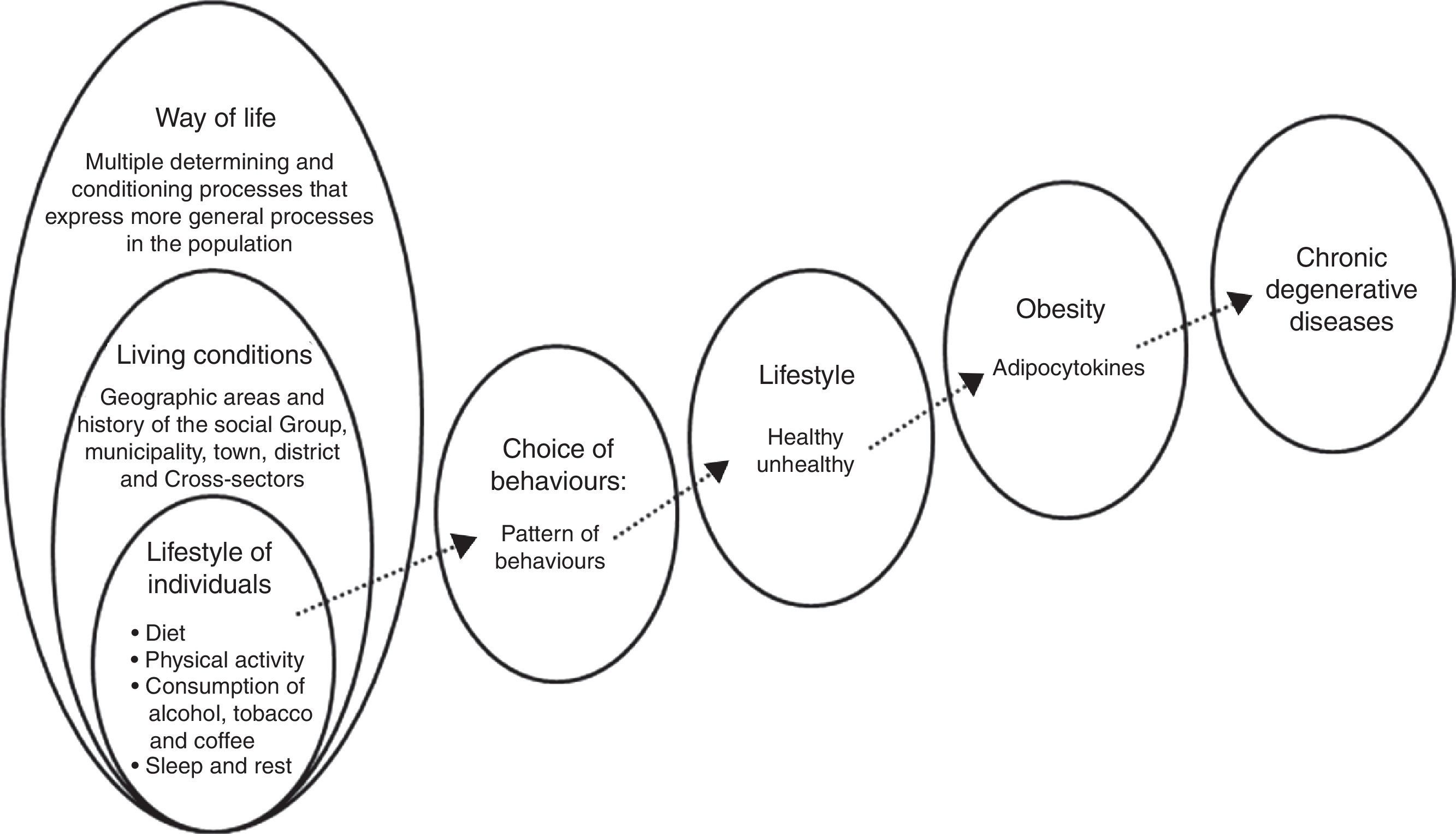
Overweight and obesity are two of the most important public health problems in Mexico. There is a clear association between lifestyle and obesity. This relationship means that unhealthy lifestyles can modify people's physiological response through adipocytokines, proinflammatory factors which are closely related to chronic degenerative diseases.
Obesity causes low-grade chronic inflammation. Adipose tissue, in addition to its function of storing energy reserves in the form of triglycerides, has important functions as an endocrine organ, producing a variety of molecules called adipocytokines such as IL-1, IL-6, IL-8, IFNγ, TNFα, leptin and resistin. The production of these molecules by adipocytes, coupled with the destruction of these cells, induces the inflammation to become chronic, and influences other systems by altering their functions, which leads to different diseases. Understanding the relationship between the different components of lifestyle and the production of adipocytokines involved in the development of chronic degenerative diseases, will allow us to address the problem and hence reduce the morbidity and mortality caused by these diseases.
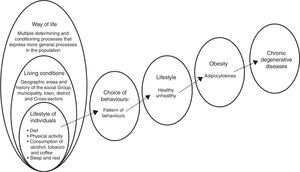
El sobrepeso y la obesidad constituyen unos de los problemas más importantes de salud pública en México. Existe una clara asociación entre estilo de vida y obesidad. Esta relación permite que los estilos de vida inadecuados modifiquen la respuesta fisiológica de los individuos, en donde los factores proinflamatorios denominados adipocitocinas estén estrechamente relacionados con enfermedades crónico-degenerativas.
La obesidad cursa con una inflamación de baja intensidad, donde el tejido adiposo, además de su función de almacenamiento de reservas energéticas en forma de triglicéridos, tiene importantes funciones como órgano endócrino, productor de una gran variedad de moléculas denominadas adipocitocinas como son: la IL-1, IL-6, IL-8, IFNγ, TNFα, leptina y resistina, entre otras. La producción de estas moléculas por los adipocitos aunada a la destrucción de estas células, permite que la inflamación se vuelva crónica e incida en otros aparatos o sistemas alterando su funcionamiento, lo cual conduce a diferentes enfermedades.
La comprensión de la relación que existe entre los diferentes componentes del estilo de vida y la producción de adipocitocinas involucradas en la evolución de enfermedades crónico-degenerativas, permitirá abordar el problema y con ello, reducir la morbi-mortalidad de estas patologías.
Since Hippocrates’ day, lifestyle has been considered to be an important dimension with regard to people's health. In his classic treatise, “On Airs, Waters, and Places”, Hippocrates considered that an entire set of environmental factors influenced human well-being, and that moderation, exercise, proper nutrition and clean surroundings were important measures for achieving good health.1 Lifestyle has gained importance from different areas of knowledge, such as sociology, anthropology, medicine and psychology. In general, lifestyle can be summarised as the result of a person's behaviours in relation to food, physical activity, smoking, and consumption of alcohol and caffeine, as well as sleep and rest, among other aspects.2
The World Health Organization (WHO) states that lifestyle is a general way of life, which is a result of the interaction between living conditions and individual patterns of behaviour, in which an individual's personal characteristics and sociocultural environment have great influence. The WHO considers that a sedentary lifestyle and physical inactivity cause two million deaths each year.2
Some of the most common health problems have been linked to lifestyle, such as obesity, which is the result of a series of behaviours that have caused a high prevalence of obesity worldwide, and particularly in Mexico. These behaviours are characterised by a poor diet that contains too many fats and simple carbohydrates (flour and sugar), a lack of physical activity and alcohol consumption, which adds empty calories.3
In a word, a poor diet insufficient activity, consumption of alcohol, tobacco and other drugs, in addition to a lack of sleep and rest, affect the metabolic processes through which the body gains weight. This is often followed by obesity, which is known to be accompanied by low-grade inflammation that causes various disorders (Fig. 1).
The aetiology of obesity is complex because there are several factors which are involved. In addition to those related to lifestyle, there are genetic, neuroendocrine-related, metabolic, immunological, environmental, social and cultural factors.
Excess visceral fat is known to be associated with metabolic syndrome, which is characterised by insulin resistance, hyperglycaemia, dyslipidaemia and hypertension.4 This syndrome redefines a series of changes and risks in people who are obese, as the pathophysiological changes that occur in this condition modify a number of processes that end up affecting the individual.
Physiology of obesityObesity can be defined as having too much body fat, characterised by an increase in adipose tissue that is out of proportion with the deposits of protein or carbohydrates.5
This condition arises as a result of the balance between a person's caloric intake and energy expenditure. The causes that condition a positive energy balance (i.e. an excess of energy consumed versus energy used in terms of the body's basal metabolism, physical activity and the thermic effect of food) seem to stem from a combination of different factors, such as lifestyle, environmental, genetic and neuroendocrine-related factors.5
Thus, obesity usually develops as a result of a chronic imbalance between intake and expenditure.6
However, it has been observed that weight gain may be of a different magnitude than that expected based on a calorie imbalance, because some metabolic factors are involved in regulating energy balance. In addition, maintaining one's body weight throughout life seems to require control mechanisms through afferent or efferent signals, which keep the body's total energy reserves constant.5
There is a close relationship between metabolic pathways and inflammation, and macrophages and adipocytes, being cells which are directly involved, share the production of a large number of molecules called adipocytokines. Members of the family of adipocytokines include interleukin-1 (IL-1), interleukin-6 (IL6), interleukin-8 (IL8), interleukin-12 (IL-12), interferon-δ (IFN-δ), tumour necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), leukaemia inhibitory factor (LIF), monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein (MIP-1), leptin and resistin.6 Leptin is one of the mediators that are common to both the immune system and the neuroendocrine system.
Some authors hold that the death of adipocytes attracts macrophages to adipose tissue to eliminate the dead cells. The death of adipocytes, which is rare in healthy people, as well as in rats and mice, is very common in obese individuals and has been linked to adipocyte hypoxia due to the expansion of adipose tissue.7 In this regard, it was recently shown that adipocyte hypoxia may actively participate in the development of obesity-related inflammation, increasing adipocytokine production, promoting the expression of proinflammatory genes and leading to the death of adipocytes.8,9 Some of the dead or dying adipocytes are surrounded by macrophages and form crown-like structures, such as those observed in the adipose tissue deposits of humans and mice.10,11
Inflammation and obesityInflammation is generally considered to be a protective mechanism; in this case, however, obesity is accompanied by some degree of inflammation called chronic low-grade inflammation or parainflammation. This inflammation differs from normal inflammation in that there are no typical signs of inflammation, but it is similar in that it shares the disorders generated by typical inflammation mediators and signalling pathways.5
In obesity-related proinflammatory states, the increased size of adipocytes plays a decisive role, because, to the extent that it increases adipose tissue, the production of adipocytokines increases, and this triggers a series of inflammation-related pathophysiological processes.12
One of the processes involved in inflammation is when neutrophils, eosinophils, monocytes and lymphocytes infiltrate adipose tissue. Adipocyte hypertrophy occurs in obesity, and this leads to increased production of a number of proinflammatory adipokines/chemokines/cytokines by adipocytes and other cells present in the adipose tissue.8 The increase in these molecules triggers local effects on the endothelium that lead to an increased production of vascular and intercellular cell adhesion molecules, (VCAM and ICAM) as well as vascular permeability. This allows fluid (which contains molecules such as those in complement protein) and cells such as polymorphonuclear and mononuclear phagocytes and other cells to escape to the extravascular compartment.8
The adipocytokines include leptin, which activates endothelial cells and promotes the accumulation of macrophages in adipose tissue, which in turn release proinflammatory molecules that perpetuate the inflammatory process. Another adipocytokine is resistin, which induces the expression of adhesion molecules (VCAM-1 and ICAM-1) in vascular endothelial cells and promotes the synthesis and secretion of proinflammatory cytokines such as TNF-α, IL-6 and IL-12.13
In the inflammatory process, macrophages in adipose tissue release chemoattractants for macrophages, which cause the chronic nature of inflammation.14,15 In this process it seems that MCP-1, also known as CCL2 and its receptor CCR2, performs a fundamental role.16,17 The accumulation of macrophages in adipose tissue plays an important role in increasing inflammatory mediators (IL-8, IL-6, IL-1, TNF-α, among others), which, coupled with a high degree of oxidative stress, hypoxia and lipolysis in adipocytes, causes an increased production of adipocytokines, which will result in metabolic and immunological diseases.18
Chronic low-grade inflammation, or parainflammation, has been shown to be a risk factor in patients with type 2 diabetes mellitus (T2DM). In fact, high levels of adipocytokines in the human population have been associated with T2DM, regardless of the degree of insulin resistance and obesity. Prospective studies have linked cytokines, proinflammatory chemokines, as well as other inflammatory markers, such as predictors of T2DM, to white blood cell counts. Studies have also focused on predicting cardiovascular risk with levels of proinflammatory cytokines, for example the ADVANCE study, in which the plasma levels of IL-6 significantly improved the prediction of macrovascular events and death in patients with T2DM.19
Inflammation in insulin-sensitive tissuesAdipose tissue, the liver, muscles and the pancreas are sites of inflammation in obesity or T2DM. In animal models and in humans with obesity or diabetes, it has been observed that macrophages infiltrate these tissues. These cells are crucial in the production of proinflammatory cytokines such as TNF-α, IL-6 and IL-1β are, which act in an autocrine and paracrine manner by promoting insulin resistance, interfering with insulin signalling in peripheral tissues through the activation of c-Jun N-terminal kinase (JNK) and nuclear transcription factor kappa B (NF-κB). These pathways are activated in multiple tissues in obesity and type 2 diabetes, and they also play a central role in promoting inflammation in various tissues. Hotamisligil et al.14 were the first to show an increase in the expression and production of TNFα in adipose tissue in obese humans and its direct link to insulin resistance. In this manner, adipose tissue inflammation has been considered to be a crucial event in causing metabolic syndrome, T2DM and cardiovascular atherosclerosis.
Adipose tissue has recently been linked to a marked accumulation of immune cells in the vascular stroma during obesity. Not only are enlarged adipocytes known to produce adipocytokines and chemokines, but macrophages in adipose tissue are also crucial in the production of adipocytokines, and their concentration is related to systemic inflammation, insulin resistance and metabolic syndrome.21
Subcutaneous fat versus visceral fatAbdominal obesity is the key component of metabolic syndrome, with a predominance in the accumulation of intra-abdominal visceral fat, measured indirectly in medical practice by waist circumference. Subcutaneous fat and visceral fat have also been associated with metabolic risk. Similarly, this syndrome has been associated with the ectopic distribution of lipids in the liver and skeletal muscle, which is involved in insulin resistance and contributes to the complications associated with metabolic syndrome. Subcutaneous fat and visceral fat differ in phenotypic, physiological and functional aspects. The inflammatory profile shows specific differences, with an increased number of macrophages, T lymphocytes and proinflammatory molecules in visceral fat versus subcutaneous fat in obese individuals.20
LiverNon-alcoholic fatty liver disease (NAFLD) often accompanies abdominal fat, and its prevalence increases in parallel with T2DM, which includes a wide range of damage: from benign steatosis to steatohepatitis (non-alcoholic), which can progress to cirrhosis or hepatocellular carcinoma. Inflammation plays an important role in the development of these conditions. NAFLD and subsequent insulin resistance in obese individuals are associated with an increased expression and overproduction of inflammatory mediators such as TNFα, IL-6 and IL-1β.21
PancreasIf T2DM, which is related to insulin resistance, develops during obesity, this involves the failure of pancreatic β cells to offset the insulin resistance, thus causing chronic hyperglycaemia. In the pancreatic islets of patients with T2DM, an inflammatory process has been observed, with the presence of amyloid deposits, an increase in dead β cells and macrophage infiltration, as well as an increase in proinflammatory cytokines and chemokines.22 The local expression and release of IL-1β is increased in the pancreatic islets, such that this cytokine is considered to be a master regulator of inflammation in T2DM, by increasing the local expression of proinflammatory cytokines and chemokines, promoting the recruitment of immune cells in the islets. This local inflammation can reduce the production of insulin and trigger the apoptosis of β cells, reducing the mass of functional pancreatic islets and thus a progression of T2DM.
ConclusionsThe incidence of obesity in the world continues to grow at an alarming rate. This involves serious health consequences and mars health achievements made over the past decades. Lifestyle is a behavioural pattern made up of an individual's habits. Failing to change bad habits may greatly affect quality of life in the future, given the relationship between an unhealthy lifestyle and chronic degenerative diseases.
Obesity has a multifactorial aetiology in which cellular, hormonal, nutritional, and genetic factors interact with sociocultural and environmental influences. Some of the genes related to obesity are known, and some hormones have been identified to be involved in the modulation of eating habits and in obesity, such as leptin, which, among other functions, sends afferents to the central nervous system to suppress the appetite. Other hormones, like resistin and visfatin, are associated with specific metabolic processes and are closely linked to immunological, and therefore inflammatory, processes.
Metabolism and immunity are closely related, since the proper functioning of both depends on homeostasis. Any dysfunction can lead to chronic metabolic disorders, such as obesity, T2DM and some chronic cardiovascular diseases. Obesity is considered a low-grade inflammatory disease, or parainflammation. To date, a large number of proinflammatory cytokines common to inflammation and obesity have been found: IL-1, IL-6, IL-8, TNF-α, leptin and resistin, among others. Adipose tissue, the liver, muscles and the pancreas are sites of inflammation in obesity or T2DM. Inflammation associated with obesity is further related to fatty liver processes, some cancers and metabolic syndrome, among others.
The more that is known about the risk posed by unhealthy lifestyles and their relation to different diseases such as T2DM, hypertension, stroke and some cancers, the more it may help to identify the best strategies to prevent, manage and treat obesity.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.
The authors would like to thank Professor Josefina Bolado from the Department of Translation of Scientific Texts at the Research Division of UNAM's School of Medicine for proofreading this text.