We describe an unusual case of leukemoid reaction secondary to congenital infection associated with prematurity as a finding in the study protocol. The haematological and clinical evolution of the newborn was satisfactory.
Se describe un caso atípico de reacción leucemoide secundario a proceso infeccioso congénito asociado a prematuridad como hallazgo de su protocolo de estudio. La evolución hematológica y clínica del recién nacido fue satisfactoria.
Neonatal leukemoid reaction is defined as a leucocyte count of >50×109/L or a neutrophil count of >30×109/L in the neonatal period. The first cases were reported by Holland and Maurer in 1963, and were associated with infection, severe anaemia, bronchopulmonary dysplasia, use of antenatal steroids, prematurity, and chromosomal abnormalities. Incidence of neonatal leukemoid reaction in neonatal intensive care units (NICU) ranges from 1.3% to 15%.1 It has also been associated with a neonatal response to hypoxia, and can mimic a blood smear finding of leukaemia, accompanied by cytopaenia with left shift leucocytosis. Bone marrow aspiration can be used to distinguish between these conditions.2
Neonatal sepsis is one of the most common causes of morbidity and mortality in NICUs. Suspicion is based on an analysis of risk factors, such as preterm birth, premature rupture of membranes 18h or more before onset of labour, and chorioamnionitis. Optimal initial treatment consists of a broad spectrum antibiotic (ampicillin plus an aminoglycoside).3 The study and diagnosis of this pathology is based on the 2005 International paediatric sepsis consensus conference guidelines, and should include systemic inflammatory response syndrome variables, criteria for suspicion or confirmation of infection, and/or more organ dysfunction variables4 (see the articles by James et al. for a more detailed explanation of these criteria). A blood count finding of neutropaenia has recently been described as the best marker of neonatal sepsis, as neutrophilia and leucocytosis are also caused by conditions such as maternal hypertension, asphyxia, and haemolytic disease.3
Premature infants are relatively immunocompromised due to their immature immune system, and are susceptible to viral, bacterial and fungal infection.5
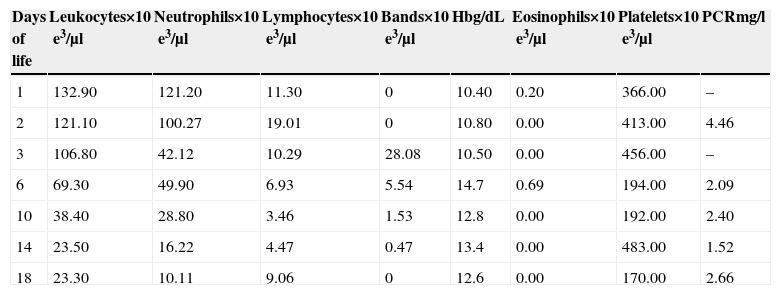
Case reportThe patient was a female neotate from the third pregnancy of a healthy, 26-year mother with a family history of systemic hypertension. She had had 5 prenatal visits, and had taken folic acid and multivitamin supplements 8 months before pregnancy. The mother presented with threatened preterm labour, premature rupture of membranes lasting 48h, treated with cefotaxime and amikacin, with no steroids. A caesarean section under general anaesthesia was performed due to maternal chorioamnionitis. Standard physical examination for premature infants showed Apgar 2/5/7, Capurro 31 weeks, weight 910g, length 35cm. Porcine surfactant at a dose of 200mg/kg was administered 15min after delivery. The infant was transferred to the PICU under mechanical ventilation. Standard doses of ampicillin and amikacin were administered, parenteral nutrition was started at 24h, umbilical catheters were placed, human immunoglobulin (750mg/kg) was administered, and vital signs were closely monitored. Blood count: leucocytosis (Table 1). Clinical course: no evidence of acute neurological injury; fever for the first 3 days; mechanical ventilation for 13 days, planned extubation and switch to head box oxygen, inotropic support with dobutamine at day 5, and hyperglycaemia management at day 3. Enteral feeding with human milk started at 48h of life was not tolerated, manifested by vomiting and abdominal bloating. Feeding improved at day 5, and was gradually increased. At day 6, bone marrow aspiration was performed, and was positive for active infection (with no change in cell morphology). Antibiotic regimen was continued for 14 days, blood culture and C reactive protein (CRP) were both negative. The infant remained hospitalized to monitor growth and development, and was finally discharged with a weight of 1950g.
Complete blood count.
| Days of life | Leukocytes×10e3/μl | Neutrophils×10e3/μl | Lymphocytes×10e3/μl | Bands×10e3/μl | Hbg/dL | Eosinophils×10e3/μl | Platelets×10e3/μl | PCRmg/l |
|---|---|---|---|---|---|---|---|---|
| 1 | 132.90 | 121.20 | 11.30 | 0 | 10.40 | 0.20 | 366.00 | – |
| 2 | 121.10 | 100.27 | 19.01 | 0 | 10.80 | 0.00 | 413.00 | 4.46 |
| 3 | 106.80 | 42.12 | 10.29 | 28.08 | 10.50 | 0.00 | 456.00 | – |
| 6 | 69.30 | 49.90 | 6.93 | 5.54 | 14.7 | 0.69 | 194.00 | 2.09 |
| 10 | 38.40 | 28.80 | 3.46 | 1.53 | 12.8 | 0.00 | 192.00 | 2.40 |
| 14 | 23.50 | 16.22 | 4.47 | 0.47 | 13.4 | 0.00 | 483.00 | 1.52 |
| 18 | 23.30 | 10.11 | 9.06 | 0 | 12.6 | 0.00 | 170.00 | 2.66 |
Leukemoid reaction, considered to be an inflammatory response or reflex, is uncommon in neonates. We performed a literature search of clinical case reports and cohort studies in extremely low-weight premature neonates, and could find no physiological explanation for this response. Physiological leucocytosis is found in the early neonatal period; the normal range is from 9000 to 30,000/mm3, and infection rarely causes an elevation of more than 100,000/mm3. Isik reported a case of congenital cytomegalovirus (CMV) with 120,000/mm3,6. In our patient, levels increased to 132,900/mm3. Risk factors were preterm birth, premature rupture of membranes more than 18h before onset of labour, and documented chorioamnionitis; clinical manifestations were intolerance of enteral feeding, hyperglycaemia and need for endotrophic support. A single case of leukemoid reaction due to herpes simplex encephalitis in a 29-week preterm infant has been reported.7 Another suggested cause is the use of prostaglandins in neonates with congenital cyanotic heart, reported by Arav-Boger.8
The excessive immune response has not been fully clarified, since the immune system of preterm neonates is immature, and therefore the function of both the innate and adaptive immune systems is limited. In addition, reserves of neutrophils and monocytes are low, cytokine production is diminished, and T cells proliferate poorly, leaving them susceptible to infection, mainly bacterial and viral. In addition to this, both granulocyte and granulocyte-macrophage colony stimulating factors are also diminished.9 Antenatal steroids have an immunosuppressive effect,8 and prenatal administration of steroids increases neutrophil levels (steroid were not given to our patient).10
In his review, Morag classifies leukemoid reaction as early-onset (<72h of life) and late-onset (>72h of life). These onset times correspond to the division between early and late sepsis. Among his findings, Morag associated sepsis and necrotizing enterocolitis with late-onset leukemoid reaction.11 Calhoun, in a case series, reports no association between hyperviscosity and leukemoid reaction in the neonate population,12 in contrast to other, older, patient populations. Hsiao concludes that extremely low-weight neonates with leukemoid reaction required longer duration of ventilatory support, a high incidence of bronchopulmonary dysplasia, and a lower mortality rate.13 Similar tends in mortality were also reported by Rastogi.14 Zanardo, meanwhile, associates maternal chorioamnionitis with an increased risk for both leukemoid reaction and bronchopulmonary dysplasia.15 Our patient was discharge with no complications.
The physiological and immune mechanisms underlying this response have never been fully clarified. In recent years, some researchers have commented that Toll-like receptors are expressed in bacterial, viral and fungal infections, and the activation of these proteins by different antigens drives proinflammatory cytokine production. Toll-like receptors are a potentially promising approach to the prevention and treatment of infectious diseases in neonates,16 and could play an important role in modulating leukemoid reaction in the neonatal period.
Conflict of interestThe authors declare that they have no conflict of interests.