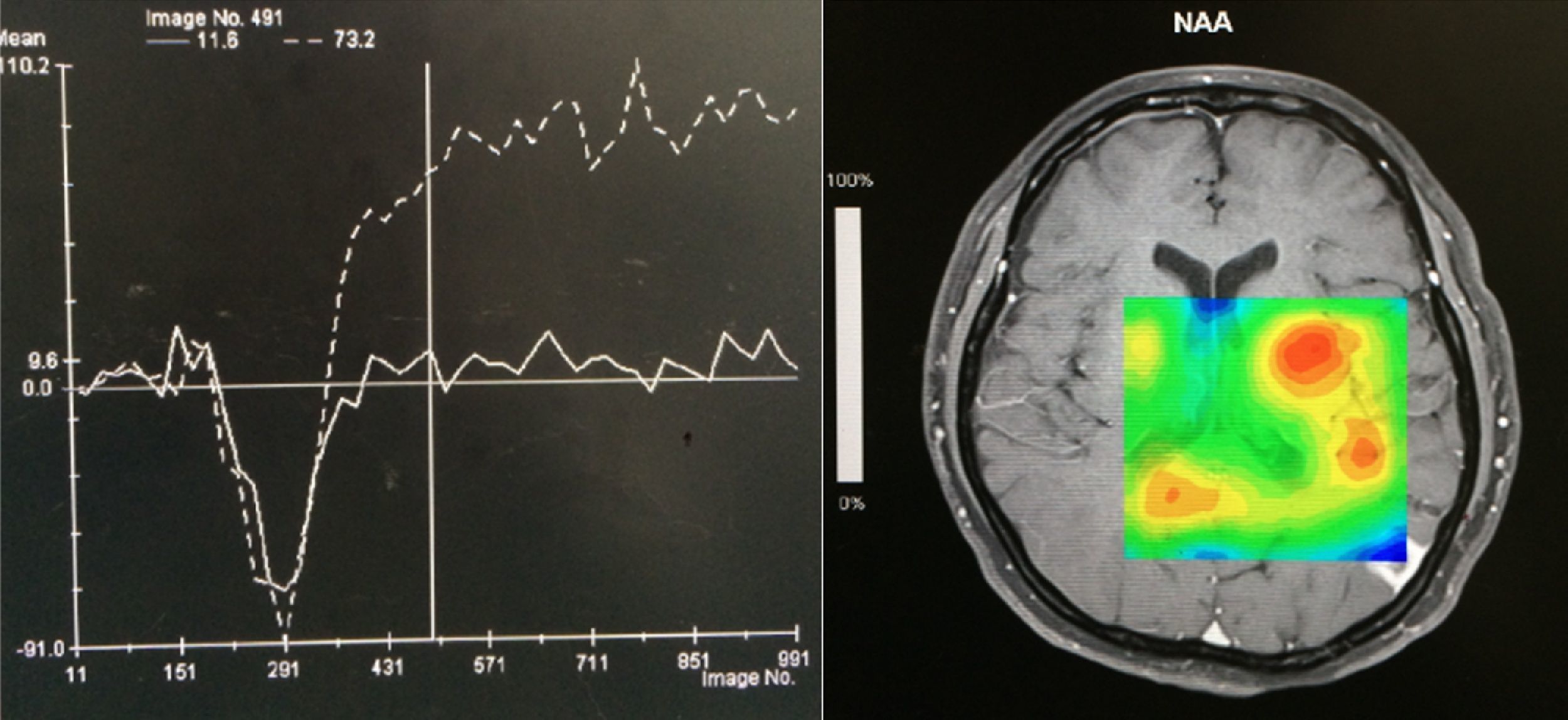
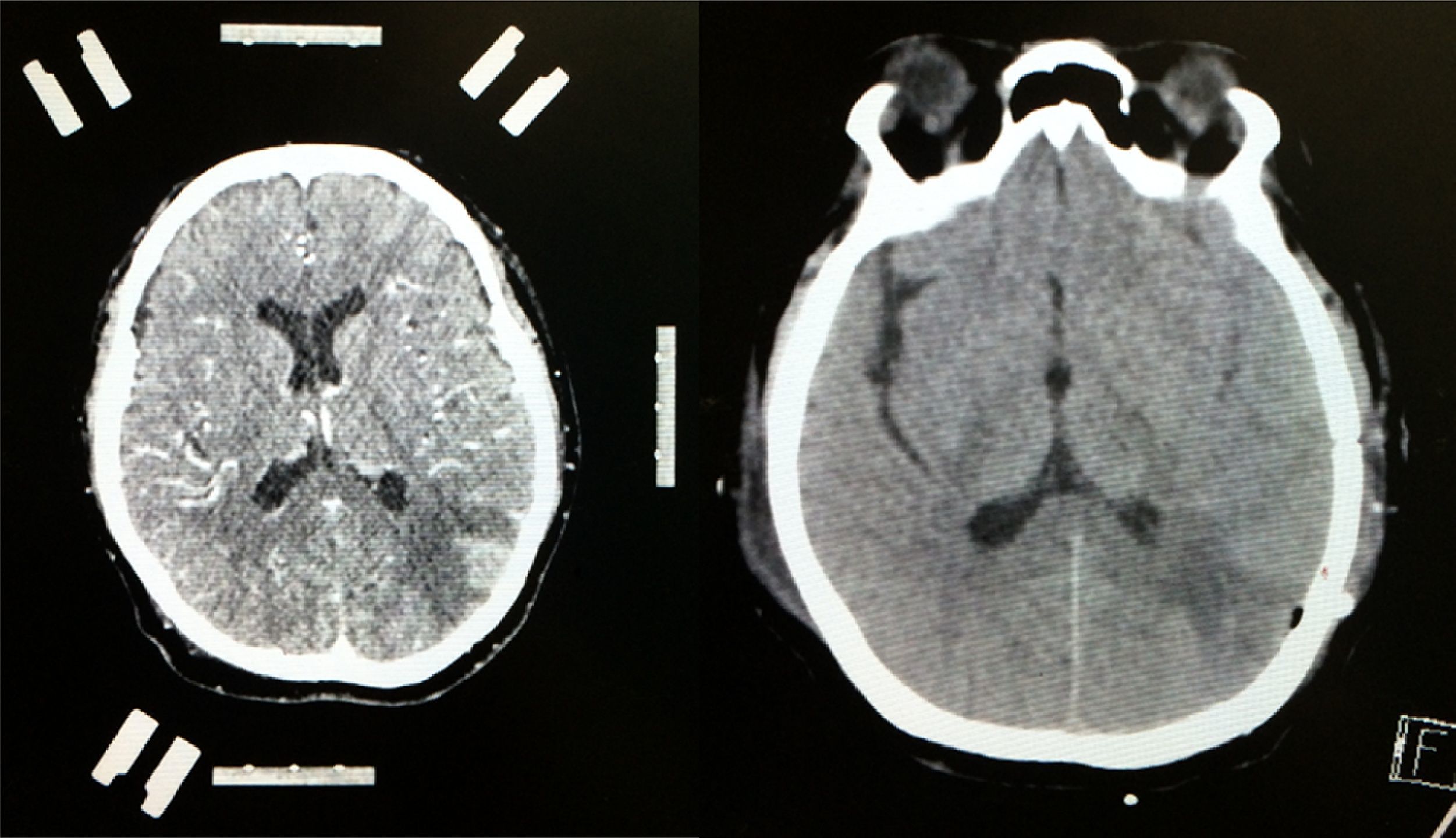
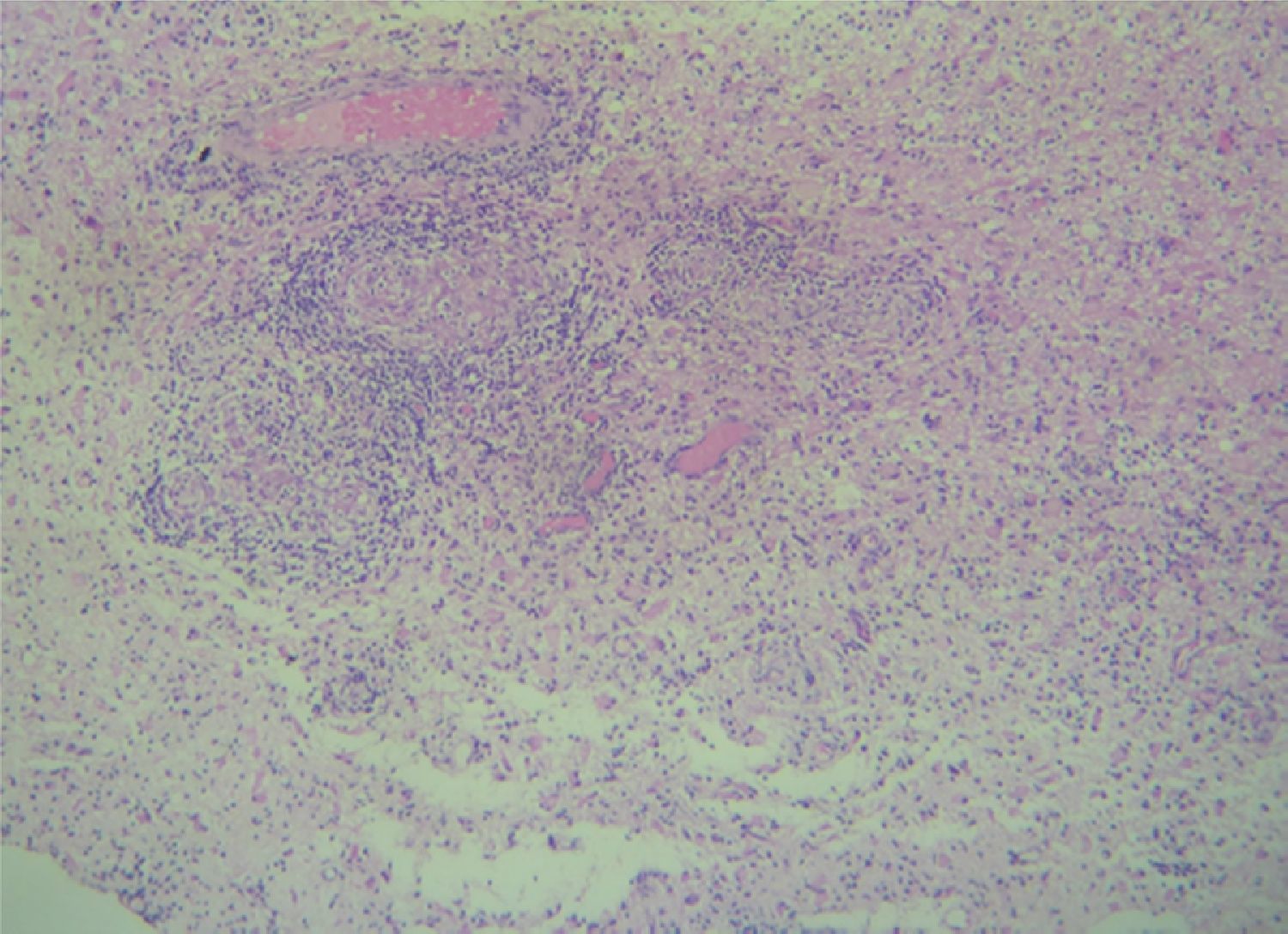
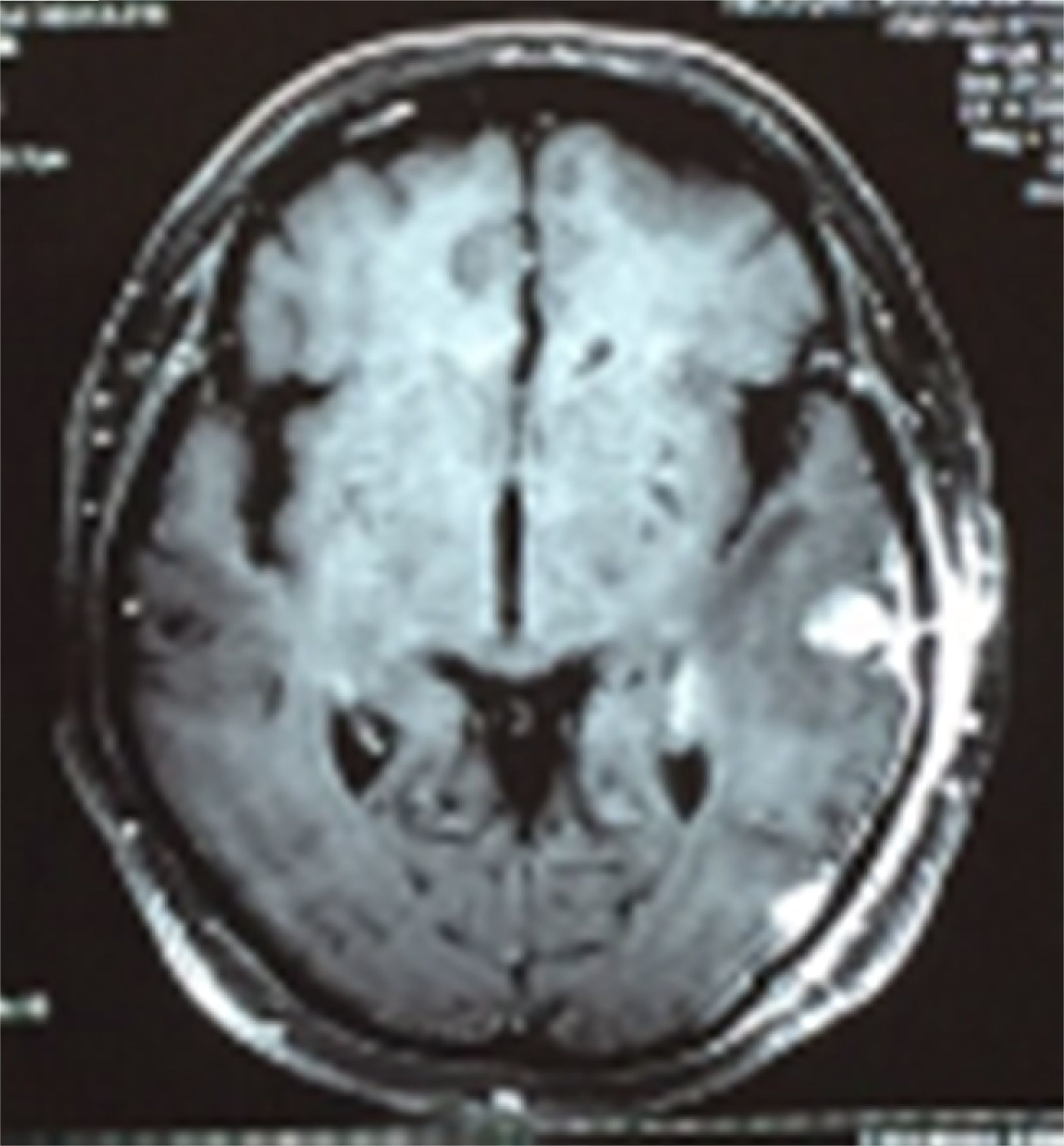
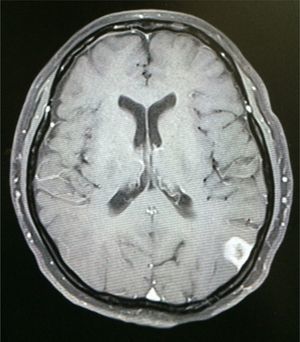
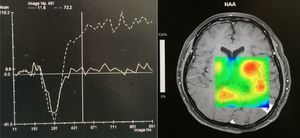
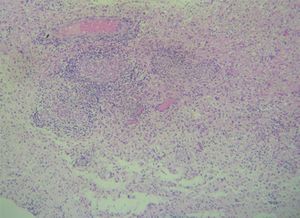
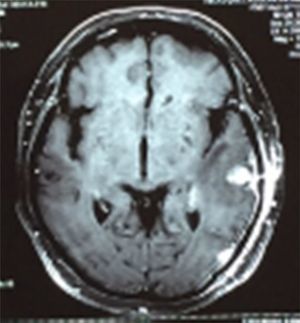
Case reportA 50-year-old female patient with a history of controlled systemic arterial hypertension and poor diet, with no history of sexual risk behaviour. She attended our offices due to alterations in language, mixed aphasia and moderate holocranial headache, with no attenuating or exacerbating factors, no predominance at a certain time of day, and subsiding spontaneously. She referred one episode of seizures without specifying the characteristics, but they were apparently tonic-clonic. Neurological examination revealed mixed aphasia subsiding with the administration of steroids. No visual alterations were found during dilated fundus examination, electronic visual field or confrontation examination, and the rest of the cranial nerves were normal. The complete blood count revealed a WBC count of 13,300cells/mm3: segmented: 75%, lymphocytes: 17%, monocytes: 5%, eosinophils: 0.5%. Magnetic resonance imaging (MRI) of the brain revealed a tumour lesion measuring 19mm×17mm×15mm in the left parietal lobe with a ring-like, popcorn-like contrast medium enhancement pattern, cortical, regular borders and hypointense areas inside the lesion (Fig. 1). Given the suspicion of tumour lesion, she was treated with steroids and experienced clinical improvement. FLAIR sequence showed perilesional oedema extending towards the left temporal lobe. Diffusion-weighted imaging showed areas of necrosis and haemorrhage, simulating a lesion with a probable neoplastic process with malignant characteristics, compatible with high-grade astrocytoma, but spectroscopy showed high levels of lactate and lipids (Fig. 2). According to protocol, the patient underwent stereotactically-guided total resection of the tumour lesion (Fig. 3). As it was located in an eloquent area, the patient remained conscious during the intraoperative period to ensure the integrity of her language function. The entire lesion was resected; it was whitish with defined borders and implanted in the dura mater, from where it was removed. There was considerable gliosis and vessels adjacent to the tumour lesion. An intraoperative histopathological study reported areas of necrosis and gliosis, abundant chronic and acute inflammatory infiltrate, and numerous plasma cells; no spirochaetes were observed directly. Given the suspicion of neurosyphilis and the definitive study by anatomical pathology, a Warthin–Starry stain immunohistochemical analysis was performed and the result was positive, detecting spirochaetes (Fig. 4). The study protocol was completed with serum RPR: 32 dil, serum VDRL: 32 dil, FTA-ABS: 3+. The rest of the laboratory tests (liver profile, coagulation profile, glucose, urea, creatinine and urine test) and serological tests (HIV, Brucella, Salmonella and hepatitis B) were normal. She was started on antibiotic treatment with 2,400,000 units of IM benzathine penicillin for 5 weeks, low doses of steroids and management of nutritional support; she was discharged from the hospital 7 days after the surgical procedure. At her one-month follow-up appointment, there were no language alterations and expectant management was maintained. After 6 months, the patient presented with a new language disturbance, articulating words but unable to follow orders. The MRI revealed a new left temporal tumour lesion compatible with sensory aphasia (Fig. 5). There were no other abnormalities during examination. Serum RPR showed 16 dil; no control serum treponemal tests were conducted. There were no other blood biometry alterations. Due to new clinical evidence and imaging techniques confirming neurosyphilis relapse, management was restarted with 4million units of penicillin G sodium IV every 4h, which was prolonged for 4 weeks due to a notable reduction in the lesion found on the control MRI. To prevent Jarisch–Herxheimer reaction, dexamethasone was administered every 8h, initially at 4mg and then at a reduced dose. When treatment was complete, the syphilis serological tests showed a decrease in titres (RPR: 4dl and VDRL: 6dl); CSF study normal. Six months after the second treatment regimen, the patient presented no abnormalities, with fluid language and no evidence of relapsing tumour lesions.
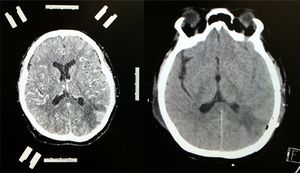
Contrast-enhanced CT scan of the left side of the brain showing stereotactic frame and fiducials; contrast medium enhancement of left parietal region showing tumour lesion with considerable perilesional oedema. Follow-up non-contrast CT scan of the right side of the brain showing total resection of the lesion with hypodense image denoting gliosis in left parietal lobe.
Neurosyphilis is a central nervous system (CNS) condition caused by T. pallidum. It includes cases with clinical manifestations (symptomatic neurosyphilis) and those cases in which no symptoms occur (asymptomatic neurosyphilis). It only presents in the tertiary phase (tabes dorsalis and progressive general paralysis).
The first descriptions of the disease date back to the end of the 15th century.3 The primary mechanism by which T. pallidum is spread is through sexual relations. Non-sexual, direct and indirect transmission are possible through primary or secondary lesions. Other less common routes of transmission are through transfusions, bites or cuts, and through transplacental route.
When T. pallidum enters the body by one of the forms of infection, and after a 10–90 day incubation period, primary syphilis is developed, called primary chancre, which consists of a reddish ulceration with indurated borders and adenopathies in the regional lymph nodes. Bacteriaemia may occur, which is often subclinical or has non-specific symptoms.4
The first stage ends after 6–12 weeks when Treponema spreads into the blood, causing lymphadenopathies and generalised exanthema. This second stage resolves spontaneously after four weeks.5 Then T. pallidum invades the CNS in up to 70% of untreated patients. Afterwards there is a latent period called latent syphilis. This period is divided into two phases by the International Classification of Diseases: early latent syphilis (under two years duration) and late latent syphilis (greater than two years of duration).4 After this phase it can regress to the second phase, or continue its progression to the third phase, affecting the cardiovascular system and CNS.
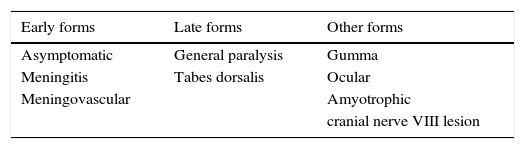
Clinically, there are diverse forms of clinical presentation, including tabes dorsalis, as well as other neurological diseases (vascular or meningeal syphilis).4 The different forms of presentation are presented in Table 1.
Up to 40% of patients with asymptomatic neurosyphilis present alterations in CSF (cerebrospinal fluid), including pleocytosis and increased CSF protein levels. It tends to disappear spontaneously, although CSF abnormalities persist.6,7
Meningeal neurosyphilis is the earliest, under 1 year, and is generally associated with skin lesions. It can present with intracranial hypertension, due to hydrocephalus, or focal signs due to acute meningitis of the vertex (paresis, paralysis, seizures, etc.). The last presentation with acute basilar meningitis specifically affects the cranial nerves VII and VIII, followed by II and III.8,9
Meningovascular neurosyphilis is the most common form of presentation, which may occur after up to 12 years of infection. It has a prodromal period lasting weeks or months, with headache, vertigo, personality changes and insomnia. Then vascular symptoms occur, similar to other cerebrovascular conditions, except that it affects patients under 50 years of age. The region most affected is the MCA (middle cerebral artery).6–9
In this condition, progressive general paralysis used to be the main reason for admission to psychiatric institutions, but this is now uncommon. It occurs 15–20 years after infection. It is insidious in nature that generally starts with non-specific psychiatric symptoms, irritability, difficulty concentrating, emotional alteration and delirious ideas. The final phases include dementia, tremor, dysarthria, seizures, paresis and alteration in sphincter control.7,9
Tabes dorsalis is the most delayed form of presentation, occurring 18–25 years after the first infection. There are clinical manifestations of episodes of shooting pain in the lower limbs, abdominal pain, paraesthesia, hypoaesthesia and alterations in tendon reflexes. Ataxia and urinary incontinence may also occur. There is absence of pleocytosis in the CSF and negative VDRL (venereal disease research laboratory) serology.6–9
CNS gumma forms a granulomatous nodule. It may present at any stage (generally stage III) and appear in any part of the body. In the CNS, gummas form from the pia mater; macroscopically they are observed as soft, well-defined lesions of varying size. Microscopically, they appear as a non-specific, chronic inflammatory infiltrate composed of lymphocytes and plasma cells. T. pallidum can be observed using immunofluorescence or silver staining. The manifestations presented will depend on the size and location of the lesion (medullary or brain).7,9
Ocular syphilis may also occur, manifesting with uveitis, keratitis or chorioretinitis. Argyll Robertson pupil or anisocoria often present. Luetic optic neuritis is associated with meningitis, and peripapillary atrophy occurs as a result. Syphilitic amyotrophy is a hypertrophic pachymeningitis that frequently presents in the (cervical) medulla. It presents with extrinsic compression, radicular involvement, atrophy of the upper extremities, pyramidal syndrome and posterior cord involvement. The other form is involvement of cranial nerve VIII, due to basilar meningitis, which presents cochlear and vestibular damage, previously a frequent cause of Ménière's disease.5,7–9
The different forms of clinical presentation are listed in Table 1. They are divided into early forms, which include meningeal and vascular involvement, and late forms, which include brain parenchyma involvement (progressive general paralysis and tabes dorsalis). The time to onset of these clinical forms depends on the time of primary infection. The disease may present in many clinical forms, manifesting complexly, and the late presentations may occur earlier, or occur simultaneously. These presentations are explained by increases in HIV (human immunodeficiency virus) patients and due to the inadequate use of antibiotics prophylactically and in treatment.6–10
Diagnosis is made according to clinical manifestations, accompanied by abnormal biochemical findings and CSF serology tests. CT scan and MRI are only useful in diagnosing cases of gumma or meningovascular forms.11,12
Studying CSF in cell counts shows mild lymphocytic pleocytosis (5–100cells/field) except in meningitis, in which it may be higher, and in tabes dorsalis, in which it tends to be normal in most cases. Glycorrhachia is normal, except in meningitis where it is decreased. Protein levels are moderately increased (45–200mg/dl) in all forms except in tabes dorsalis, in which they are often normal. Moreover, there may be oligoclonal bands and immunoglobulins (Ig) with increased Ig/albumin ratios.12,13
The serological test that confirms the disease continues to be rabbit testicular inoculation, capable of detecting up to 10 Treponema per inoculate, but this is not carried out in daily practice. The main diagnostic method continues to be antibody detection tests in blood and CSF. There are two types: reagin tests, which are non-specific, show the presence of anti-lipid antibodies; while specific treponemal tests detect antibodies to Treponema. Rapid plasma reagin (RPR) and VDRL tests are used most frequently, and less often tetrahydropteric acid (THPA) levels and the fluorescent treponemal antibody (FTA) detection test or the fluorescent treponemal antibody absorption (FTA-Abs) test.14,15
In early syphilis, FTA is the first test to be positive; in secondary forms all the tests show strong positivity. In the latent and tertiary forms, 70% of reagin tests are positive and 80–100% of treponemal tests are positive, especially FTA-Abs.11–13
Thus a negative FTA test excludes the diagnosis of neurosyphilis and positive FTA requires lumbar puncture to rule out T. pallidum CNS involvement. There may be false positives in dilutions less than 1/8 and in diseases in which there are alterations in immunoglobulin serum levels, such as rheumatoid arthritis, haemolytic anaemia or cirrhosis of the liver, and in pregnancy.11
In CSF, the VDRL test is very specific for the diagnosis of neurosyphilis, with a value close to 100%, but it has a very low sensitivity. This makes it the test of choice for the diagnosis of neurosyphilis, although due to its low sensitivity a negative result does not exclude the disease. FTA-Abs in CSF is much more sensitive than VDRL, but there are many false positives due to the passing of antibodies through the blood–brain barrier; THPA was created to prevent this. This assay detects the intrathecal production of Treponema. A result of 70–500 suggests probable active neurosyphilis and a figure greater than 500 is a confirmed diagnosis. Several more accurate diagnostic methods are being created, such as polymerase chain reaction (PCR).11,13,14
Due to the varying manifestations and results in serological studies, there are two criteria to diagnose the disease. For defined neurosyphilis: positive treponemal tests in blood, positive VDRL in CSF. For probable neurosyphilis: positive treponemal tests in blood and in CSF, presence in CSF >5 leukocytes/mm3 or >45mg/dl of proteins.12,13
Imaging techniques such as CT scan and MRI of the brain are very useful in the diagnosis of this pathological condition. In gummatous neurosyphilis, singular or multiple nodular lesions present around the meninges, which are associated with nearby oedema. On MRI, T1- and T2-weighted sequences show hypointense and hyperintense images respectively, with ring-like reinforcement, heterogeneous characteristics and defined borders. On most occasions, they are found cortically and near the brain meninges, as mentioned above. Spectroscopy is quite helpful as it guides us to rule out tumour processes from infectious processes; in neurosyphilis, lactate and lipids are elevated. Differential diagnosis must consider conditions such as lymphoma, toxoplasmosis, tuberculosis, metastasis, meningioma or glioblastoma.16–19
The anatomical pathology study will show a granulomatous process that may be accompanied by abundant plasma cells, lymphocytes, epithelioid cells and fibroblasts, with central gummatous necrosis, eosinophils and spirochaetes.18,19
It is always recommended to start treatment in patients with confirmed neurosyphilis or those in whom there is a probability of presenting the condition. The object of treatment is to obtain adequate treponemal levels to thereby eliminate this disease from the patient. The form of presentation, dose and duration of treatment may vary in patients with immunodeficiencies, and even more in HIV patients.13 HIV patients are more likely to present the early forms, such as acute syphilitic meningitis and meningovascular syphilis.16
The treatment of choice in patients with symptomatic neurosyphilis and HIV infection is crystalline penicillin G at a dose of 12–24million units daily for a minimum of 14 days. Some authors recommend completing treatment with intramuscular benzathine benzylpenicillin. Jarisch–Herxheimer reaction, an acute systemic manifestation that occurs in the first few hours and which is more common in the early stages of syphilis, may present during treatment. There must be a resolution of abnormalities in the CSF, WBC count and protein levels; this is the best method for determining treatment efficacy and these determinations should be performed at the end of treatment and every 6 months in the following 2–3 years. Normal WBC count will be obtained within one year, and protein levels will return to normal within 2 years. CSF-VDRL should simultaneously disappear together with serological dilutions.15,16,19
ConclusionsThanks to the widespread use of penicillin, gummatous neurosyphilis is currently very rare. Therefore, its clinical presentation and imaging techniques are confused with other conditions, such as neoproliferative disorders and neoplasms. Our case report emphasises that cerebral syphilitic gumma must be recognised in non-immunocompromised or non-HIV patients with reactive syphilis serology, but normal VDRL treponemal tests (THPA and FTA-Abs) and WBC count in CSF. We were able to use imaging techniques such as MRI and, especially, spectroscopy sequence to differentiate neoplastic lesions from infectious lesions, helping us to correctly and appropriately treat the condition. Penicillin is the first-line antibiotic of choice, with good clinical, serological and imaging results, and penicillin derivatives may be used in case of allergic reactions.
Conflict of interestThe authors declare that they have no conflict of interests.