In recent decades there has been a very significant increase in obesity in most developing countries. In addition to environmental, genetic and hormonal factors, nutritional and maternal environment factors influencing critical periods of foetal development have acquired increasing significance since the thrifty phenotype theory was described by Harles and Barker and epidemiological studies demonstrated that perinatal conditions may modify individuals’ future metabolic responses via genomic reprogramming. Perinatal programming corresponds to a critical and accelerated period of developmental plasticity from preconception through early postnatal life. This characteristic may also have a long-term influence on metabolic health and obesity. Epigenetic modifications favour the survival of the individual in critical periods when nutritional restriction is established, but exerts long-term risks, as metabolic programming tracks into infancy and adulthood and induces fat mass accumulation, particularly if energy consumption is exceeded. Although the mechanisms are not yet fully understood, it is evident that hormonal factors such as insulin and leptin may influence the programming of hypothalamic circuits for energy balance regulation. Nutritional interventions in animal models at critical stages of development have demonstrated that microenvironmental modifications might induce a permanent modulation of the progeny genome expression via epigenetic mechanisms. A transgenerational transmission of obesity has been proposed.
En las últimas décadas se ha presentado un incremento muy importante de obesidad en la mayor parte de los países desarrollados. Además de los factores ambientales, genéticos y hormonales, existen otros factores a considerar y que han cobrado una mayor relevancia desde la teoría del fenotipo ahorrador propuesta por Harles y Barker, como son los factores nutricionales y matroambientales, que influyen en períodos críticos del desarrollo como la etapa fetal, modificando de esta forma la respuesta metabólica futura del individuo por reprogramación genómica. La programación perinatal corresponde a un período de plasticidad del desarrollo que va desde la etapa preconcepcional hasta la vida posnatal temprana. Esta característica también lo hace un período altamente susceptible a una programación anómala. Las modificaciones epigenéticas favorecen la sobrevida del individuo en períodos críticos, sin embargo, también predispone a cambios metabólicos adversos a lo largo de la vida, expresándose en enfermedades crónicas, como es el caso de la obesidad y sus múltiples comorbilidades. Si bien los mecanismos exactos no son conocidos hasta el momento, se le ha adjudicado participación a factores hormonales como insulina y leptina en la programación hipotalámica de los circuitos de la regulación del balance energético. Intervenciones nutricionales en modelos animales en etapas críticas del desarrollo ha permitido observar que existe la capacidad de modular la expresión del genoma de forma permanente en los descendientes mediante mecanismos epigenéticos, lo cual sugiere fuertemente que existe una transmisión transgeneracional de la obesidad.
In the past three decades, the prevalence of being overweight and obese has increased significantly in most developed countries becoming a serious public health issue not only in the adult population, but also progressively and alarmingly from the earliest stages of childhood, along with the associated comorbidities that comprise metabolic syndrome. Among the most important factors giving rise to the development of obesity are genetic and hormonal factors and metabolic influence. Today foetal environment and neonatal nutrition factors are assuming even greater relevance.1,2
Clinical and epidemiological studies have gathered contemporary scientific evidence that enables us to demonstrate that changes in nutrition in both foetal and neonatal life, either due to maternal malnutrition, obesity, gestational diabetes, or accelerated catch-up growth, are associated with a higher presence of adipose fat in adulthood. There have been attempts to explain this phenomenon from several perspectives, mostly using perinatal nutrition as a predisposing factor for early metabolic programming without yet being certain of this knowledge.
Since 1992 the primary theory related to metabolic programming was proposed by Hales and Barker, better known as the “thrifty phenotype” theory, which explained how malnutrition in utero could result in changes in foetal development with the purpose of adapting and surviving in an environment lacking in nutrition. There are other theories which suggest that there are several developmental advantages with which the individuals may have a higher likelihood of changing their metabolic programming, as with the theory by Singhal and Lucas, who propose that accelerated postnatal catch-up growth may be even more important, mainly in newborns who are small for their gestational age and who in a specific period of time had a higher risk of long-term metabolic complications.
Other theories have been derived from these two main hypotheses, even fusing the two, including those that essentially propose that a poor combination of adverse prenatal nutrition and normal nutrition or overnutrition in the postnatal period may have even larger adverse effects on metabolic programming and its diverse comorbidities during adulthood.3
During foetal development there are several specific windows of sensitivity in which environmental factors have a large influence. Based on this, the foetus has the ability to adapt to adverse environmental factors by optimising the future metabolic responses by reprogramming its genome. This reprogramming favours early survival, but causes a predisposition towards chronic diseases in later stages of life.2 Being overweight during critical periods of developing during foetal or neonatal life has been demonstrated to increase the risk of obesity during childhood or adulthood. Although not all the mechanisms are fully understood, circulating hormones such as insulin and leptin play a critical role in developing and programming the hypothalamic circuits that regulate the energy balance.1 Malnutrition during critical periods of development, both in utero as well as postnatal, have been associated as a risk factor for obesity and other metabolic diseases throughout life, possibly affecting the neuron circuits responsible for regulating the energy balance.
There is increasing evidence that suggests that the perinatal environment may be critical in the development of the central nervous system and its mechanisms for regulating the energy balance and metabolism. Both genetic and environmental factors in postnatal life enable body composition regulation to be determined throughout life, and maternal and paternal nutrition and gestational health participate in a very decisive way.3
The nutrition interventions that have mostly been observed in animal models in different critical stages of development demonstrate how nutrition in the pregnant mother has the ability to permanently modulate the expression of different genetic profiles in their descendants through epigenetic mechanisms, through DNA methylation, histone modifications, or non-coding RNA modifications. Therefore, as we might imagine, these epigenetic processes can have very important implications, which for our current topic may increase the risk of being obese or presenting other comorbidities.4
The obesity phenotype has increased significantly in the past 2 generations (15–35.7%), which suggests that environmental or epigenetic factors beyond genetic factors play a role in the obesity epidemic, meaning there may be a transgenerational transmission of obesity as the result of changes to the epigenome.5
Changes in metabolic programming influence the structure of organs, cell responses, and genetic expression that have an impact on both metabolism and its physiology. The changes can be immediate or life-long and vary in the different cells and the different periods of life.5,6
Epigenetic changes include DNA methylation modifications, covalent histone modifications, and covalent microRNA expression modifications. Epigenetic regulation of genetic transcription mostly happens through DNA methylation, which is highly dynamic during embryogenesis. In postnatal and adult life, DNA methylation is susceptible to intrinsic and extrinsic factors.5
Abnormal DNA methylation is associated with inappropriate gene silencing, with changes in epigenetic markers that are associated with several human diseases.
The epigenome is highly dynamic and changes in response to the availability of nutrients, physical activities, and even paternal characteristics. Associations between maternal weight gain and gestational diabetes and methylation of the leptin gene in the placenta and umbilical cord blood have already been described. In addition, increased carbohydrates in the mother during the first months of gestation have been associated with the methylation of genes related to adipogenesis. An association between epigenetic changes and programming to develop obesity have been demonstrated in studies on rats.6,7
These epigenetic changes have been detected in factors regulating growth, adipogenesis, brain circuits controlling appetite, satiety, and rewards, and glucose homeostasis.6–8
The objective of this review is to demonstrate that the metabolic plasticity that living beings have to anticipate environmental conditions such as malnutrition (food amount and quality) can supply the underlying pathophysiological mechanisms of diverse chronic diseases such as obesity and its comorbidities, where it is increasingly evident that the factor involved and the period of development may provide a higher or lower risk of affecting the individual in a different way.
DefinitionA quarter century ago, Barker and Osmond from the University of Southampton, England, proposed the concept of foetal programming by associating the presence of chronic disease in young adults in England and Wales during the second world war in whom an increased incidence and prevalence of cardiovascular disease had been observed, using a common history of a low birth weight, and suggesting that this was a manifestation of intrauterine stress and a possible risk factor for these comorbidities.9
Barker and Hales proposed the “thrifty phenotype” hypothesis. They suggested that foetuses exposed to suboptimal conditions in utero suffer a programming of the developmental process in response to adverse intrauterine conditions such that it enabled them to adapt and survive, in addition to anticipating possible suboptimal conditions in postnatal life. If, contrary to what was expected, the postnatal conditions are optimal and resources abundant, the body prepared for an adverse environment and scarce nutrients (as a foetus) is confronted with a different environment for which it is not adapted, and therefore, by encountering an incongruence in its programming is more susceptible to developing chronic diseases.10
The concept of foetal programming, which was recognised as a period of developmental plasticity, exists in other critical periods of development that go beyond just life as a foetus, such as the preconception period and early postnatal life.
Prenatal nutritionMaternal malnutritionThere are three main models of maternal malnutrition that essentially result in low birth weights, and this may be due to the fact that the mother has been subjected to a low protein diet, general food restriction with a decrease in daily calorie requirements, and uterine artery ligature (which simulates uteroplacental insufficiency in pregnant women).
Studies conducted during the Dutch famine are particularly illustrative for assessing stress in utero. During the four months the famine lasted (December 1944–April 1945), the daily ration given to individuals was from 400 to 800 calories. Observations revealed that the malnourished foetuses had an atherogenic lipid profile, increased cardiovascular risk, and decreased cognitive function. Glucose tolerance was altered in all the foetuses exposed to the famine, but it was particularly evident in those who were at the end of gestation.11–13 Studies resulting from this historic event demonstrated that the risk of obesity at 19 years of age in children of mothers in the first half of pregnancy, exposed in the winter during the Dutch famine, demonstrated a significantly higher risk than unexposed foetuses; whereas those who were exposed to the famine during the third trimester and early postnatal period showed a lower risk of obesity.14
Stress has the ability to modify the energy balance, availability of nutrients, and cell signalling pathways. These pathways are also modified in metabolic syndrome and in diabetes mellitus. Maternal malnutrition can increase retroperitoneal fat and increase the proportion of large adipocytes in the visceral fat. In protein-restricted rats, insulin-mediated glucose uptake and insulin-dependent lipolysis are reduced in adipocytes, indicating insulin resistance.11
Accelerated weight gain in the postnatal period is not only a risk factor for obesity in premature individuals or those with a low birth weight, it is also a risk factor for healthy weight newborns.12
There are numerous changes that can occur due to malnutrition in utero and which can contribute to the presence of an obesity phenotype in adulthood. In female animals, particularly those with a relevant prior history of having been born from malnourished mothers, the development of hyperphagia and a predilection towards high-fat foods has been observed. Both male and female animals from protein-restricted mothers during pregnancy usually show a behaviour with a preference for high-fat foods versus control group animals, in addition to showing a considerable decrease in physical activity.15
Maternal malnutrition accompanied by postnatal overnutrition predisposes the offspring to an increased expression of molecules that positively regulate the appetite, such as neuropeptide Y (NPY) and agouti-related protein, and a decreased expression of molecules related with satiety and increased energy consumption such as proopiomelanocortin.16 These alterations can induce changes in body composition, causing an increase in the waist-to-hip ratio for a certain body mass index (BMI) and increased visceral fat, as has been described in studies conducted in humans. Animal studies showed that a maternal low-protein diet during pregnancy and breastfeeding was associated with an increase in adipose tissue size of their offspring. Analysis of adipose tissue in adult rats makes it possible to observe that there is a high expression of the PPAR γ and C/EPBα genes, which translated into an increased preadipocyte proliferation rate, which in adults also presents decreased miRNA-483-3p expression, which promotes adipocyte hypertrophy.17,18
Maternal malnutrition can also influence the development of the musculoskeletal system as well as its metabolic capacities, where, in particular, alterations in GLUT-4-mediated glucose transport and insulin resistance have been observed.19,20
A clinical trial was conducted in Guatemala where the individuals recruited were assigned to a protein-rich supplement or a protein-free supplement during pregnancy, while breastfeeding, and in early infancy. The subjects assigned the protein-rich supplement gave birth to babies who weighed more, grew taller, and had lower serum glucose levels than the other group.21,22
In studies conducted in animal models of malnutrition gestations, the rats offsprings presented with a low-grade inflammation associated with obesity, characterised by elevation of certain inflammatory factors in plasma such as tumour necrosis factor alpha (TNFa), interleukin 6 (IL6), as well as the expression of proinflammatory mediators in white fat tissue. A study conducted in rodents demonstrated how even ensuring an isocaloric diet with 40% less protein per kilogram of bodyweight made the offspring gain 35% less bodyweight than expected, as well as proportionally smaller organs, as was the case with the pancreas, liver, muscle, and spleen. Nevertheless, certain organs such as the brain were volumetrically unaffected in appearance. In particular, unbalanced or insufficient perinatal nutrition can induce epigenetic changes in the genome of the descendants, which results in long-term changes in the transcriptional control of adipogenesis and/or inflammatory processes.23
Maternal overnutritionAlthough there is a paternal epigenetic influence on the bodyweight and metabolic function of descendants, the maternal influence has a much more significant association with both birth weight as well as the presence of obesity in adulthood.23,24
Excessive calorie intake and obesity is arising as a health issue, mainly in industrialised countries, where, in general, obesity is reported at a frequency between 15% and 20% among women of reproductive age. In the United States, the incidence of obesity in pregnant women ranges from 18.5% to 38.3%. Prenatal BMI is a strong predictor of birth weight, and obese mothers tend to have children who are large for the gestational age, between 1.4 and 1.8 times more frequently, which predisposes their children to obesity in adulthood. This all takes on greater importance if we consider than more than 40% of women gain more weight than recommended during pregnancy.25
It has been demonstrated in animals such as rats and sheep that maternal obesity at the time of conception increases adipogenesis in the foetus, which lasts through adulthood and causes them to present a greater amount of white adipose tissue. These findings were initially described in animal models where most variables can be very strictly controlled.26,27
One of the most studied models from the perspective of maternal overnutrition and its metabolic effects on descendants is gestational diabetes. In certain ethnic groups, especially the Pima, the risk of being obese in the children of mothers who had gestational diabetes can be up to ten times higher than in the general population. They even present altered glucose tolerance from early ages such as during childhood and early adolescence.28
It has been observed in rats with gestational diabetes that their offspring had a higher predisposition to hyperphagia, obesity, and other metabolic diseases. It has also been observed that rats who have a significant prior history of malnutrition in utero, structural and functional changes in the hypothalamus, including an increased immunopositivity for neuropeptide Y, agouti-related protein, and galanin, and a decreased immunopositivity for proopiomelanocortin and α-melanocyte-stimulating hormone (α-MSH) in the arcuate nucleus. These observations highlight the importance of the influence of feeding during the breastfeeding period which undoubtedly has large effects on metabolic programming.29
The children of obese mothers have a higher amount of body fat, higher leptin levels, and insulin resistance. Maternal obesity is a risk factor for descendants, as having a high-fat and hypercaloric diet in general during pregnancy has side effects in the offspring, such as metabolic syndrome, hyperinsulinaemia, insulin resistance, and increased body fat deposits.3,30 The effect of maternal overnutrition with high-fat diets has been associated with anxious behaviour in offspring, apparently related to an increased expression of corticosteroid receptors, influenced by an inflammatory process in the central nervous system (hippocampus and amygdala).31
In studies conducted in rats, in addition the to the metabolic effects, hyperinsulinaemia has also been demonstrated to have the ability to modify the formation of neuron circuits related to regulating hunger and satiety in the central nervous system, inducing a preference for predominantly hypercaloric foods composed of fat and sucrose.31
There is also evidence that a high-fat maternal diet can alter the epigenome, such that these effects can be transmitted to future generations (Table 1).32,33
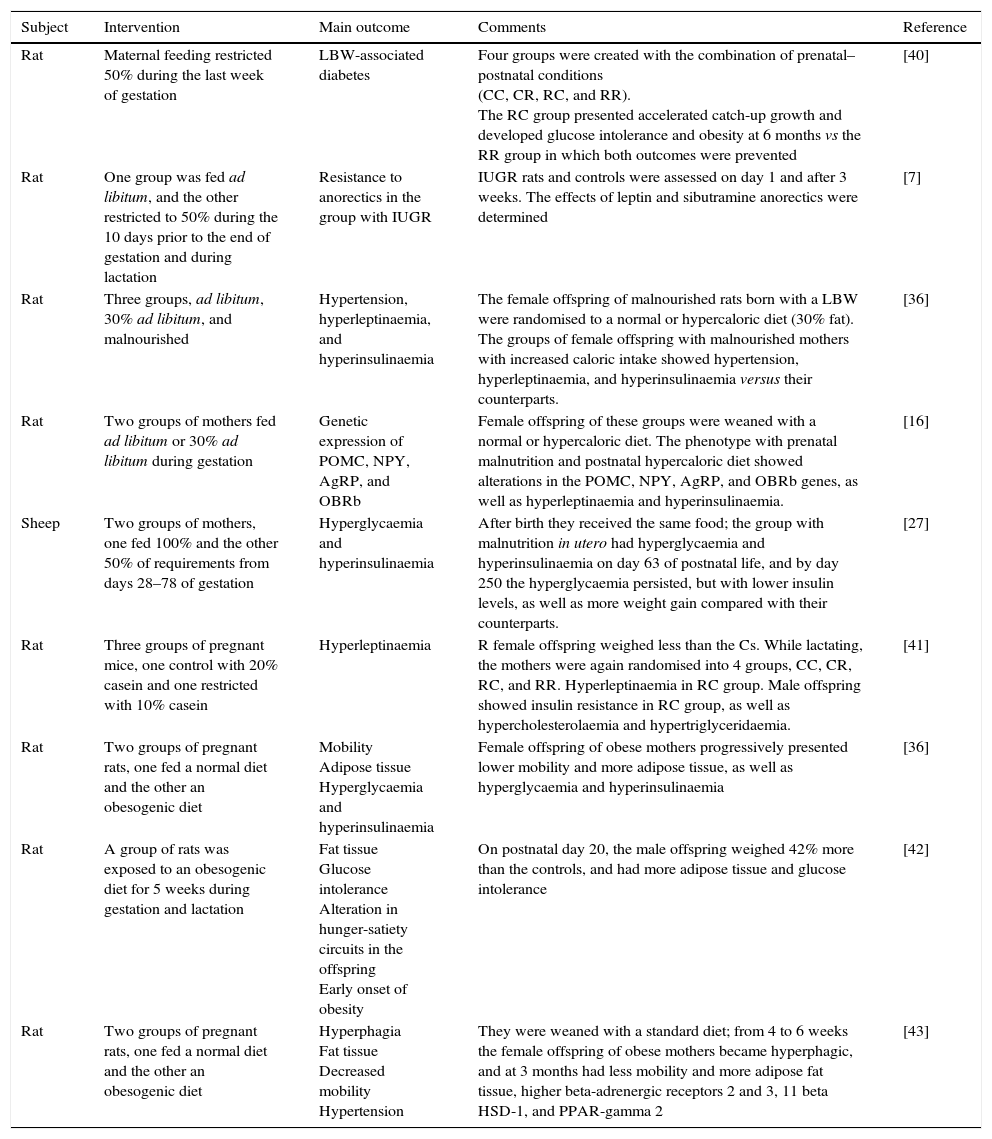
Studies on animals and maternal nutrition and the risk of obesity in the offspring.
| Subject | Intervention | Main outcome | Comments | Reference |
|---|---|---|---|---|
| Rat | Maternal feeding restricted 50% during the last week of gestation | LBW-associated diabetes | Four groups were created with the combination of prenatal–postnatal conditions (CC, CR, RC, and RR). The RC group presented accelerated catch-up growth and developed glucose intolerance and obesity at 6 months vs the RR group in which both outcomes were prevented | [40] |
| Rat | One group was fed ad libitum, and the other restricted to 50% during the 10 days prior to the end of gestation and during lactation | Resistance to anorectics in the group with IUGR | IUGR rats and controls were assessed on day 1 and after 3 weeks. The effects of leptin and sibutramine anorectics were determined | [7] |
| Rat | Three groups, ad libitum, 30% ad libitum, and malnourished | Hypertension, hyperleptinaemia, and hyperinsulinaemia | The female offspring of malnourished rats born with a LBW were randomised to a normal or hypercaloric diet (30% fat). The groups of female offspring with malnourished mothers with increased caloric intake showed hypertension, hyperleptinaemia, and hyperinsulinaemia versus their counterparts. | [36] |
| Rat | Two groups of mothers fed ad libitum or 30% ad libitum during gestation | Genetic expression of POMC, NPY, AgRP, and OBRb | Female offspring of these groups were weaned with a normal or hypercaloric diet. The phenotype with prenatal malnutrition and postnatal hypercaloric diet showed alterations in the POMC, NPY, AgRP, and OBRb genes, as well as hyperleptinaemia and hyperinsulinaemia. | [16] |
| Sheep | Two groups of mothers, one fed 100% and the other 50% of requirements from days 28–78 of gestation | Hyperglycaemia and hyperinsulinaemia | After birth they received the same food; the group with malnutrition in utero had hyperglycaemia and hyperinsulinaemia on day 63 of postnatal life, and by day 250 the hyperglycaemia persisted, but with lower insulin levels, as well as more weight gain compared with their counterparts. | [27] |
| Rat | Three groups of pregnant mice, one control with 20% casein and one restricted with 10% casein | Hyperleptinaemia | R female offspring weighed less than the Cs. While lactating, the mothers were again randomised into 4 groups, CC, CR, RC, and RR. Hyperleptinaemia in RC group. Male offspring showed insulin resistance in RC group, as well as hypercholesterolaemia and hypertriglyceridaemia. | [41] |
| Rat | Two groups of pregnant rats, one fed a normal diet and the other an obesogenic diet | Mobility Adipose tissue Hyperglycaemia and hyperinsulinaemia | Female offspring of obese mothers progressively presented lower mobility and more adipose tissue, as well as hyperglycaemia and hyperinsulinaemia | [36] |
| Rat | A group of rats was exposed to an obesogenic diet for 5 weeks during gestation and lactation | Fat tissue Glucose intolerance Alteration in hunger-satiety circuits in the offspring Early onset of obesity | On postnatal day 20, the male offspring weighed 42% more than the controls, and had more adipose tissue and glucose intolerance | [42] |
| Rat | Two groups of pregnant rats, one fed a normal diet and the other an obesogenic diet | Hyperphagia Fat tissue Decreased mobility Hypertension | They were weaned with a standard diet; from 4 to 6 weeks the female offspring of obese mothers became hyperphagic, and at 3 months had less mobility and more adipose fat tissue, higher beta-adrenergic receptors 2 and 3, 11 beta HSD-1, and PPAR-gamma 2 | [43] |
LBW: low birth weight; IUGR: intrauterine growth restriction; POMC: proopiomelanocortin; NPY: neuropeptide Y; AgRP: agouti-related peptide; R: restricted, C: control, O: obese.
Postnatal feeding has a large influence on both postnatal growth and the speed of catch-up growth; therefore, early postnatal overfeeding can accelerate growth and also exacerbate metabolic risks.12
The largest risk in modifying metabolic programming occur in during critical periods of development, which certainly have their main effect in earlier stages of life such as organ development. Although they are already structurally present during the early postnatal period, they are still in the process of maturation which has not yet finished; as is the case of the islets of Langerhans and neurons, where undoubtedly a change in their programming could cause chronic diseases and alterations in the hypothalamic hunger and satiety circuits in adults.34
Currently early overfeeding, particularly in newborns who are very small for their gestational age, continues to be a dilemma for clinical management. Aggressive feeding can contribute to brain development, while in contrast there is a latent risk of inducing chronic diseases such as obesity, diabetes, cardiovascular diseases and other comorbidities.35
According to the predictive–adaptive hypothesis, an animal can adapt specifically to its environment early (in utero or immediately after birth). In metabolic and energy balance terms, a foetus who was malnourished in utero is always programmed to store when it is available; therefore, postnatal overfeeding will be sufficient to store more energy than necessary in the form of fat, which will consequently increase the risk of obesity in its postnatal life.3
Accelerated postnatal growth is associated with high levels of leptin, which during infancy is mainly obtained through breastfeeding. Chronic leptin elevation can, in the long term, lead to the development of leptin resistance accompanied by hyperphagia and increased energy accumulation.36
Studies in rats in whom the amount of carbohydrates was modified and supplemented to their food in early postnatal life showed an increase in the secretion of insulin. It is believed that these high insulin levels, when chronic, have the ability to cause a permanent modification of the hunger and satiety centres in the hypothalamus. This translates clinically and in the long term into hyperphagia, increased weight gain, and other metabolic changes in adulthood.37
In humans, it has been observed that rapid weight gain immediately after birth is a very important risk factor for developing obesity throughout life. For every 100g of weight that a child gains in the first week of life, the associated risk of obesity in adulthood increases 28%, even in children who had a normal birth weight.38
Animal models allow altered metabolic programming to be put into context in humans, which started to be observed more often through uncommon growth patterns starting in the 1940s, when breastfeeding decreased in favour of infant formulas, and with its earlier weaning. In these children a higher mean weight gain (224g) was observed during the first year of birth compared to those who were breastfed and weaned after 16 weeks of life. In addition to gaining more weight in their first years of life, the development of obesity and cardiovascular risk could be observed in adulthood. Studies evaluating the perinatal diet and obesity-associated risk, showed that exclusive breastfeeding is a protective factor against obesity, while infant formula feeding and their contents are associated with faster weight gain and a higher risk of future obesity.39,40
The energy distribution in human milk is 42% carbohydrates, 52.9% fats, and 5.1% protein. Typically, feeding supplementary and complementary to breastfeeding is usually done in the form of foods that have a high carbohydrate density, mainly based on simple sugars. This type of feeding modifies the energy contribution, which can increase carbohydrates from 10% to 30% and decrease the amount of fats. Based on what was observed in animal models, it is very likely that the early introduction of grains, fruit, or fruit juices in humans in infancy could expose individuals to elevated proportions of carbohydrates during a critical period of development in early postnatal life, predisposing them to obesity and other long-term comorbidities.34
There is still some controversy regarding the benefits of exclusive breastfeeding and the ideal duration of this type of postnatal feeding in terms of the risk associated with being overweight or obese, particularly for all the factors that are usually involved. Nevertheless, the cumulative evidence is in favour of exclusive breastfeeding as a protective factor against being overweight or obese.39
Effect of breastfeeding on being overweight and obeseThe type of feeding in the neonatal and infancy stage, either breast milk or manufactured formulas, is one of the most analysed factors regarding the development of overweight or obesity. There is an association between the type of feeding during the neonatal stage and differences in body composition in early infancy, with studies finding faster weight gain in infants fed with manufactured formulas.44 On the other hand, it has been observed that breastfed children show less weight gain and body fat. It has been indicated that the protective effect of breastfeeding is dose-dependent, with a lower prevalence of obesity for more time spent breastfeeding during the first year of life.37 There is a widespread belief that children fed with manufactured formulas have advantages in growth; however, it has been demonstrated that in the first 12 months of life children fed breast milk and formulas grew at similar rates in terms of number of centimetres gained per months, and only a lower weight gain was observed in those fed with breast milk. It is difficult to establish whether the effect of being breastfed is due to factors of the breast milk itself or to non-beneficial properties of the formulas. It has been found that feeding with formulas enriched with macronutrients is harmful for metabolic and cardiovascular conditions later in adolescence.39 Breastfeeding has been associated with other beneficial effects for metabolic and cardiovascular risk programming, such as the observed decrease in the incidence of type 2 diabetes mellitus in high-risk groups, or in the prevalence of lower blood pressures in adult patients with a history of being born prematurely and breastfed in comparison with those fed manufactured formulas.40
The composition of breast milk differs from that contained in infant formulas. Breast milk contains growth factors, enzymes, hormones, and cytokines that make it unique and useful for gastrointestinal maturation and protection against infectious and metabolic diseases. The hormones that are found in breast milk include: leptin, ghrelin, adiponectin, insulin-like growth factor 1 (IGF-1), obestatin, and resistin. These hormones play a role in energy balance and have an impact on fat deposits. Satiety in breastfed children is regulated by changes in the milk composition during feeding, since at the end of each drink there is a higher fat supply, and leptin is released, stimulating satiety. Adiponectin is the most specific protein for adipose tissue, and it is inversely correlated with fat tissue and positively correlated with insulin sensitivity; therefore, it is considered a protective hormone against obesity and it is found in breast milk. Infant formulas contain a higher amount of protein, contributing to accelerated weight gain, increased insulin secretion, glucose production in the liver, and an increase in IGF-1. The increased insulin and IGF-1 secretion stimulate preadipocyte differentiation and induce adipogenesis. Higher plasma concentrations of insulin have been found in children fed with formula that in those fed with breast milk. This increases the risk of becoming obese in the future. Breast milk, on the other hand, induces lower levels of insulin, which decrease the accumulation of excess fat in the body. Infant formulas contain an omega-6/omega-3 ratio that stimulates adipocyte growth and differentiation, and promotes greater inflammation in the baby's body. This inflammation plays a significant role in the progression of obesity.36–38
In general terms, it has been proposed that breastfeeding is associated with a 4–20% reduction in the risk of developing obesity.38 Most authors agree that breastfeeding should be preferred over manufactured formulas, not only because of the potential effect on reducing the incidence of obesity, but also because of the other known beneficial effects on other systems.38
Some of these studies have not found a correlation between the type of milk (breast milk and formulas) and the percentage of body fat, BMI, and being overweight at later ages, and in other studies the protective effect of breast milk disappears when adjusting for other confounding variables, such as ethnic group and socio-economic status.37 Furthermore, it has been indicated that both prolonged breastfeeding and milk from diabetic and obese mothers can limit the protective effect of breast milk on obesity, and may even be associated with a higher incidence of atherosclerosis.36 Therefore it is essential that women reach the reproductive age in the best health condition.
Meta-analyses of observational studies indicate that breastfeeding decreases the risk of childhood obesity by 20% versus the use of manufactured formulas, after adjusting for confounding biological and sociodemographic variables.37 Based on this, in addition to the immunological advantages, the possible protective effect of breastfeeding on obesity should be considered as another important point for recommending and promoting breastfeeding during the first months of life.39
International organisations such as the WHO (World Health Organisation) and ESPGHAN (European Society for Paediatric Gastroenterology, Hepatology and Nutrition) recommend breastfeeding for a minimum of 6 months, and it should be exclusive in the first 4–6 months of age.36,44
Family participation is important for achieving collaboration in acquiring healthy eating habits. It is not recommended to feed babies every time they cry; sucking is per se a stimulus that brings pleasure, such that newborns can be comforted by being fed even when they are not hungry. This behaviour can promote a psychological relationship and dependence on the eating-pleasure pairing, which can become a life-long inappropriate source of stability.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.