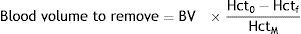Even though blood transfusion saves thousands of lives worldwide, it causes complications in some patients, and must therefore be correctly administered. As there is no universally accepted consensus on blood transfusion in surgical patients, we have reviewed the latest studies and gathered the best available evidence on blood management strategies. In this study, we discuss indicators for transfusion of erythrocytes and other blood products, haemostatic agents for cardiothoracic and orthopaedic interventions where it is imperative to regulate blood loss, and alternatives in specific situations such as Jehovah's Witnesses patients. Finally, we put forward an algorithm for the preoperative management of surgery patients with low haemoglobin levels.
Si bien la transfusión de sangre ha salvado miles de vidas en el mundo, algunos otros han sido víctimas de sus potenciales complicaciones, por lo cual debe ser correctamente empleada. No existe aún consenso aceptado universalmente sobre la transfusión sanguínea en el paciente quirúrgico, por lo cual se brinda esta revisión construida con los estudios más actuales y con mejor evidencia al respecto. Se mencionarán los indicadores para transfusión de eritrocitos y otros hemoderivados, la preparación preoperatoria, manejo transoperatorio y postoperatorio, agentes hemostáticos a emplear en cirugías cardiotorácicas u ortopédicas donde es imperante regular las pérdidas sanguíneas, así como alternativas en situaciones específicas como pacientes testigos de Jehová. Por último, se brinda un algoritmo para el abordaje de pacientes con hemoglobina baja y deban ser sometidos a cirugía.
Although blood transfusions have saved thousands of lives worldwide, they are not without their complications. In general surgery, blood transfusions are given to improve oxygen delivery to tissues, based on the patient's physiological requirements. Under normovolaemic conditions, the body responds to a loss of haemoglobin by increasing the cardiac output. In a normal heart, increased lactate production and an oxygen extraction ratio of 50% occur at a haemoglobin level of approximately 3.5–4g/dL, while in coronary stenosis, the anaerobic state occurs at a haemoglobin level of between 6.0 and 7.0g/dL. For this reason, the threshold for preoperative packed red blood cell (RBC) transfusion has been discussed at length in numerous studies and clinical trials.1 In a review of 16 reports of the surgical outcome of a series of patients of the Jehovah's Witness faith, only 1.4% of 1404 procedures resulted in death due to anaemia.2 In another review of 61 Jehovah's Witness patients, all deaths with the exception of 3 patients that died following cardiac surgery, attributable to anaemia occurred in patients with a haemoglobin level below 5.0g/dL.3 On the basis on these and other studies in which haemoglobin levels of less than 8.0g/dL have been associated with a 2.5 increase in risk for mortality, the blood transfusion threshold in surgical patients with no cardiovascular disease is now considered to be 8.0g/dL. Other authors mention the importance of taking the anaesthesia technique into consideration, as it can reduce cardiac output. In addition to this, surgery itself often causes acute haemorrhage to a greater or lesser extent, and therefore raised the transfusion threshold to 10.0g/dL.1,4 The debate on the appropriate haemoglobin threshold for transfusion continues to this day. In an attempt to clarify this issue, we give a brief description of the different schools of thought and the most recent evidence-based transfusion guidelines.
To transfuse or not to transfuseSome groups advise against preoperative transfusion in haemodynamically stable patients with low haemoglobin levels due to the considerable number of potential adverse effects, such as alloimmunization, transfusion reactions, fluid overload, infection, electrolyte imbalance. In view of this, efforts have been made to develop and implement new methods that elevate haemoglobin levels, prevent adverse reactions, and contribute to patient safety. Generally speaking, these can be divided into 2 categories: autologous blood reinfusion, and techniques aimed at limiting blood loss. Strictly speaking, these approaches can be divided into a series of strategies dubbed “pillars of blood management”: (1) optimising haematopoiesis and appropriate management of anaemia; (2) minimising bleeding; (3) harnessing and optimising physiological tolerance of anaemia using all available modalities while specific treatment is initiated. Some examples of these strategies include: autologous transfusion, haemodilution, blood substitutes, intra- and postoperative red cell salvage, haematopoietic factors, haemostatic medication, antifibrinolytics, fibrin sealants, and conjugated oestrogens.
Another challenge for surgeons arises when, either on religious grounds or due to lack of blood compatible with a particular blood phenotype, allogeneic transfusion is not an option and other solutions must be found. First and foremost, these patients must be treated by a multidisciplinary team that can ensure their safety while respecting their beliefs. In a report published by clinicians from the “Soonchunhyang Bloodless Center” in Korea, a teaching hospital specialising in performing surgery without allogeneic blood transfusions (94% of its patients are Jehova's Witnesses), 1407 patients scheduled for various types of surgery were grouped by Hb level (≤7.0g/dL and >7.0g/dL). The patients were given erythropoietin or darbepoetin together with intravenous or oral iron supplements after quantifying their levels of iron, ferritin, folic acid and vitamin B12. Overall mortality was 0.8% (11 patients) in the ≤7.0g/dL group, and 0.2% in the >7.0g/dL group. These results suggest that blood substitutes can safely be used in both these groups of patients when risk factors, comorbidities and risk of bleeding are taken into account, and each patient is correctly prepared for each type of surgery.4–6
The foregoing report describes elective surgery; however, whole blood is still used in modern practice for particularly serious and emergency situations, such as patients undergoing surgery to treat wounds received in combat. In this context, a study carried out in the French military hospital in Kabul between 2006 and 2009 included 15 patients receiving overall 66 units of fresh whole blood (at a mean of 3 units per patient). All blood units had been tested for HIV and other pathogens using rapid testing techniques. No complications were reported in either donors or recipients. Average time from collection to transfusion was 140±197min (mean 43min). Mortality among recipients was 27%. It is important to stress that this technique is reserved exclusively for the military or extreme emergencies.7 In these cases, fresh whole blood is essential for oxygen transport and adequate coagulation, since studies have shown that storing whole blood interferes with the metabolism, biochemistry, morphology and activity of red blood cells, and therefore affects the quality of the blood. It has also been reported that the longer the blood is stored, the greater the detrimental effect on coagulation. This is mainly caused by a gradual decrease in the pH of transfused RBC while the plasma buffer is overpowered due to the acidic overload.8 Because of these changes, the mortality rate is significantly increased in comparison with packed red blood cells, which can be stored for up to 6 weeks.9
Allogeneic transfusion is emerging as a risk factor for many common complications in transfused patients, such as pulmonary lesion and hospital-acquired infection, and is a predictor of morbidity and mortality in critical patients. As a result, the notion of blood transfusion as a revitalising treatment capable of improving a patient's overall status and speeding up recovery has been replaced by the more rational view that use other, less risky, methods must be used. Furthermore, the increased demand in an ageing population, and a drop in the number of blood donors have made it even more imperative to take a different approach to transfusion. Studies have shown that most (up to 94%) transfusions performed in surgery patients can be attributed to one or a combination of the following factors: low preoperative haemoglobin levels, excessive intra- and postoperative blood loss, the type of surgery performed, and finally, inappropriate transfusion protocols. In view of the negative results of inappropriate transfusions, some experts have suggested including blood transfusion among surgery quality indicators.
The contribution made to research into blood transfusion by Jehova's Witness patients must be acknowledged. Treating these patients can at times present a major challenge, particularly in situations often associated with major, acute blood loss and/or severe anaemia that require immediate blood transfusion. It is important to remember that although Jehova's Witnesses refuse blood transfusion, they do not reject other treatment strategies, and much is still to be discovered in the field of blood transfusion alternatives.6,10
Indicators for transfusionVarious guidelines on blood transfusion for every type of patient have been published. All stress that blood products must only be given when it is physiologically necessary, and not on the basis or arbitrary haemoglobin or haematocrit levels. In 2011, a study was conducted into whether RBC transfusions mentioned in published studies were really needed. The authors reviewed 494 articles, finding that transfusion was appropriate in just 11.8%. In 59.3% it was inappropriate for the specific status of each patient, and in 28.9% the suitability of the transfusion could not be determined.11 Hb levels in this latter group of patients in which the need for transfusion was “unclear” were 10.0g/dL. Nevertheless, the necessity of blood transfusion in haemodynamically stable patients with Hb levels between 6.0g/dL and 10.0g/dL is still hotly disputed. Under current guidelines, blood transfusion is not indicated in the treatment of patients with Hb levels of >10.0g/dL, and should only be considered in patients with Hb levels of <6.0g/dL, and possibly, although not wholly justifiably, in ischaemic heart disease patients with Hb levels of <8.0g/dL.
Whenever possible, physiological tissue oxygen and ischaemia indicators should be used to guide the decision to transfuse blood products. In 2012, the American Association of Blood Banks (AABB) published their clinical practice guidelines on the transfusion of RBC, with recommendations graded according to their level of evidence:
- (1)
The AABB recommends adhering to a restrictive transfusion strategy. In adult and paediatric intensive care unit patients, transfusion should be considered at haemoglobin concentrations of 7g/dL or less. Quality of evidence: high; strength of recommendation: strong.
- (2)
In hospitalized, haemodynamically stable patients with pre-existing cardiovascular disease, the AABB suggests adhering to a restrictive transfusion strategy. Transfusion should be considered at a haemoglobin concentration of 8g/dL or less or for symptoms (chest pain, orthostatic hypotension or tachycardia unresponsive to fluid resuscitation, or congestive heart failure). Quality of evidence: moderate; strength of recommendation: weak.
- (3)
In hospitalized, haemodynamically stable patients with acute coronary syndrome, the AABB cannot recommend for or against a liberal or restrictive RBC transfusion threshold. Further research is needed to determine the optimal threshold. Quality of evidence: very low.
- (4)
In hospitalized, haemodynamically stable patients, transfusion decisions should be influenced by symptoms as well as haemoglobin concentration.
These recommendations are applicable in most postoperative patients, and also in non-surgical patients, with the exception of patients with acute coronary syndrome. Transfusion should also be restricted in patients following an autologous blood transfusion programme.
To facilitate compliance with these recommendations, situations in which RBC transfusion would be appropriate have been clearly defined:
- (a)
Treatment of symptomatic anaemia.
- (b)
Prophylaxis in life-threatening anaemia.
- (c)
Restoration of oxygen-carrying capacity in case of haemorrhage.
- (d)
RBCs are also indicated for exchange transfusion in diseases such as sickle cell anaemia, severe parasitic infection (malaria, babesiosis), severe methemoglobinaemia and severe hyperbilirubinaemia in newborns.
Dose and administration:
- •
One unit of RBC will raise the haemoglobin level of an average-size adult (70kg) by ∼1.0–1.5g/dL (or raise HCT by ∼3%).
- •
Transfuse slowly for first 15min.
- •
Complete transfusion within 4h.
RBC transfusion is NOT routinely indicated for pharmacologically treatable anaemia such as:
- (a)
Iron deficiency anaemia (due to nutritional deficiency or chronic blood loss).
- (b)
Vitamin B12 or folate deficiency anaemia.
Carson et al. carried out a systematic Cochrane review of 19 studies with a total of 6264 patients in order to determine haemoglobin thresholds for RBC transfusion. The authors conclude that restrictive transfusion strategies were associated with a statistically significant reduction in mortality in hospitalised patients, but not in 30-day mortality. They go to emphasise that giving less blood is in fact safer, and that RBC transfusion is not essential until Hb concentrations fall below 7.0 or 8.0g/dL.6,12,13
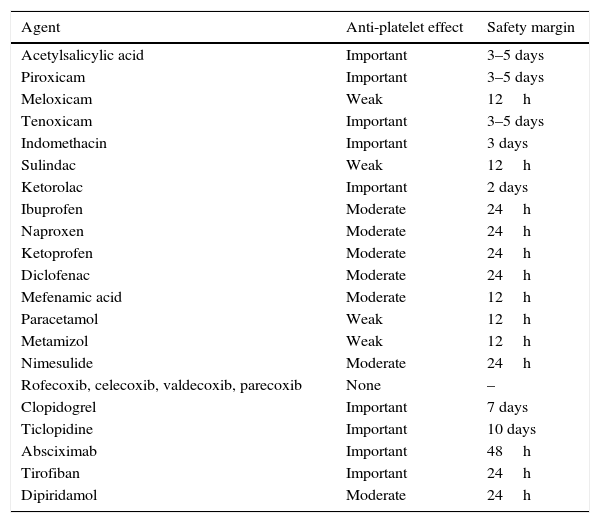
Preoperative preparationA well-documented clinical history and thorough physical examination are needed to determine potential risk factors for decompensation, bleeding or thrombosis. Particular attention should be paid to the patient's personal and family history in respect of the following factors: mucocutaneous bleeding, ecchymosis or frequent spontaneous bruising, previous surgery requiring transfusion, use of haemostatic agents to control unforeseen bleeding, postoperative bleeding, menstrual cycle and characteristics, and use of prescription or over-the-counter medication that can affect coagulation, such as: vitamin K, low molecular weight heparins, warfarin, acenocoumarol, anisindione, bromadiolone, brodifacoum, diphenadione, chlorophacinone, pindone, NSAIDs, clopidogrel, dipyridamole, ticlopidine, and IIb/IIIa inhibitors (Table 1). Pentoxifylline and some cephalosporins and penicillins can inhibit platelet aggregation and increase the risk of bleeding. Acetylsalicylic acid (ASA) has also been widely studied due to its antiplatelet action derived from irreversible inhibition of COX-1, which prolongs the action of ASA to 7–10 days (platelet lifetime). However, 3 or 4 days after administration, the number of platelets is sufficient to ensure adequate haemostasis. Other NSAIDs also inhibit platelet aggregation by a mechanism similar to that of ASA, but with 2 major differences: firstly, COX-1 inhibition is reversible, so platelet function is restored once the drug has been eliminated. Secondly, NSAIDs differ greatly in their capacity to inhibit COX-1 and consequently in their anti-platelet aggregation action.14 In this regard, in the recent update of their blood conservation clinical practice guidelines The Society of Thoracic Surgeons and The Society of Cardiovascular Anaesthesiologist recommend (class of recommendation 1, level of evidence B) that drugs that inhibit the platelet P2Y12 receptor should be discontinued at least 3 days prior to coronary revascularization.
Antiplatelet effect of NSAIDs and other agents.
| Agent | Anti-platelet effect | Safety margin |
|---|---|---|
| Acetylsalicylic acid | Important | 3–5 days |
| Piroxicam | Important | 3–5 days |
| Meloxicam | Weak | 12h |
| Tenoxicam | Important | 3–5 days |
| Indomethacin | Important | 3 days |
| Sulindac | Weak | 12h |
| Ketorolac | Important | 2 days |
| Ibuprofen | Moderate | 24h |
| Naproxen | Moderate | 24h |
| Ketoprofen | Moderate | 24h |
| Diclofenac | Moderate | 24h |
| Mefenamic acid | Moderate | 12h |
| Paracetamol | Weak | 12h |
| Metamizol | Weak | 12h |
| Nimesulide | Moderate | 24h |
| Rofecoxib, celecoxib, valdecoxib, parecoxib | None | – |
| Clopidogrel | Important | 7 days |
| Ticlopidine | Important | 10 days |
| Absciximab | Important | 48h |
| Tirofiban | Important | 24h |
| Dipiridamol | Moderate | 24h |
It is recommended that coagulation tests including bleeding time be performed prior to surgery using either the Ivy (normal values: 3–10min) or Duke (normal values: 5–5min) methods. Although these methods have less sensitivity (64.2%) and specificity (99.1%) for predicting intraoperative bleeding, a normal finding in a correctly performed test indicates that the drug given has not significantly affected primary haemostasis. Another useful study is the PFA-100 platelet function analyser, an automated testing system that uses sequential platelet activators. The sample is first tested using collagen/epinephrine. A closure time of <180s rules out the presence of any significant platelet dysfunction. If the closure time is >180s, a further test is performed using collagen/adenosine diphosphate (ADP). A normal finding (<120s) on this test indicates the probability of aspirin-induced platelet dysfunction. Prolongation of both closure times (collagen/epinephrine >180s and collagen/ADP >120s) indicates one of the following platelet dysfunctions:
- (1)
Anaemia (haematocrit <28%).
- (2)
Thrombocytopaenia <100×109L−1.
- (3)
Significant platelet dysfunction (other than that caused by aspirin).
Wherever possible, minimally invasive procedures are preferred, such as laparoscopy or interventional radiology. Other strategies, such as preoperative embolisation, can be effective in inhibiting blood flow and reducing intraoperative bleeding.15,16 Some vitamins and herbal supplements can also affect haemostasis: (a) cranberries, fish oil, ginger, ginkgo biloba, grape seed extract reduce platelet aggregation; (b) camomile and dandelion root inhibit coagulation; (c) vitamin K, and to a lesser extent vitamin E, modify coagulation.17
Management of anaemiaAnaemia is an independent predictive factor for morbidity and mortality.18 Recommendations on the management of preoperative anaemia have now been changed to discourage the use of RBC transfusion. The Network for Advancement of Transfusion Alternatives (NATA) recently recommended measuring haemoglobin levels 4 weeks prior to elective orthopaedic surgery. A comprehensive treatment approach should be taken with these patients to achieve normal preoperative haemoglobin concentration.19 Therapy will always depend on the aetiology of the anaemia and the resources available. When haemoglobin is less than 6g/dL, mean corpuscular volume (MCV) is mainly used to suggest aetiology and guide treatment strategies. An MCV of over 80fl will suggest anaemia secondary to other pathology such as cancer or kidney disease, or even acute bleeding. An MCV of less that 80fl, meanwhile, will support a diagnosis of iron deficiency anaemia, and iron supplement therapy must be started immediately to correct the imbalance. The most widely used haematinics are oral or intravenous iron supplements (intramuscular preparations, which must be administered by trained personnel and are associated with local pain, pigmentation and even sarcoma, are not recommended), folic acid, vitamin B12, and erythropoiesis-stimulating agents. Iron supplement dosage is based on a formula used to calculate total iron deficiency (iron deficiency=(target Hb−actual Hb)×2.4×kg weight+500) and on the cause of chronic iron loss. Haemoglobin levels recover quite rapidly, and less allergenic, faster-acting preparations are currently available, such as iron sucrose and ferumoxytol, which can deliver 510mg iron in 17s. Treatment response is evaluated by quantifying reticulocyte levels, which should be measurable at 4 and 7 days after start of treatment. Haemoglobin levels and haematocrit improve after 1 or 2 weeks of iv administration, but can take several weeks to return to normal levels. Anaemia is fully resolved within 4–6 weeks. Adjuvant therapy with erythropoiesis-stimulating agents such as erythropoietin (EPO) improves the effectiveness of iron therapy and reduces dose requirements. Evidence has shown that intravenous iron with or without adjuvant EPO can reduce the need for transfusion in surgical patients. Combined administration of these agents 2–4 weeks before cardiac surgery increases red cell mass in patients with preoperative anaemia, in patients refusing blood transfusion, and in those at high risk for postoperative anaemia (class of recommendation IIa, level of evidence B). However, chronic use of EPO is associated with thrombotic cardiovascular events in cardiac and/or renal failure patients.6,16,20
Pre-donationIn elective procedures with high risk of significant blood loss and need for transfusion, preoperative autologous donation can be considered. In this procedure, a few units of the patient's blood are collected and stored during the weeks preceding the procedure and re-infused to the patient perioperatively if needed. However, some patients included in pre-donation programmes will need haematinics to prevent onset of anaemia. The procedure must be timed appropriately to ensure that the patients are not anaemic at the time of surgery due to pre-operative autologous donation. Additionally, storage of pre-donated blood units is similar to allogeneic blood units, and hence may suffer from the same deleterious effects of storage and from blood transfusion errors. Recombinant human erythropoietin may be considered to restore red cell volume in patients undergoing PAD before cardiac procedures (class of recommendation IIb, level of evidence A).6,16 The ideal method for autologous red blood cell collection is double red cell apheresis obtained within the 42-day period prior to start of elective surgery. Patients should be given haematinics alone or in combination with erythropoietin to ensure a speedy recovery.
Intraoperative managementPatient blood management strategies during surgery generally focus on minimising blood loss, collecting and re-infusing shed blood, and improving tolerance of anaemia. Vital signs should be closely monitored, and unnecessary hypovolaemia and tachycardia should be avoided. Various options are available to limit blood flow to the site of surgery, thereby limiting blood loss: patient positioning to elevate the site of surgery, use of tourniquet, embolisation or infusion of local vasoconstrictive agents. Hypotensive anaesthesia is another approach to limiting blood loss, although hypotension must be closely monitored by a team of experienced anaesthetists to ensure adequate perfusion of vital organs. Other methods for improving intraoperative haemostasis at the site of bleeding are: electrocautery and argon-beam coagulation, for cleaner cuts together with more effective haemostasis; topical haemostatic agents containing a mixture of thrombin, fibrinogen, calcium, collagen, gelatine, and cellulose act as a fast-acting tamponade of bleeding vessels; antifibrinolytic agents such as tranexamic acid and epsilon aminocaproic acid (lysine analogues), which act by preserving blood clots formed at sites of bleeding. These strategies have been shown to be safe and effective in reducing bleeding and transfusions in surgical patients (class of recommendation 1, level of evidence A). Williams-Johnson et al. showed that these agents, in addition to being safe and effective in reducing bleeding and transfusion rates, also reduce mortality in various patient populations.6,16,21 Aprotinin, a polypeptide derived from bovine lung tissue, is an enzyme inhibitor that degrades proteins such as human trypsin, plasmin, plasma and tissue kallikrein (fibrinolysis inhibitor), reduces transfusional requirements but has been associated with a 49–53% increase in risk of 30-day mortality, and a 47% increase in risk of renal dysfunction in adult patients, (class of recommendation III, level of evidence A). Systemic infusion of certain coagulation factors (activated factor VII, prothrombin complex, factor XIII, and fibrinogen) are also effective in achieving haemostasis, but their safety profile remains unclear. Specifically, recent studies have questioned the efficacy of high-dose recombinant activated factor VII in reducing bleeding at the expense of an increased risk of thromboembolic events.
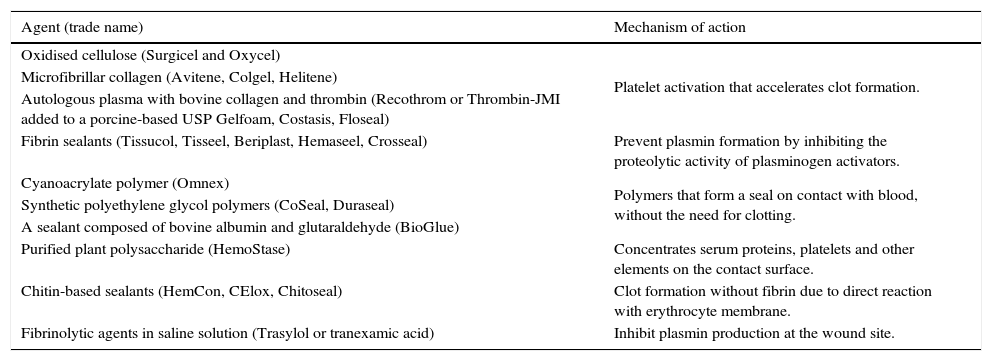
Another systemic approach to minimising blood loss is to avoid hypothermia (if not otherwise indicated), as hypothermia can adversely affect platelet function. Table 2 shows the local haemostatic agents currently used in surgery that have been given a class of recommendation of II and level of evidence B for cardiac surgery, and are also broadly recommended in orthopaedic surgery.16,22,23
Local haemostatic agents used in surgery, by mechanism of action.
| Agent (trade name) | Mechanism of action |
|---|---|
| Oxidised cellulose (Surgicel and Oxycel) | Platelet activation that accelerates clot formation. |
| Microfibrillar collagen (Avitene, Colgel, Helitene) | |
| Autologous plasma with bovine collagen and thrombin (Recothrom or Thrombin-JMI added to a porcine-based USP Gelfoam, Costasis, Floseal) | |
| Fibrin sealants (Tissucol, Tisseel, Beriplast, Hemaseel, Crosseal) | Prevent plasmin formation by inhibiting the proteolytic activity of plasminogen activators. |
| Cyanoacrylate polymer (Omnex) | Polymers that form a seal on contact with blood, without the need for clotting. |
| Synthetic polyethylene glycol polymers (CoSeal, Duraseal) | |
| A sealant composed of bovine albumin and glutaraldehyde (BioGlue) | |
| Purified plant polysaccharide (HemoStase) | Concentrates serum proteins, platelets and other elements on the contact surface. |
| Chitin-based sealants (HemCon, CElox, Chitoseal) | Clot formation without fibrin due to direct reaction with erythrocyte membrane. |
| Fibrinolytic agents in saline solution (Trasylol or tranexamic acid) | Inhibit plasmin production at the wound site. |
There are also other strategies for avoiding allogeneic transfusion, such as collecting the blood lost during the surgery (cell salvage), which is then washed and/or filtered and re-infused into the patient. Although promising, the technique has been associated with the reintroduction of tumour cells, fat droplets, amniotic fluid, bacteria, or pharmaceutical agents present in the surgical field. Cell salvage requires high-end filtering devices to be installed in the operating room, although several studies have indicated that leucocyte depletion filters are also able to remove unwanted cells and particulate materials. Another strategy is normovolaemic haemodilution, which relies on removing a part of the blood volume from the circulation and replacing it with colloid or crystalloid solutions before onset of bleeding. The blood lost during the surgery, therefore, is diluted, and the total amount of blood loss from the surgical wound is reduced. The collected blood is kept in the operating room at ambient temperature and is re-infused back to the patient within at least 6h (class of recommendation I, level of evidence C). Gross proposed a formula for calculating blood volume to be removed in allogeneic blood transfusion and haemdilution procedures.24
BV=blood volume in litres (body surface m2×2.5); Hct0=haematocrit at start; Hctf=end haematocrit permitted; and HctAV=average start and end haematocrit.Postoperative managementThe postoperative period is as important as the surgery itself, and close monitoring is essential to detect complications that require re-intervention, transfusion, or urgent treatment. In patients undergoing complicated procedures, postoperative laboratory tests are strongly recommended over the first 48h, and then every 24h after that. Blood can also be salvaged from wound drainage and later re-infused. Optimal oxygenation strategies will ensure delivery of sufficient oxygen to wound tissue or sacrificed tissue when blood flow has been restricted to reduce intraoperative bleeding, irrespective of the transfusion method used (allogeneic, autologous or haemodilution). Prophylaxis of upper gastrointestinal bleeding by means of proton pump inhibitors is recommended. If anaemia (previously absent) is shown on blood tests, additional physical examination, patient interviews and laboratory tests should be performed to locate “hidden” source of bleeding. If this can be ruled out, and the patient remains haemodynamically stable, a multidisciplinary approach should be taken to identify the cause (immune, alloimune or microangiopathic haemolytic anaemia, disseminated intravascular coagulation), administer treatment, and as far as possible avoid re-intervention that would increase the risk for morbidity and mortality (unless this is unavoidable, or suspicion of bleeding is strong). The decision to transfuse should be based on the patient's clinical status, on the cause of the anaemia, and on haemodynamic and blood oxygen parameters. RBC transfusion is justified in patients with >3g/dL decrease in Hb in less than 24h for no apparent reason (after ruling out all possible complications), and therefore with no clear treatment strategy. In patients with an Hb loss of <3g/dL in 24h with a clearly identified, and therefore treatable cause, haematinic agents or erythropoiesis stimulating agents should be given. Several weeks of therapy will be needed to normalise Hb levels, but this strategy will avoid the risks associated with transfusion. It is important to bear in mind that in the postoperative period transfusion should only be given when strictly necessary due to acute blood loss or haemodynamic instability. Transfusion for other less pressing reasons (to hasten recovery, treat fatigue, general malaise, or to shorten hospital stay) should be avoided, as the risks of blood transfusion will most probably outweigh the benefits.
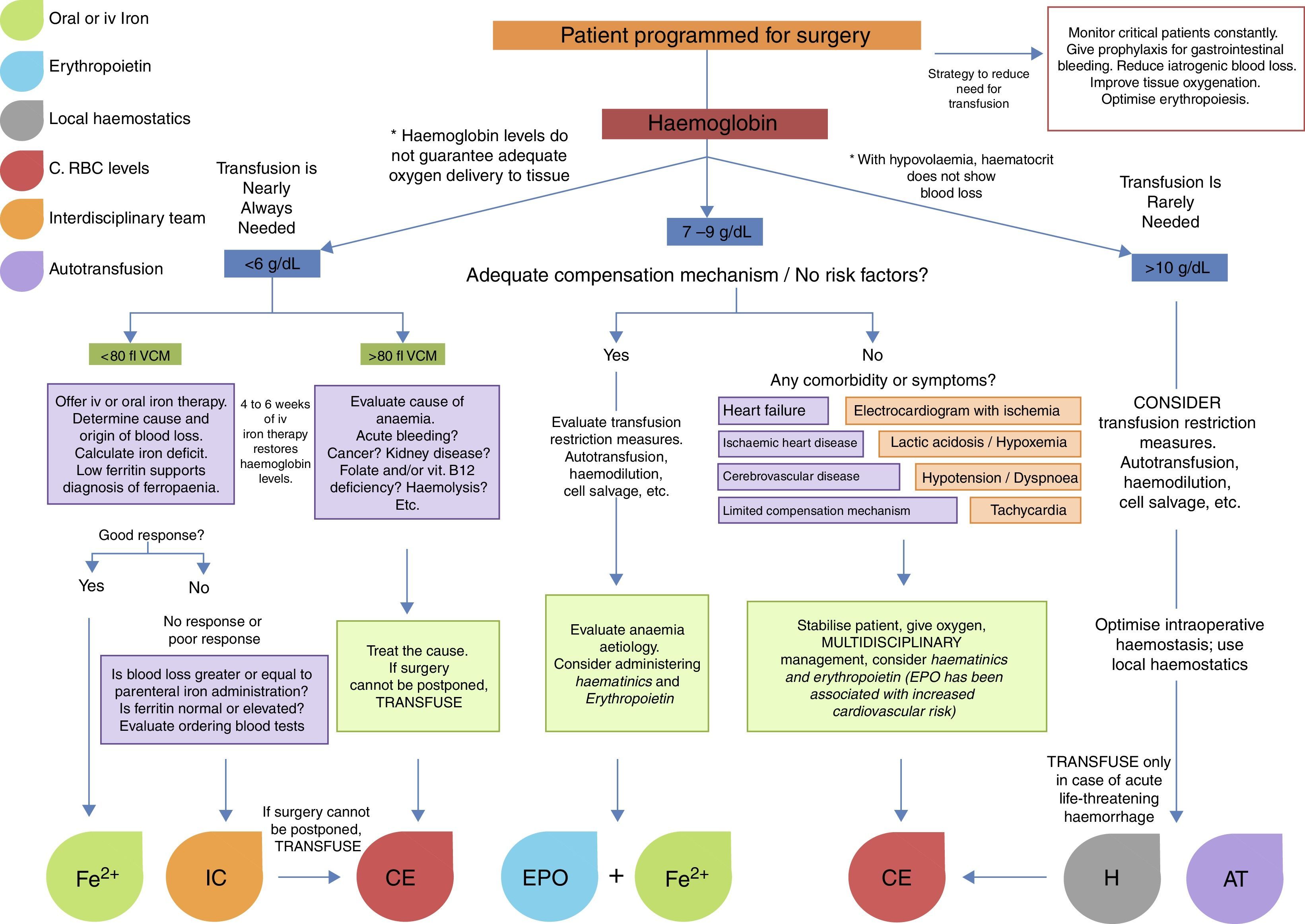
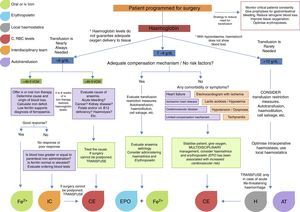
Fig. 1 summarises the treatment strategy applied at each stage of surgery.24–26
Indications for fresh plasma, cryoprecipitates and platelet concentratesWe have so far only discussed transfusion of red blood cells, but evidence for the use of other blood products should also be addressed. According to international guidelines, the prophylactic use of fresh-frozen plasma in surgery in the absence of clotting disorders is not recommended, as it does not reduce blood loss, and exposes patients to unnecessary risks and immunological complications, (level of recommendation IIIA). The following is a list of current specific indications for transfusion of fresh-frozen plasma.6,16,27,28
- (1)
Replacement of multiple coagulation factor deficiencies (in patients with bleeding or prior to an invasive procedure).
- (2)
Reversal of over-anticoagulation due to warfarin or acenocoumarol if no prothrombin complex concentrate or vitamin K is available (iv dose 5–10mg/day, needing 12–24h to bring INR to below 2.0) in patients with bleeding or prior to urgent invasive procedures.
- (3)
Disseminated intravascular coagulation or consumption coagulopathy in patients with bleeding.
- (4)
Dilutional coagulopathy (massive transfusion).
- (5)
Replacement of specific factor (factor IX) or protease (ADAMTS-13) deficiencies in thrombotic thrombocytopaenic purpura (TTP).
- (6)
When no factor concentrates are available.
- (7)
Plasmapheresis.
- (8)
In the presence of uncommon factor deficiencies.
The preoperative waiting period in patients anticoagulated with unfractionated or low-molecular-weight heparin is 6 and 12h, respectively. Administration of these agents should restart 12 after surgery due to risk for re-thrombosis.
In a recent update of a systematic review of randomised controlled trials on the effectiveness of plasma transfusion in a variety of therapeutic and prevention settings, Yang et al. concluded that there is no statistical evidence to recommend fresh-frozen plasma over clotting factors concentrate.29 Fresh-frozen plasma transfusion is associated with multiple adverse effects, usually caused by incorrect prophylactic or therapeutic use. Approximately 30–50% of fresh-frozen plasma transfusions are prophylactic, with or without a planned procedure. Of these, 50% do not conform to guidelines. In its guidelines for fresh-frozen plasma transfusion, the AABB cites clear evidence for its use in massive transfusion and in warfarin anticoagulation-related intracranial haemorrhage, but not in other contexts.28 One strategy for reducing the adverse effects of plasma transfusion that has proved successful in developed countries is to restrict collection to male donors that have never been transfused, excluding multiparous women due their association with a greater risk of antibodies. The risks most commonly associated with plasma transfusion are:
- (1)
TRALI (transfusion-related acute lung injury).
- (2)
TACO (transfusion associated circulatory overload).
- (3)
Anaphylactic and/or allergic reactions.
- (4)
Infectious disease transmission.
- (5)
Febrile non-haemolytic transfusion reaction.
- (6)
Red blood cell alloimmunization.
- (7)
Haemolytic transfusion reaction.
Fresh-frozen plasma transfusion has even been associated with nosocomial infection in surgery patients. For these reasons, it is imperative to use plasma products appropriately in order to limit risks and increase benefits.30
Platelet transfusion in the intraoperative or immediate preoperative period is indicated in patients with bleeding, thrombocytopaenia, or primary or secondary platelet dysfunction. Absolute indication for platelet transfusion in any context is severe thrombocytopaenia (less that 30×103/mcL) with active haemorrhage, or less than 10×103/mcL with or without bleeding. However, in surgical patients these indications change in accordance with the type and site of surgery, the presence of pre-existing clotting disorders, and the patient's clinical status. Transfusion should be considered when platelet levels are below 50×103/mcL, or less than 100×103/mcL in the case of brain surgery (level of recommendation 2C). During massive transfusion, and in disseminated intravascular coagulation, platelet levels should be maintained at 75×103/mcL to prevent reaching levels of 50×103/mcL (level of recommendation 2C). In procedures involving cardiopulmonary bypass, platelet transfusion is recommended in patients with non-surgery related postoperative bleeding or other clotting disorders (level of recommendation 2C). In patients with anaemia and thrombocytopaenia of less than 20×103/mcL, without bleeding, increasing haematocrit by 30% can reduce the risk of bleeding. One platelet concentrate per 10kg of body weight should be given. Half-life in circulation will range from a matter of hours to 5 days, depending on the status of the patient.
Use of cryoprecipitates is restricted to 2 types of patients: type A haemophiliacs or patients with type 2 and 3 Von Willebrand disease for whom Factor VIII concentrate, with or without FvW, is not available, depending on the case; and patients with primary or secondary hypofibrinogenaemia (with liver disease), when fibrogen levels fall below 100mg/dL (level of recommendation 2C).
Surgery in cancer patients is challenging in both medical and transfusional terms. Surgery is usually more invasive, the surgical site is usually highly vascularised, and the clinical characteristics of the patients are far from ideal (pre-existing anaemia, advanced age, malnutrition, comorbidities, etc.). Because of this, indications for transfusion in these patients are less clear, and rely more on the experience of the surgeon than on guidelines. Nevertheless, the foregoing recommendations for transfusion are applicable in cancer patients. Al-Refaie et al. reported that postoperative RBC transfusion in cancer patients is associated with a high rate of 30-day mortality, more complications, and longer hospital stay. In view of this, strategies to improve transfusion safety are recommended. These include leucocyte depletion filters, which have been proven to reduce the number of circulating tumour cells, of which only between 1/104 and 1/108 are capable of metastasis. Other options include autotransfusion, haemdilution and RBC irradiation (25Gy).24–26Fig. 2 shows an algorithm for the management of patients with low haemoglobin levels scheduled for surgery.
Conflict of interestThe authors declare that they have no conflict of interests.