Red cell distribution width (RDW) is the percentage of the erythrocyte volume variation and has been identified as a biological marker in patients with cardiovascular disease. Increased levels have been associated to worse clinical outcomes and it is suggested that it could be useful as a prognostic risk factor in this group of patients.
MethodsThis was an observational, prospective, longitudinal and analytic study with the objective of determining the correlation between RDW and in-hospital mortality in ST elevation myocardial infarction (STEMI) patients. 61 patients were included. We analyzed the correlation between RDW and in-hospital mortality as well as that between RDW and the GRACE risk score at hospital admission. Pearson correlation was determined in both cases by using IBM SPSS statistics software.
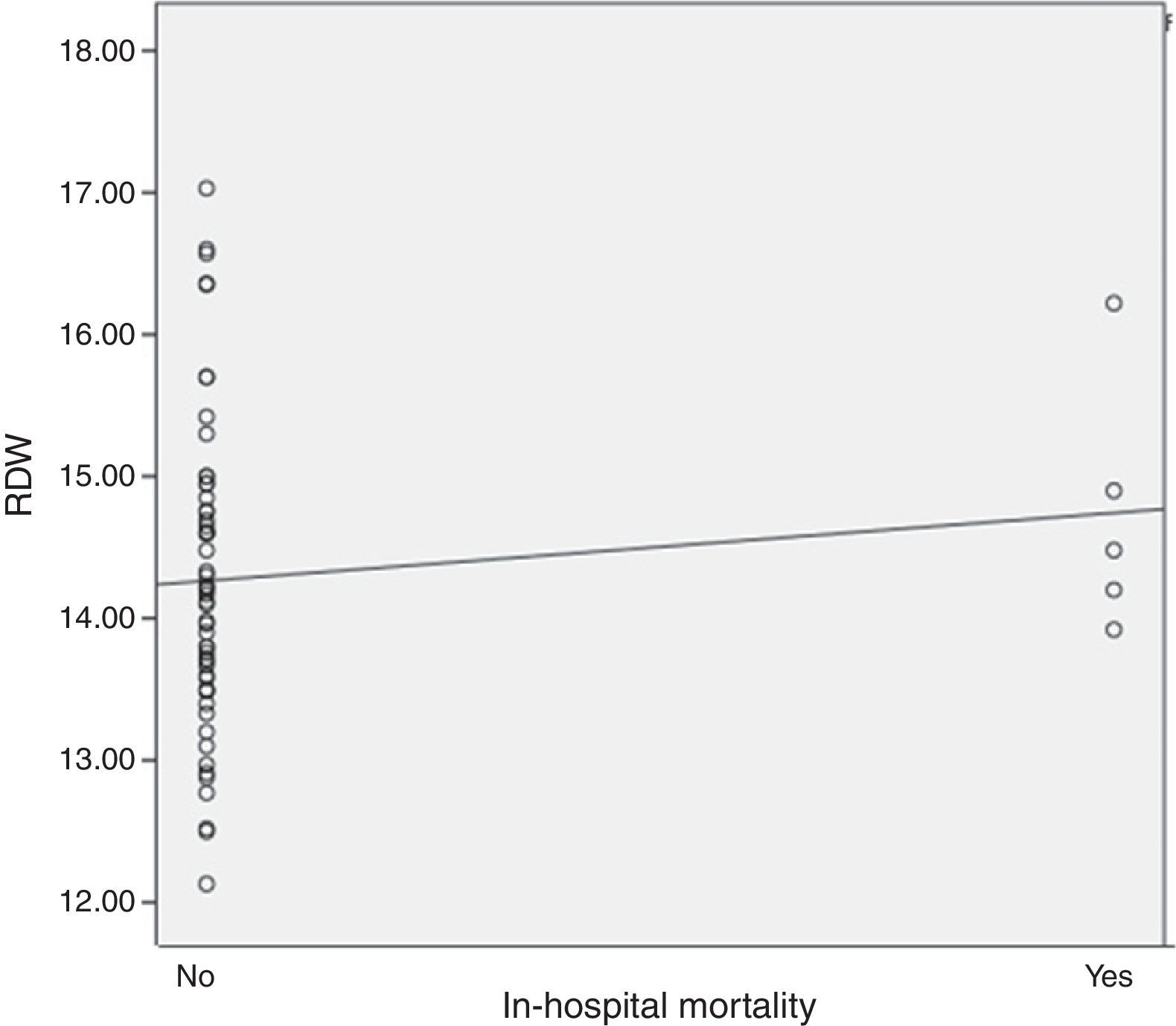
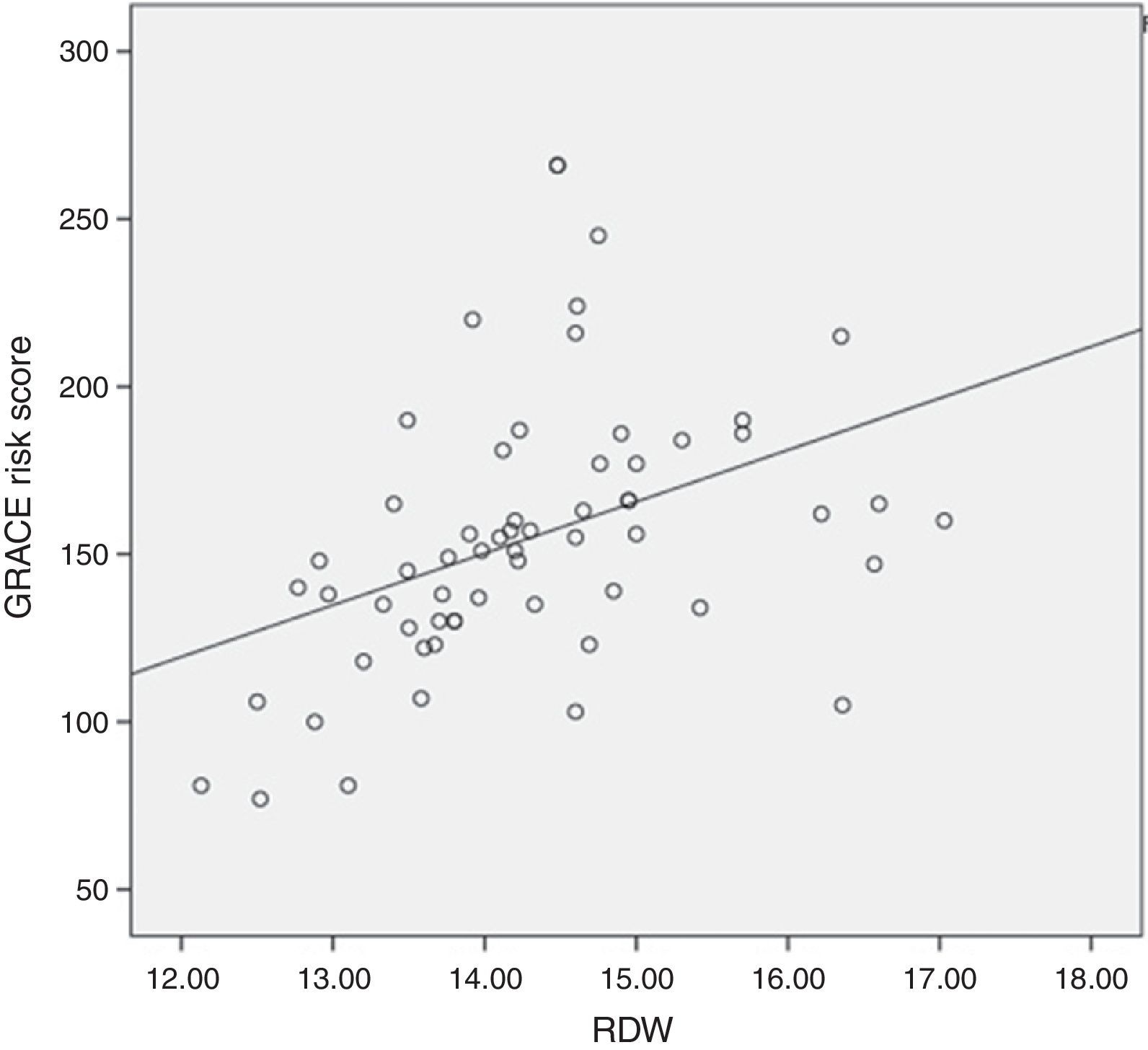
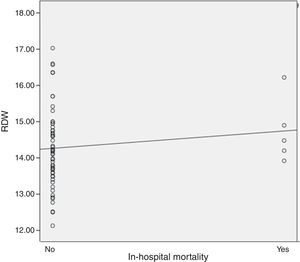
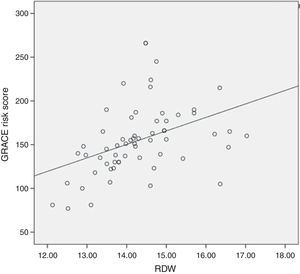
Results61 STEMI patients were included, 77% (47) male and 14% (14) female. Average age was 61.8±11.7 years. Average GRACE risk score was 154.9±40.3. Average RDW was 14.3±1.07. In-hospital mortality presented in 5 (8.1%) cases. It was found, as expected, a positive correlation between in-hospital mortality and the GRACE risk score (r=0.314, P=0.05). Regarding the primary end-point of the study, it was found a positive correlation between RDW and in-hospital mortality (r=0.343), however there was no statistical significance. Regarding the secondary end-point we observed a positive statistically significant correlation between RDW and the GRACE risk score at hospital admission (r=0.410, P=0.01).
ConclusionsRDW is a biological marker of easy acquisition that does not generate additional cost to neither the patient nor the health institutions. High RDW levels could be useful to predict in-hospital mortality in STEMI patients, as well as to give additional value to established risk scores such as the GRACE.
El ancho de distribución eritrocitaria (ADE) es el porcentaje de variación del volumen de los eritrocitos y ha sido identificado como un biomarcador en pacientes con enfermedad cardiovascular. Niveles elevados han sido asociados a peores resultados clínicos y se sugiere que podría ser útil como marcador pronóstico de riesgo en este grupo de pacientes.
MétodosSe realizó un estudio observacional, prospectivo, longitudinal y analítico con el objetivo de determinar la correlación entre el ADE y la mortalidad intrahospitalaria en pacientes con infarto al miocardio con elevación del segmento ST (IAMCEST). 61 pacientes fueron incluidos. Analizamos la correlación entre el ADE y la mortalidad intrahospitalaria así como aquella entre el ADE y la escala de riesgo GRACE al ingreso hospitalario. Se determinó la correlación de Pearson en ambos casos utilizando el programa SPSS statistics de IBM.
ResultadosSe incluyeron 61 pacientes con IAMCEST, 77% (47) hombres y 14% (14) mujeres. La edad promedio fue de 61.8±11.7 años. La puntuación promedio en la escala de GRACE fue 154.9±40.3. El ADE promedio fue de 14.3±1.07. La mortalidad intrahospitalaria se presentó en 5 (8.1%) casos. Se encontró, como era esperado, una correlación positiva entre la mortalidad intrahospitalaria y la puntuación en la escala de GRACE (r=0.314, P=0.05). Respecto al objetivo primario, se identificó una correlación positiva entre el ADE y la mortalidad intrahospitalaria (r=0.343), sin embargo, no fue estadísticamente significativa. Respecto al objetivo secundario, se identificó una correlación positiva y estadísticamente significativa entre el ADE y la puntuación en la escala de GRACE al ingreso hospitalario (r=0.410, P=0.01).
ConclusionesEl ADE es un marcador biológico de fácil adquisición, el cual no genera costos adicionales para el paciente ni para los servicios de salud. Niveles incrementados del ADE podrían ser de utilidad para predecir la mortalidad intrahospitalaria en pacientes con IAMCEST, así como aportar valor adicional a las escalas de riesgo establecidas, tales como la de GRACE.
Although the classical risk factors for cardiovascular disease (CVD) are very important, identification of potential novel risk factors could help clarify CVD pathophysiology, offers novel targets for intervention, and lead to improved risk stratification. Erythrocytes, or red blood cells (RBCs), are constituents of clots and thrombi formed in vivo, but little is known about whether inherent properties of RBCs could affect the risk for CVD.1
Classical in vitro studies of the function of the coagulation system are performed in plasma, without erythrocytes. However, RBCs are constituents of clots and thrombi formed in vivo and may play a prothrombotic role in blood coagulation by increasing blood viscosity and forcing platelets toward the vessel wall.1
Red blood cell distribution width (RDW) measures RBCs volume variations (anisocytosis) and is reported as part of a standard complete blood count.1–13 It is defined as the quotient of standard deviation of RBC volume and its mean volume and is expressed as a percentage according to the following formula: RDW=(standard deviation of red blood cell volume/mean cell volume)×100. (The result is multiplied by 100 in order to express it as a percentage.) Higher RDW values reflect greater variations in RBC volume.1–3 Although RDW is usually measured as a routine test, its levels have been mainly used in the differential diagnosis of several kinds of anemia and high levels indicate the presence of anisocytosis.1,4–9
Increased RDW has been associated with different CVDs such as coronary heart disease, stroke, heart failure, atrial fibrillation, peripheral artery disease, pulmonary arterial hypertension, and venous thromboembolism. RDW has also been associated with overall and cardiovascular mortality in different populations.1,3,9–11
MethodsThis was an observational, prospective, longitudinal and analytical study with the objective of determining the relation between RDW and in-hospital mortality. Sixty-one STEMI patients were included, all of them were admitted to the coronary unit of the department of cardiology at the General Hospital of México in the course of 6 months. Exclusion criteria were: chronic renal failure, hepatic failure, known hematological diseases, history of hemorrhage in the last three months that had required hospitalization and history of transfusions in the last three months. As these factors could result in substantial modifications of the RDW and play a bias in our study.
We analyzed the correlation between RDW and in-hospital mortality as well as that of RDW and the GRACE risk score (applied by means of medCalc statistical software) at hospital admission. Pearson correlation was determined in both cases by using IBM SPSS statistics software.
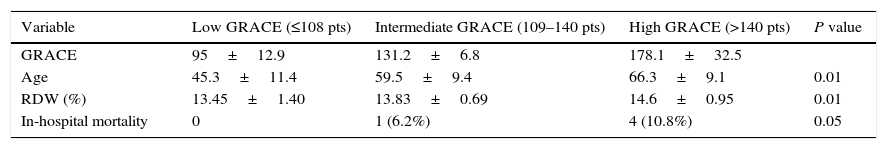
ResultsIn our study 61 STEMI patients were included, 77% (47) male and 14% (14) female. Average age was 61.8±11.7 years. Average GRACE risk score was 154.9±40.3, with 37 (60.6%) patients belonging to the high risk group, 16 (26.2%) patients to the intermediate risk group, and 8 (13.1%) to the low risk group. Treatment was pharmacological in 16 (26.6%) cases, thrombolysis in 7 (11.4%) cases, percutaneous coronary intervention (PCI) in 16 (26.2%) cases, pharmaco-invasive therapy in 19 (31.1%) cases and rescue-PCI in 3 (4.9%) cases. Average RDW was 14.3±1.07. In-hospital mortality presented in 5 (8.1%) cases; 4 in the high GRACE score group (RDW 14.6±0.95) and 1 in the intermediate GRACE score group (RDW 13.83±0.69). No in-hospital mortality cases presented in the low risk group (Table 1).
It was found, as expected, a positive correlation between in-hospital mortality and the GRACE risk score (r=0.314, P=0.05). Regarding the primary end-point of our study, it was found a positive correlation between RDW levels and in-hospital mortality (r=0.343), however there was no statistical significance (Fig. 1). Regarding the secondary end-point, it was found a statistically significant positive correlation between RDW and the GRACE risk score at hospital admission (r=0.410, P=0.01) (Fig. 2). In this study we also identified a positive correlation between RDW levels and age (r=0.392, P=0.01). There was no significant difference in RDW levels between male and female patients.
DiscussionIn our study we observed a positive correlation between RDW values and the parameters to which they were compared (in-hospital mortality and GRACE risk score), although in-hospital mortality was not statistically significant.
The factors probably involved in the increased RDW values in patients with CVD are related with pathophysiological mechanisms well established as the triggers for endothelial damage and atherosclerosis. Such mechanisms diminish the half-life of the erythrocytes, which generate an increment in peripheral mobilization of reticulocytes with the consequence of an RDW increment.1–3,6,12,13
The inflammatory response and endothelial damage manifest clinically as alterations in several parameters assessed in the GRACE risk score, which also result in RDW increment.1–3,6,12,13 Oxidative stress, inflammation, and released cytokines secondary to inflammation damages the iron metabolism, reduces the life span of erythrocytes, inhibits erythrocyte maturation, accelerates the migration of reticulocytes into the peripheral circulation, reduces the bone marrow response to erythropoietin, and increases RDW.1–3,5,6,9,12 This is particularly relevant when anemia and iron deficiency have been recognized as treatable co-morbid conditions.6,12
7 of 9 parameters assessed in the GRACE risk score (age, heart failure, history of myocardial infarction, increased heart rate, ST segment deviation, creatinine serum levels and elevated cardiac biomarkers),14 may be related with the mechanisms also implicated in the increment of RDW values.
Age of patients (the issue that generates a major negative impact in the GRACE risk score) was positively correlated with RDW in our study. Higher RDW has been associated with worse outcome in the elderly, in patients with heart failure or ischemic heart disease and in those undergoing percutaneous coronary revascularization, irrespective of baseline hematocrit.13 Although the pathophysiological mechanisms involved in this alteration are not well described, it is well known that there is an increased endothelial damage and accumulated risk factors for CVD as the patients grow older, which could explain, at least partially, these observations.
Heart failure is a condition that increases mortality in patients with myocardial infarction and its presence, as assessed by BNP and NT-proBNP serum levels, has been related with incremental RDW values.1,2,4,6,7 Therefore some authors have observed an increased RDW as a predictor of mortality in patients with heart failure2,4,6,7; even after adjustment for hematocrit.6,11 The severity of heart failure in STEMI patients, as assessed by the Killip and Kimball scale, is included in the GRACE risk score and explains, partially, the positive correlation between RDW and the final score in this risk scale observed in our study.
History of myocardial infarction could be related to some factors that increase RDW levels. Re-hospitalization of patients because of re-infarction was prognosticated by high levels of RDW during hospitalization, independent of revascularization and other laboratory parameters.3,9,11 Factors like increased oxidative stress, endothelial damage and atherogenic burden were related to the non-reflow phenomenon and the absence of collaterals in one study.10 Erythrocytes contain large amounts of free cholesterol, and pathological changes in the erythrocyte membrane and spread of inflammatory cascade causes erythrocyte aggregation in the atheromatous plaque and the storage of free cholesterol in the RBC membrane.1,2,9 This decreases RBC rheology, which impairs blood flow through the microcirculation, resulting in the diminution of oxygen supply at the tissue level.2 A positive correlation between high RDW and high total cholesterol levels that was contained by the erythrocyte membrane in myocardial infarction patients has been demonstrated.9 All of the aforementioned factors can be related not only to the risk of re-infarction, but also to the extension of the damaged myocardial tissue, the electrocardiogram (ECG) changes and the elevation of cardiac biomarkers.
ECG ST-segment deviation is related to the severity and to the level of the coronary arterial obstruction. Therefore factors that increase RDW, such as atherogenic burden2 and the absence of collateral vessels,10 can contribute to the presence of ST-segment deviations on the ECG.
The elevation of cardiac biomarkers is a determinant of the extension of the infarction, as well as a prognostic factor. In one study it was found an association between increased RDW and elevated troponin T levels.1 In another study a greater baseline RDW was associated with increased myocardial injury in non ST elevation myocardial infarction patients.7 Factors such as the absence of collateral vessels,10 the severity of the inflammatory damage,1–3,5,6,9,12 the atherogenic burden,2 heart failure1,2,4,6,7 and renal failure1; can potentially contribute to both increased cardiac biomarkers serum levels and higher RDW levels.
The presence of tachycardia at hospital admission in STEMI patients is a well-established risk factor for morbidity and mortality. The causes of tachycardia in these patients may be several, ongoing angina or acute heart failure can contribute in varying degrees. Erythrocyte progenitor cells are stimulated by neurohumoral and adrenergic system activation, depending on decreased erythropoiesis and, as a result, RDW levels increase.9,11 Because of this, independent of the cause, the activation of the adrenergic and neurohumoral systems is a direct cause of RDW increment.
Renal function, as assessed by the glomerular filtration rate, as well as the presence of microalbuminuria, has been observed to be positively related with the increment in RDW levels.1 The presence of renal insufficiency in myocardial infarction patients is multifactorial; heart failure, hypotension and cardiogenic or hypovolemic shock, contribute in varying degrees to its production. All of these mechanisms also may cause an increased RDW.
RDW is an epiphenomenon that reflexes the grade of severity in the alteration of multiple pathophysiological mechanisms, which lead to major clinical anomalies such as those assessed in the GRACE risk score.
Despite the aforementioned, because of RDW lack of specificity,1 its main strength may be if it adds information to the already established risk scores such as the global registry of acute coronary events (GRACE) score, used in this study, that was developed from the registry of patients within the wide spectrum of acute coronary syndrome and has been validated as a predictor of death or myocardial infarction (MI) during hospital stay and at 6 months following hospital discharge.14
Among the limitations of this study is the fact that patients were only followed during hospital stay, which could explain the lack of statistically significance in the correlation between RDW and in-hospital mortality. However, given the positive correlation observed between RDW and the GRACE risk score, we think that a long-term follow up (30 days to six months) of these patients may show a positive statistically significant correlation between RDW levels and overall mortality.
ConclusionsRDW is a biomarker whose increased levels are the result of multiple pathophysiological mechanisms that play a role in the myocardial infarction and which could be a useful tool for risk stratification in such patients. Its benefit relies upon its easy acquisition in daily clinical practice by means of a complete RBC count, which does not generate additional costs for neither the patients nor the health services. High RDW values could predict in-hospital mortality in STEMI patients, as well as give additional information to the validated GRACE risk score.
More studies with higher populations will be needed to support the use of RDW values as a biomarker in myocardial infarction patients. However, with the evidence we have so far, RDW could be implemented in the prognostic evaluation of STEMI patients, early at hospital admission.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe author declares this study has received no funding.
Conflict of interestThe author declares there are no conflicts of interest.