The nationwide epidemiological research of transfusion-transmitted diseases is relevant since the information provided by blood banks makes possible the knowledge of the distribution and seroprevalence of diseases, it also allows the indirect verification of the effectiveness of vaccination programs.
ObjectiveTo determinate the national seroprevalence of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) in Mexican blood donors in the blood banks registered at the Ministry of Health (SSA) in a 13 year period.
Materials and methodsAnalysis of the monthly reports of blood banks (n=555) sent to the National Center of Blood Transfusion (NCBT) and case detection of donors with HBV and HCV positive results (doubly reactive). Additionally, prevalence estimation for each serological marker was performed for the last year (2012) for each state.
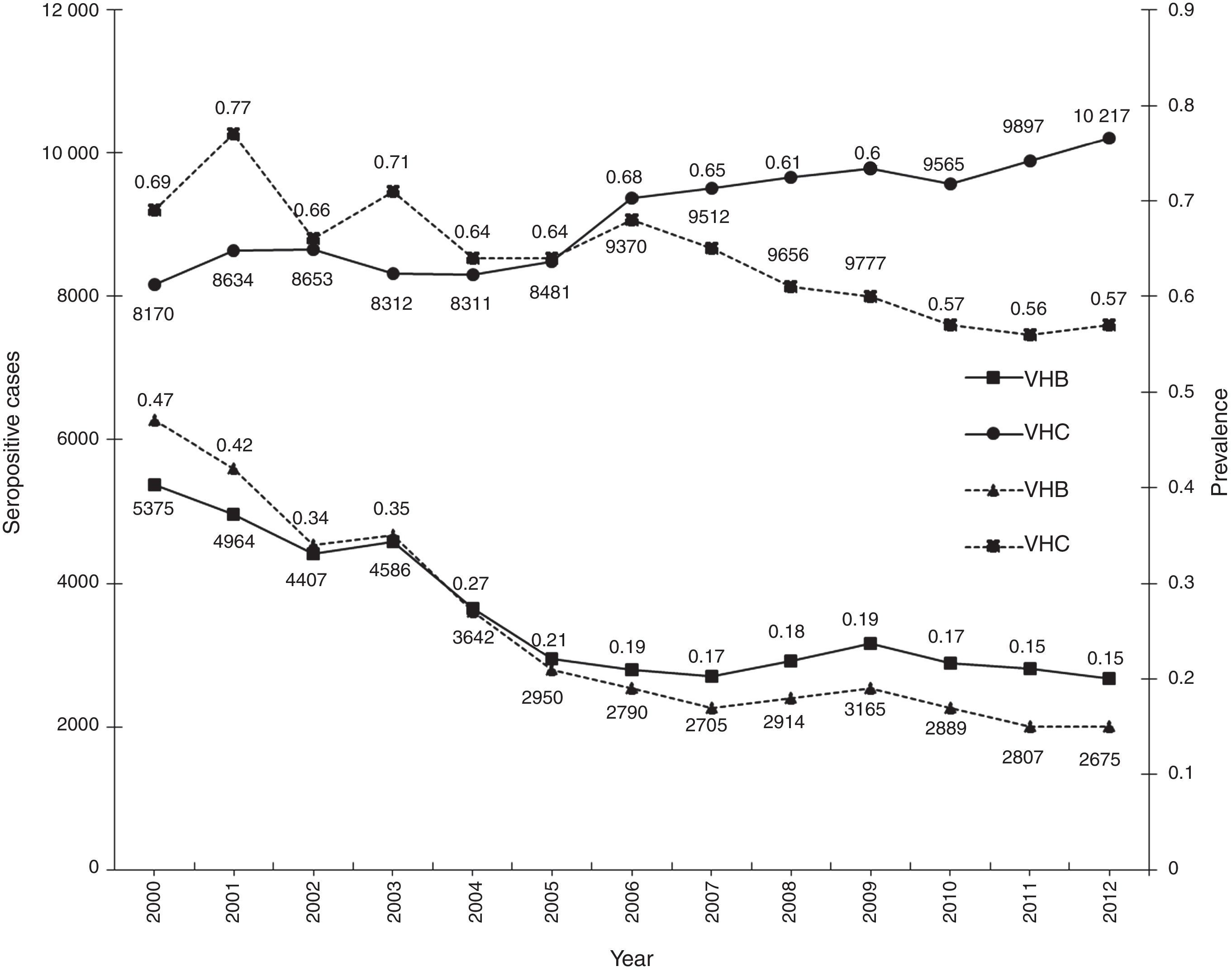
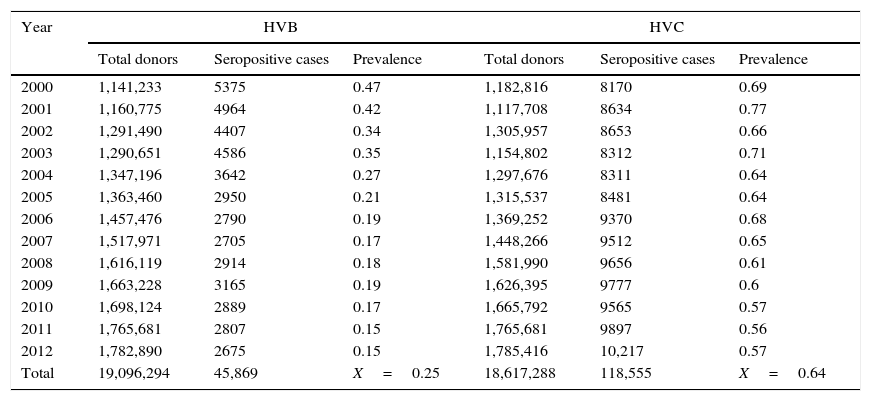
ResultsA total of 19,096,294 and 18,617,288 monthly reports with results of serological tests for HBV and HCV were respectively found in a period of 13 years (2000–2012). A decrease of 5375 (prevalence=0.47) to 2675 (prevalence=0.15) HBV seropositive cases was observed. The serologic marker for HCV showed an increase of 8170 (prevalence=0.69) to 10,217 (prevalence=0.57) seropositive cases. A higher prevalence was identified for both viruses in the adjacent states of the north border of the countries northern border in the latest report (compared to the national average).
ConclusionsA decrease in HBV prevalence to 0.15 in the 13 years period was observed, but the prevalence for HCV marker showed an increase to 0.57, this indicates that HCV remains a potential transfusion transmissible pathogen that needs effective and opportune treatment.
La investigación epidemiológica a nivel nacional de las enfermedades transmitidas por transfusión sanguínea tiene gran importancia, debido a que a través de los bancos de sangre es posible conocer la distribución y seroprevalencia de estas enfermedades, así como verificar de forma indirecta la eficacia de los programas de vacunación.
ObjetivoDeterminar la prevalencia durante 13 años a nivel nacional, de los marcadores serológicos de los virus de hepatitis B (VHB) y hepatitis C (VHC) en donantes de sangre mexicanos en los bancos de sangre registrados ante la Secretaría de Salud (SSA).
Materiales y métodosAnálisis de los informes mensuales de los bancos de sangre (n=555) y búsqueda de los casos de donantes con resultado positivo (doblemente reactivos) al VHB y VHC enviados al Centro Nacional de la Transfusión Sanguínea (CNTS) y cálculo de prevalencias para cada marcador serológico. Además, se realizaron estimaciones de prevalencias para cada marcador serológico para el año 2012 por entidad federativa.
ResultadosUn total de 19,096,294 y 18,617,288 informes mensuales con resultados de pruebas serológicas del VHB y VHC fueron obtenidos respectivamente durante 13 años (2000-2012). Un descenso de 5,375 (prevalencia=0.47) a 2,675 (prevalencia=0.15) casos seropositivos al VHB fue observado. El marcador serológico del VHC presentó un aumento de 8,170 (prevalencia=0.69) a 10,217 (prevalencia=0.57) casos seropositivos. En el último reporte (2012), los estados de la frontera norte presentaron la mayor prevalencia a ambos virus (comparado con la media nacional).
ConclusionesSe observó disminución hasta 0.15 en la prevalencia frente al VHB durante los 13 años analizados y la prevalencia para el marcador del VHC presentó un incremento hasta 0.57. El aumento de la prevalencia del VHC indica que sigue siendo un potencial patógeno transmisible por transfusión y necesita tratamiento oportuno y eficaz.
Hepatitis is an hepatic disease, most commonly caused by a viral infection. In particular, types B and C lead to chronic disease in hundreds of millions of people around the world and, together, are the most common cause of cirrhosis and cancer. It is usually the result of parenteral contact with body fluids. The most common mode of transmission of this viruses include transfusion of contaminated blood or blood components, such as platelet concentrates and plasma, invasive medical procedures using contaminated equipment and hepatitis B transmission from mother to baby at birth, between family members, and also by sexual contact.1,2 It is estimated that around the world there are 160 and 170 million cases of hepatitis B and C respectively.3,4 The number of calculated cases on hepatitis B in Mexico is approximately 1.7 and 1.4 millions for hepatitis C, of these about 80% of the patients do not know their condition.5,6 Since 1999, the HBV vaccination is obligatory in children and the availability of this vaccine for adults has prevented the emergence of new cases.7 The HCV transmission is mostly parenteral, and it is also considered the causal agent of 80–90% of post-transfusion hepatitis, blood recipients, hemophilia and hemodialysis patients are considered the most vulnerable groups.8–12 These viruses are a major concern for health institutions as well as for the Mexican Foundation for Hepatic Health (FundHepa).1,2 The quality control of blood includes serological screening of HBV and HCV since 1992, which has prevented new infections by this route.13,14 Before this year, control serology screening did not exist; therefore patients who received blood or blood components before 1995 should be tested for HBV and HCV. The Single Information System for Epidemiological Surveillance (SUIVE) of the Mexican Ministry of Health, recorder with 217,513 cases of viral hepatitis in the country from 1990 to 1999, 3.7% correspond to HBV. HCV prevalence has been reported since 2000, with a prevalence of 6%, in a population of 192,588 cases of viral hepatitis.15 It is because of this that the research of national epidemiology of borne diseases by blood transfusion is of great importance. Through blood banks it is possible to monitor the distribution and seroprevalence of these diseases and indirectly verify the effectiveness of the vaccination programs.15–18 Blood banks are opportunity fields to detect these positive cases in order to provide opportune treatment. To the best of our knowledge, there are no national studies that report the prevalence and annual distribution of seropositive cases of HBV and HCV in Mexican blood donors. The present work documents the prevalence and annual distribution of HCV and HBV seropositivity in Mexican blood donors reported from the 555 blood banks (public and private) registered at the Ministry of Health (SSA).
Materials and methodsStandardized working procedures for the selection and deferral of donors were employed in all blood banks. Each donor was selected by medical personnel based on a detailed medical history and a brief physical examination. Quantification of hemoglobin, blood pressure, temperature, pulse and heart rate was made before the donation of the blood unit. Relationship between donors with positive serology and the above parameters was not established. Diverse technologies for enzyme immunoassays that were implemented in the search for serological markers for HBV and HCV were also identified (Table 1). According to the current Official Mexican Norm NOM-253 SSA1-2012 for the disposal of human blood and blood components for therapeutic purposes; all repeatedly reactive units were discarded. The prevalence was determined from seropositivity records obtained for these two viruses.14 In order to identify the national prevalence of the HCV and HBV in Mexican blood donors, a 13 year national retrospective analysis from 2000 to 2012 of the monthly reports of an average of 555 blood banks (public and private) registered at the Ministry of Health (SSA) was performed. Blood donors who presented seropositivity to HBV, HCV or both were identified per year (Table 2). Data were entered into computer using a spreadsheet program (Excel, Microsoft Corp., Redmond, WA) and calculations of means, standard deviations and prevalences were made for seropositive cases HBV and HCV in the studied period. For the year 2012, a search of seropositive cases was conducted in all states and the prevalence for both serological markers was determined likewise.
Seropositive cases and prevalence of HBV and HCV in Mexican blood donors (2000–2012).
| Year | HVB | HVC | ||||
|---|---|---|---|---|---|---|
| Total donors | Seropositive cases | Prevalence | Total donors | Seropositive cases | Prevalence | |
| 2000 | 1,141,233 | 5375 | 0.47 | 1,182,816 | 8170 | 0.69 |
| 2001 | 1,160,775 | 4964 | 0.42 | 1,117,708 | 8634 | 0.77 |
| 2002 | 1,291,490 | 4407 | 0.34 | 1,305,957 | 8653 | 0.66 |
| 2003 | 1,290,651 | 4586 | 0.35 | 1,154,802 | 8312 | 0.71 |
| 2004 | 1,347,196 | 3642 | 0.27 | 1,297,676 | 8311 | 0.64 |
| 2005 | 1,363,460 | 2950 | 0.21 | 1,315,537 | 8481 | 0.64 |
| 2006 | 1,457,476 | 2790 | 0.19 | 1,369,252 | 9370 | 0.68 |
| 2007 | 1,517,971 | 2705 | 0.17 | 1,448,266 | 9512 | 0.65 |
| 2008 | 1,616,119 | 2914 | 0.18 | 1,581,990 | 9656 | 0.61 |
| 2009 | 1,663,228 | 3165 | 0.19 | 1,626,395 | 9777 | 0.6 |
| 2010 | 1,698,124 | 2889 | 0.17 | 1,665,792 | 9565 | 0.57 |
| 2011 | 1,765,681 | 2807 | 0.15 | 1,765,681 | 9897 | 0.56 |
| 2012 | 1,782,890 | 2675 | 0.15 | 1,785,416 | 10,217 | 0.57 |
| Total | 19,096,294 | 45,869 | X=0.25 | 18,617,288 | 118,555 | X=0.64 |
The Research Ethics Committee of the National Blood Transfusion Center approved this protocol. Additionally, the collection and presentation of data was carried out under the observance of the principles of confidentiality and discretion outlined by the Federal Law of Accountability and Access to Public Government Information.
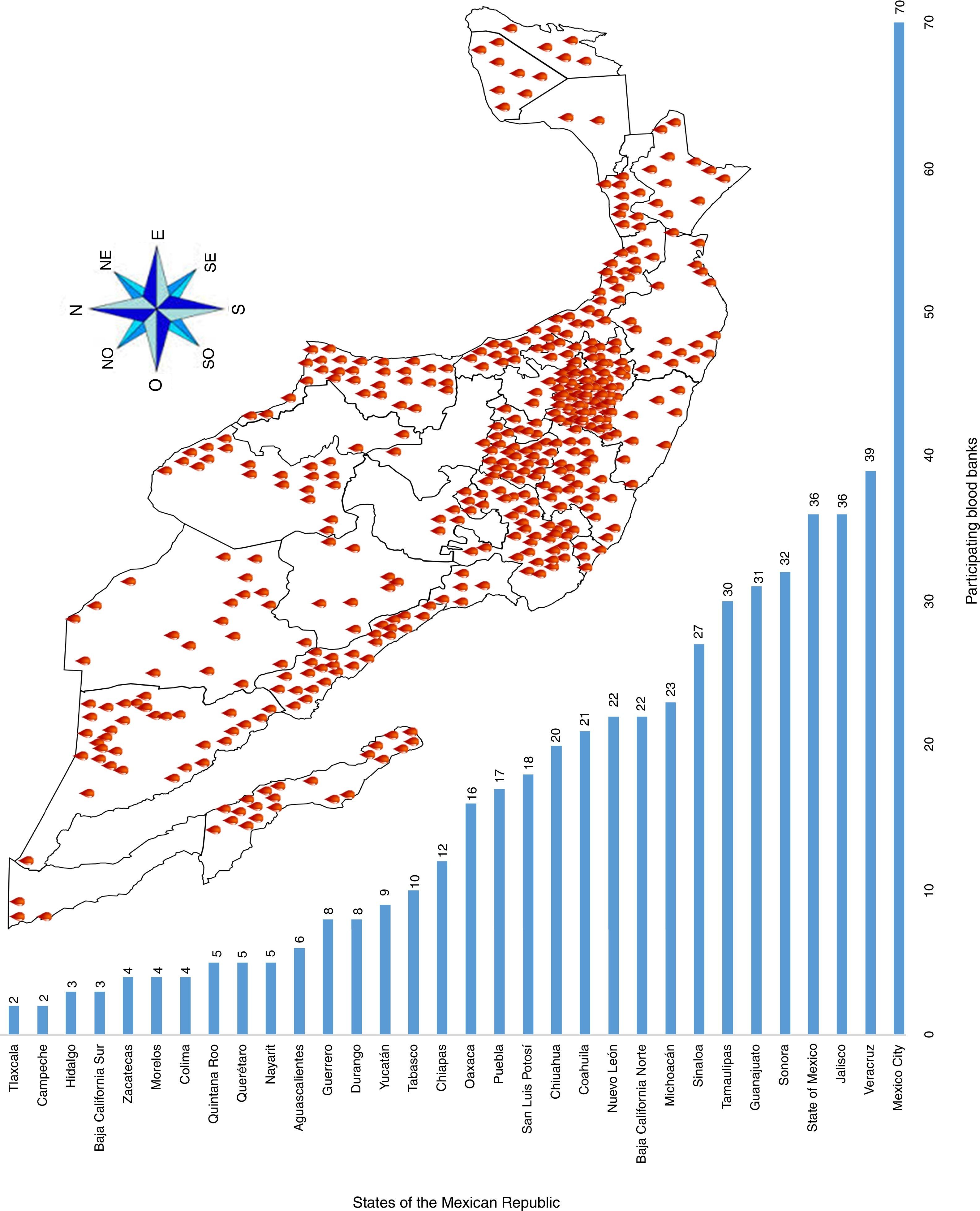
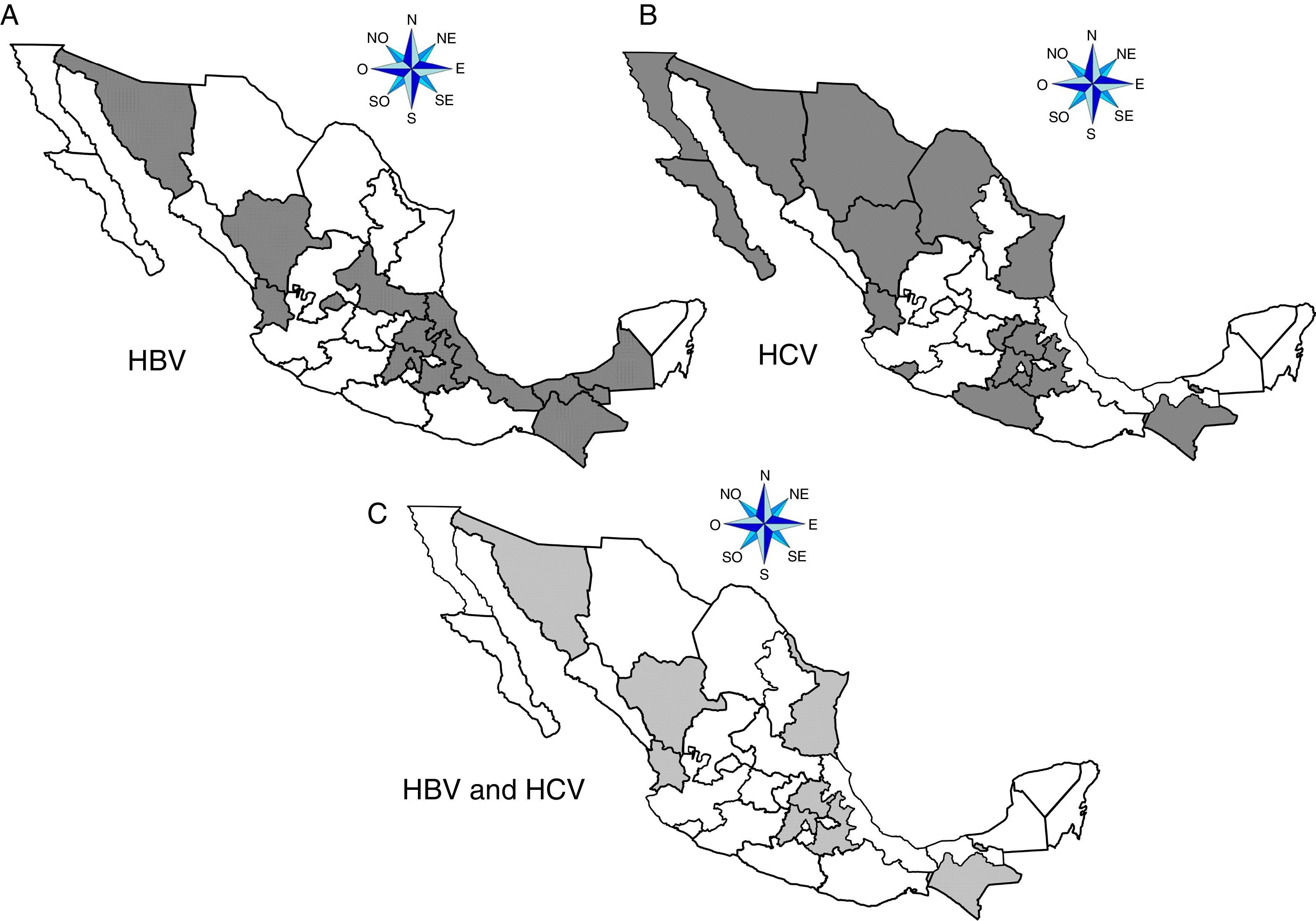
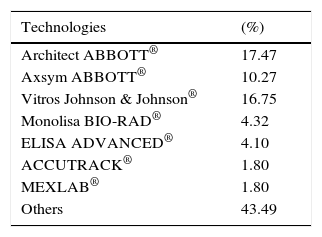
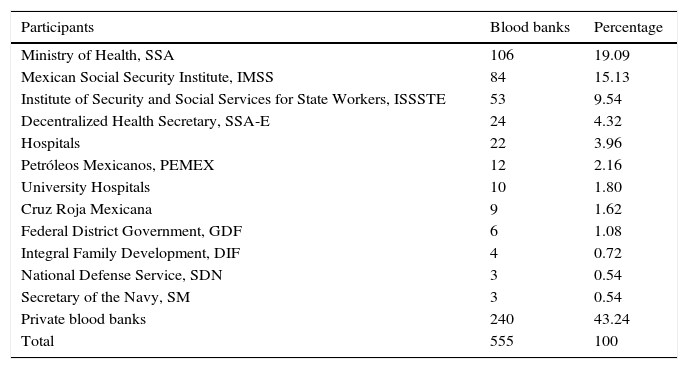
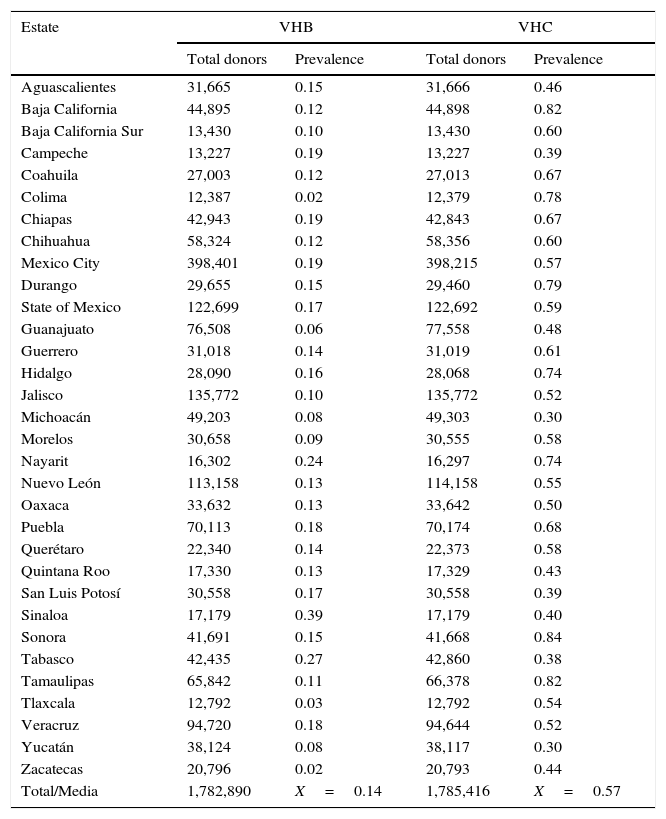
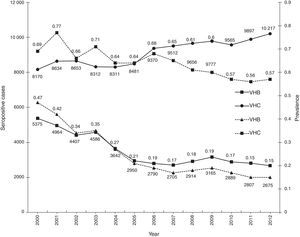
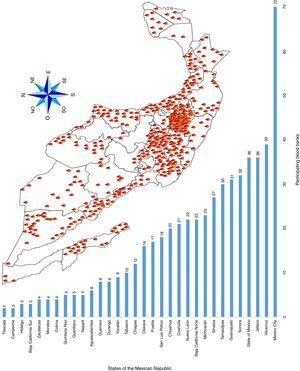
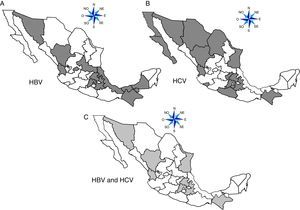
ResultsA total of 19,096,294 and 18,617,288 monthly reports of infectious serological tests for HBV and HCV blood donors in a period of 13 years from 2000 to 2012 were collected. Seropositivity of 0.24% (45,869 cases) for HBV and 0.63% (118,555 cases) for HCV in the donor population was identified. The participation of blood banks during this period was mainly private (43.24%), followed by those belonging to the SSA (19.09%), IMSS (15.13%) and ISSSTE (9.54%) (Table 3). Fig. 1 shows the results of the identification of the number of seropositive cases (doubly reactive) and prevalence for HBV and HCV in a period of 13 years. In the case of HBV, a progressive decrease in the number of seropositive cases is observed. HBV started with a prevalence of 0.47 (5375 donors) in 2000 and concluded with a prevalence of 0.15 (2675 donors) in 2012, resulting in a reduction of 52.1% and 68.9% of seropositive cases and prevalence respectively. In the case of HCV two behaviors are identified in two different periods. An initial behavior in the period from 2000 to 2005 where no change was identified in the seropositivity to HCV (X=8426.83±194.71 seropositive cases). In a second period (2006–2012) a progressive increase in the number of seropositive cases is observed (X=9559.3±507.68 doubly reactive cases). A total of 2047 (25.05%) new cases of HCV was finally identified in the 13 years studied. An initial prevalence of 0.69 (8170 donors) and a final of 0.57 (10,217 donors) was observed for this serological marker. A decrease in prevalence does not indicate a direct reduction in the number of seropositive cases, as this statistical parameter depends on the size of the population that has been analyzed. This is shown in Fig. 1; where HCV prevalence decreased from 2008 although the number of seropositive cases increased. The latest report from 2012 of the registered blood banks shows that the prevalence of both markers was of 0.14 (2675 donors) and 0.57 (10,217 donors) for hepatitis B and C respectively (Table 4). As for the state level, Aguascalientes, Campeche, Chiapas, Mexico City, Durango, State of Mexico, Hidalgo, Nayarit, Puebla, San Luis Potosi, Sinaloa, Sonora Tabasco and Veracruz were the states with the highest prevalence of HBV (compared to the national average 0.14). In contrast, Baja California Norte, Baja California Sur, Coahuila, Colima, Chiapas, Chihuahua, Durango, State of Mexico, Guerrero, Hidalgo, Morelos, Nayarit, Puebla, Querétaro, Sonora and Tamaulipas were the states that had higher prevalence (compared to the national average of 0.57) for HCV. Finally, Fig. 3 shows that Chiapas, Durango, State of Mexico, Hidalgo, Nayarit, Puebla, Sonora and Tamaulipas were the states with the highest prevalence of both infectious markers. The last report of the geographic distribution of blood banks, clearly shows that most are distributed in the northern border frontier states and concentrated in the center region of the country, shown in Fig. 2. The use of technologies to detect antibodies to HBV and HCV were mainly chemiluminescent immunoenzymatic techniques followed by colorimetric methods (Table 1).
Participants and their geographical distribution in the study of the prevalence of HBV and HCV.
| Participants | Blood banks | Percentage |
|---|---|---|
| Ministry of Health, SSA | 106 | 19.09 |
| Mexican Social Security Institute, IMSS | 84 | 15.13 |
| Institute of Security and Social Services for State Workers, ISSSTE | 53 | 9.54 |
| Decentralized Health Secretary, SSA-E | 24 | 4.32 |
| Hospitals | 22 | 3.96 |
| Petróleos Mexicanos, PEMEX | 12 | 2.16 |
| University Hospitals | 10 | 1.80 |
| Cruz Roja Mexicana | 9 | 1.62 |
| Federal District Government, GDF | 6 | 1.08 |
| Integral Family Development, DIF | 4 | 0.72 |
| National Defense Service, SDN | 3 | 0.54 |
| Secretary of the Navy, SM | 3 | 0.54 |
| Private blood banks | 240 | 43.24 |
| Total | 555 | 100 |
Annual prevalence of HBV and HCV in blood donors (2012).
| Estate | VHB | VHC | ||
|---|---|---|---|---|
| Total donors | Prevalence | Total donors | Prevalence | |
| Aguascalientes | 31,665 | 0.15 | 31,666 | 0.46 |
| Baja California | 44,895 | 0.12 | 44,898 | 0.82 |
| Baja California Sur | 13,430 | 0.10 | 13,430 | 0.60 |
| Campeche | 13,227 | 0.19 | 13,227 | 0.39 |
| Coahuila | 27,003 | 0.12 | 27,013 | 0.67 |
| Colima | 12,387 | 0.02 | 12,379 | 0.78 |
| Chiapas | 42,943 | 0.19 | 42,843 | 0.67 |
| Chihuahua | 58,324 | 0.12 | 58,356 | 0.60 |
| Mexico City | 398,401 | 0.19 | 398,215 | 0.57 |
| Durango | 29,655 | 0.15 | 29,460 | 0.79 |
| State of Mexico | 122,699 | 0.17 | 122,692 | 0.59 |
| Guanajuato | 76,508 | 0.06 | 77,558 | 0.48 |
| Guerrero | 31,018 | 0.14 | 31,019 | 0.61 |
| Hidalgo | 28,090 | 0.16 | 28,068 | 0.74 |
| Jalisco | 135,772 | 0.10 | 135,772 | 0.52 |
| Michoacán | 49,203 | 0.08 | 49,303 | 0.30 |
| Morelos | 30,658 | 0.09 | 30,555 | 0.58 |
| Nayarit | 16,302 | 0.24 | 16,297 | 0.74 |
| Nuevo León | 113,158 | 0.13 | 114,158 | 0.55 |
| Oaxaca | 33,632 | 0.13 | 33,642 | 0.50 |
| Puebla | 70,113 | 0.18 | 70,174 | 0.68 |
| Querétaro | 22,340 | 0.14 | 22,373 | 0.58 |
| Quintana Roo | 17,330 | 0.13 | 17,329 | 0.43 |
| San Luis Potosí | 30,558 | 0.17 | 30,558 | 0.39 |
| Sinaloa | 17,179 | 0.39 | 17,179 | 0.40 |
| Sonora | 41,691 | 0.15 | 41,668 | 0.84 |
| Tabasco | 42,435 | 0.27 | 42,860 | 0.38 |
| Tamaulipas | 65,842 | 0.11 | 66,378 | 0.82 |
| Tlaxcala | 12,792 | 0.03 | 12,792 | 0.54 |
| Veracruz | 94,720 | 0.18 | 94,644 | 0.52 |
| Yucatán | 38,124 | 0.08 | 38,117 | 0.30 |
| Zacatecas | 20,796 | 0.02 | 20,793 | 0.44 |
| Total/Media | 1,782,890 | X=0.14 | 1,785,416 | X=0.57 |
To the best of our knowledge, there is a lack of studies documenting the prevalence of serological markers for HBV and HCV nationwide. Some prevalence reports of these viruses are focused on small samples of the donor population.19–24 This studies are limited since they are local studies and lack national epidemiological value. The screening for detecting seropositive cases (doubly reactive) of HBV and HCV are necessary for appropriate evaluation of blood donors population, but prevention measures are certainly still the best alternative to control of infection by these viruses.25 In the case of HBV the data reported in this work shows a gradual decrease (per year) of the prevalence of HBV seropositive cases. On the other hand HCV showed an opposite result. The sample size of national population remained constant in the number of donors per year (χn=1,437,228±216,718) indicating homogeneity in the sample size of the donor population analyzed. Previous results of seropositivity at state level for the same serological markers have been previously reported, HCV is more predominant compared with HBV in the donor population; this results are similar to those reported by the states of Veracruz and Guerrero.5,15–17,24 HBV prevalence values started with 0.47 in 2000 and a gradual decline from 2001. A final prevalence of 0.15 in 2012, resulted in the reduction of 5375 to 2675 seropositive cases (47.90%); the above is probably associated with improvement in donor selection criteria and current coverage of the National Health System in the HBV vaccination. In relation to 2012, the states of the Mexican republic where the prevalence is higher than 0.16 were identified (Table 4). Inadequate identification of risk factors in the donor population or the characteristics of the population who attend to donate remain main causes for the high incidence of these infectious markers.24 A previous study showed that during the period from 1990 to 1999, 3.7% of the donor population was seropositive for HBV.5 There are no reports for HCV for the same period or the follow up of the prevalence of this marker since the year 2000. In 2000, the number of double reactive HCV cases was surpassed by 52.1% compared with HBV and at the end of 2011, there were already 7090 HCV seropositive cases. The increase in seropositivity is assumed to be caused by several reasons: the lack of a preventive vaccine, and the high cost of effective treatment, which makes the number of seropositive cases a point for attention. In 2007 it was estimated that Mexico had 192, 588 infected persons with this virus, values which currently have significantly increased.15 On the other hand it is important to note that the identified prevalences were from individuals who were selected for blood donation; however there is an important group of those individuals who were excluded because of risk factors for transfusion transmitted infectious diseases. Within the limitations of this study is the lack of additional data such as gender, age, marital status, among others. With this information vulnerable groups could be identified in the donor population. Therefore, there is a need to study those states where the prevalence is higher in order to identify risk factors that trigger the transmission of these viruses. It is important to consider that this study is limited only to the analysis of those individuals attending blood banks which were accepted for blood donation. It might also determine hepatic enzymes as in some blood banks of developed countries in those states with the highest prevalence for HCV.
The importance of this study is based on the prevalence of repeatedly reactive cases to these viruses, however it does not include the results of the confirmed cases, for possible presence of false positive and false negative results due to the use of various diagnostic technologies with different sensitivities and specificities. Therefore blood banks are areas of opportunity to establish flowcharts of confirmatory studies and reference for the care of confirmed cases. The NCBT is a center of reference only for some state centers of blood transfusion of the Mexican republic such as Chiapas, Puebla, Michoacán and Nayarit. The NCBT could be a reference for the whole country to confirm cases of HCV and HBV.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interest.
We thank Mariana Diaz-Moretin for her contribution of the English manuscript.