Iron deficiency anaemia is a public health problem that affects all age groups. In Mexico, it is a common cause of morbidity, and accounts for 50% of cases of anaemia worldwide. It is more prevalent during the first 2 years of life, during adolescence and pregnancy. It is characterised by fatigue, weakness, pallor and koilonychia. Treatment is based on dietary recommendations and oral and intravenous iron supplements. In this review article, we summarise the characteristics of iron efficiency anaemia, its metabolism, epidemiology, symptoms and diagnosis, and explore different therapeutic approaches.
La anemia por deficiencia de hierro, es un problema de salud público, ocurre en todas las etapas de la vida. En México es una causa frecuente de morbilidad, y representa el 50% de casos de anemia a nivel mundial, es más frecuente durante los 2 primeros años de vida, la adolescencia, mujeres embarazadas. Se caracteriza por presentar fatiga, debilidad, palidez de tegumentos, coloiniquia; el tratamiento se basa en recomendaciones dietéticas, suplementos de hierro vía oral y vía intravenosas. El presente artículo de revisión se resume las características de la anemia por deficiencia de hierro, metabolismo, epidemiología, cuadro clínico, diagnóstico y alternativas terapéuticas.
Iron is the second most abundant metal in the earth's crust, and is essential for life. It is a vital component of several bodily functions, primarily haemoglobin synthesis and transport of oxygen throughout the body. It is found in several different enzymes involved in maintaining cell integrity, such as catalases, peroxidases and oxygenases. Proportionally higher concentrations of iron are found in the basal ganglia of the human brain than in liver. In breastfeeding infants, parts of the brain, particularly the microglia, continue to develop, and therefore iron is vital for developing cognitive functions at this stage of life.1,2
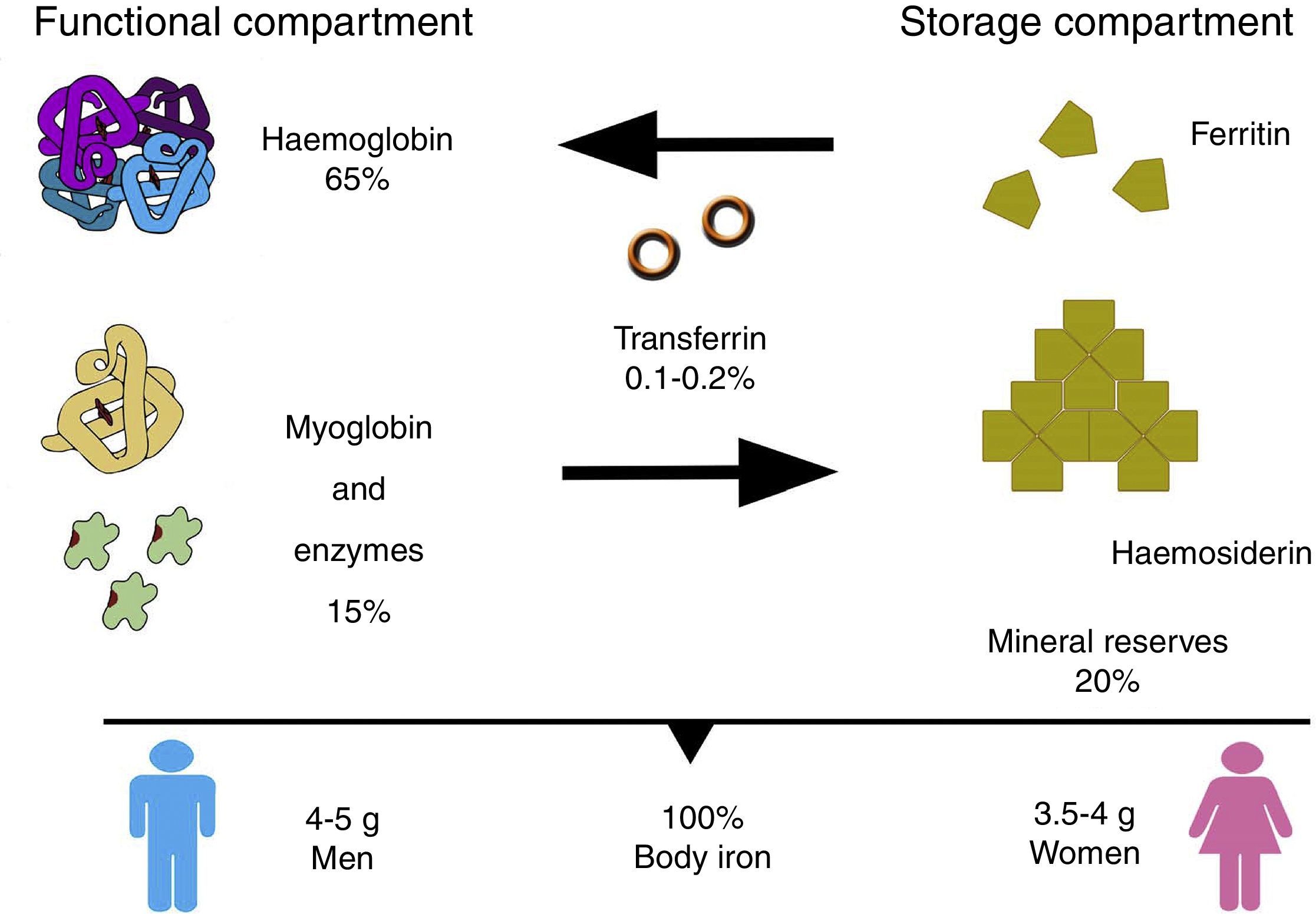
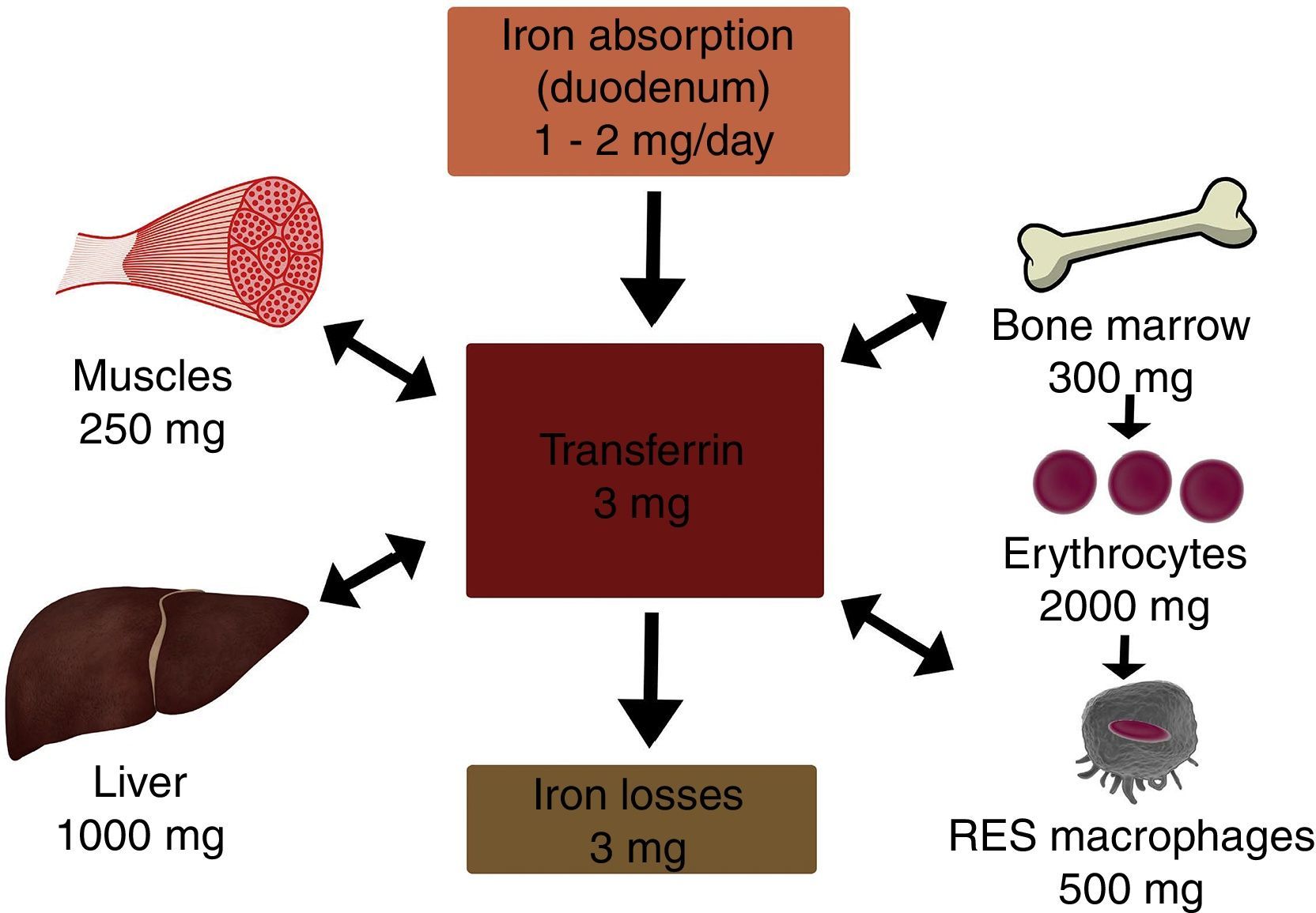
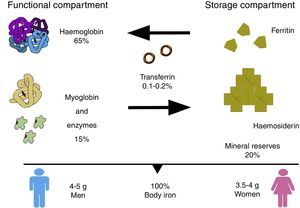
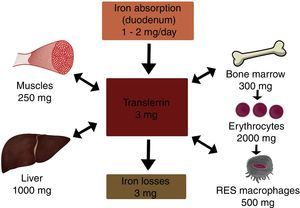
In the body, iron is distributed in 2 compartments. The first is a functional compartment formed of a number of compounds, including haemoglobin, myoglobin, transferrin and enzymes, and all of which require iron as a cofactor or a prosthetic (ion or haem) group. The second is a storage compartment, formed of ferritin and haemosiderin, which constitute the body's mineral reserves.2 The body iron content of a normal, 70kg individual is around 50mg/kg; 3.5–4g in women, and 4–5g in men. Most of the iron is distributed as follows: 65% in haemoglobin (2300mg), 15% in myoglobin and enzymes, 20% in iron stores, and only 0.1–0.2% is bound to transferrin (Fig. 1).2,3
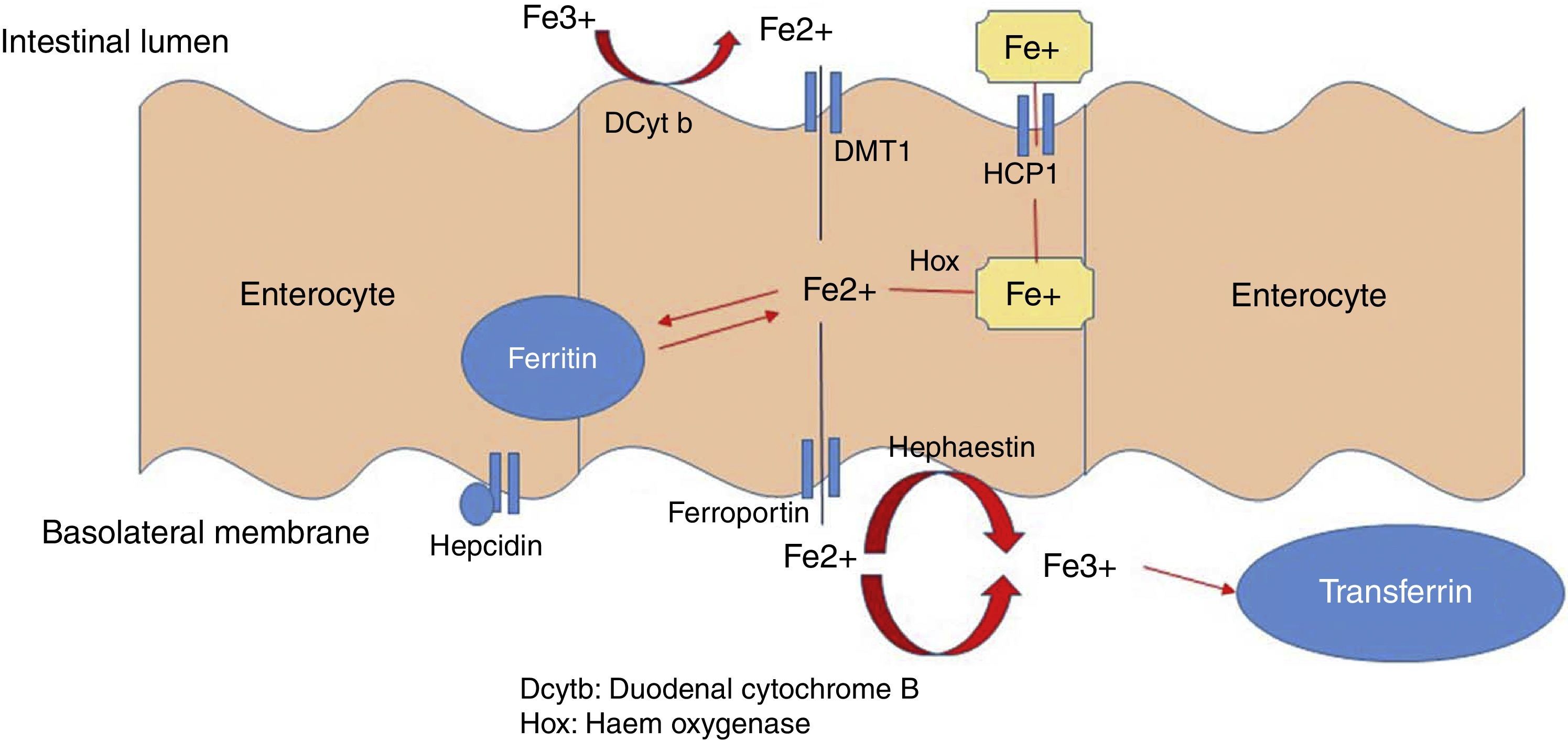
Absorption of ironA normal diet contains 6mg/1000 calories, with a daily intake of 15–20mg of iron absorbed in the duodenum and the first part of the jejunum (1–2mg/day). Iron is found in 2 forms: haem (10%) and non-haem (90% ionic). Haem iron is found in foods of animal origin (red meat, chicken, fish) in the form of haemoglobin or myoglobin; between 15% and 20% of haem iron is absorbed. Non-haem, or inorganic, iron is found in foods of plant origin, cereals, and some foods or animal origin such as milk and eggs; less than 5% of non-haem iron is absorbed.
Haem is most easily absorbed form of iron: through a process called endocytosis, iron is taken up directly by intestinal cells, where haem oxygenase (hox) breaks its ring to release ferrous iron (Fe2+).
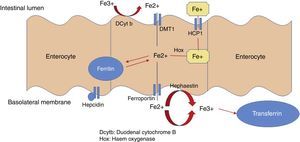
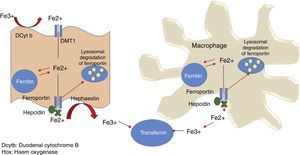
Dietary non-haem or inorganic iron is found as an oxide (Fe3+). The apical edge of the enterocyte contains a ferric reductase enzyme (duodenal cytochrome B [Dcytb]), which transforms ferric iron (Fe3+) to its soluble ferrous iron (Fe2+) form, thereby allowing it to pass through the mucous membrane of the intestine. Non-haem iron is transported by a protein called divalent metal transporter 1 (DMT1), which also carried other metallic ions such as zinc, copper and cobalt by means of a proton coupling mechanism. In the enterocyte, iron can follow 2 pathways: a small ferritin-bound portion is stored; the rest is transported through the basolateral membrane of the enterocyte by ferroportin 1 (Ireg-1), aided by the protein hephaestin, which transforms Fe2+ into Fe3+. Thus released, it passes into the systemic circulation and binds with transferrin. Enterocytes can also take up iron from the blood by means of transferrin receptor 1 (TfR), expressed on its basolateral membranes, in association with HFE (also called the hemochromatosis gene) and β2 microglobulin (Fig. 2).
Non-haem iron absorption is strongly influenced by dietary factors, such as meat or ascorbic acid (vitamin C), which improve the bioavailability of non-haem iron. A diet containing calcium (dairy products), tannins (tea), or phytates (fibre-rich diets), meanwhile, together with the administration of medicines such as tetracycline, proton pump inhibitors, and antacids, can diminish iron absorption.3–5
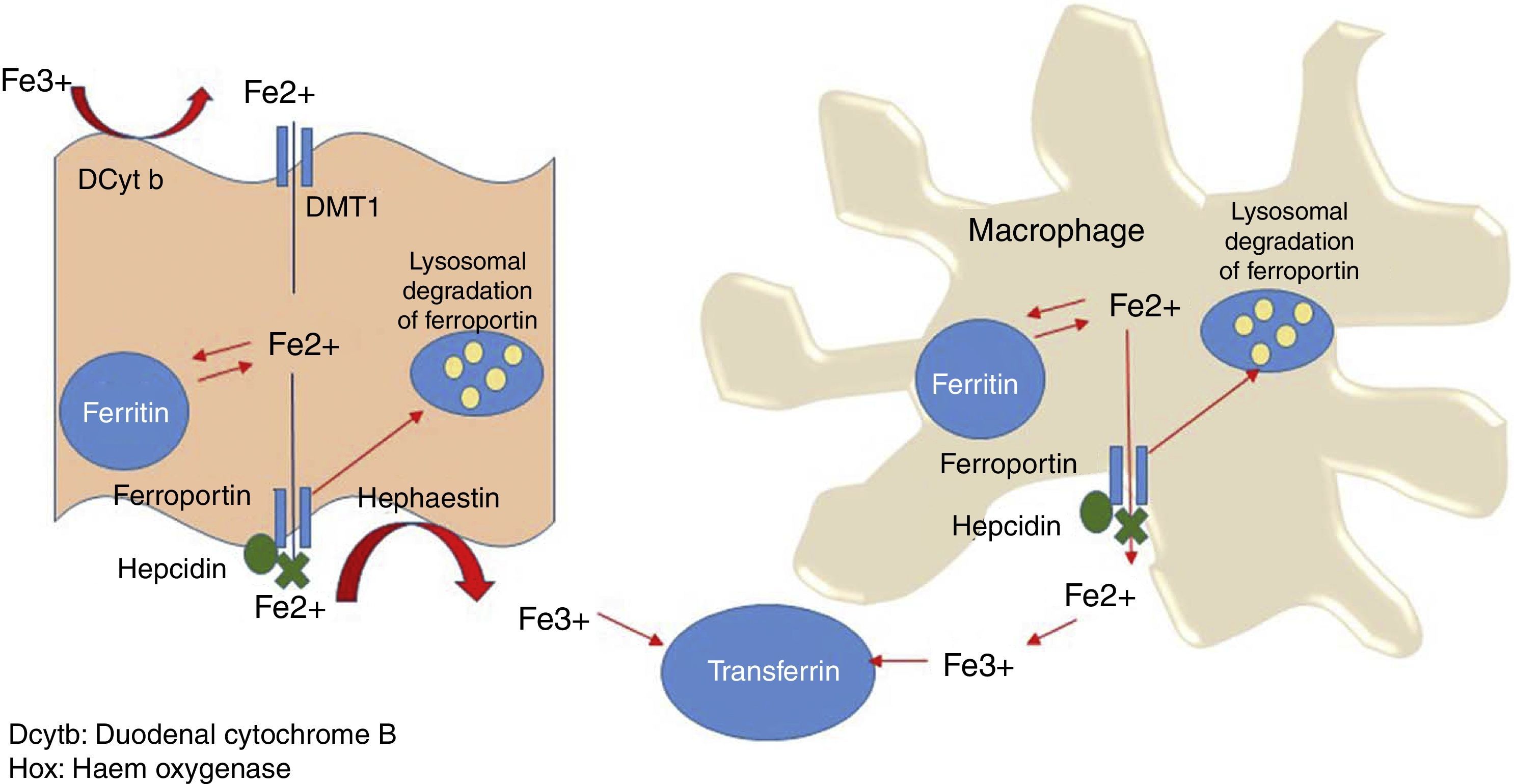
Systemic iron homeostasisHepcidin is currently thought to be the main regulator of systemic iron homeostasis, including the intestinal absorption of iron and the recycling of iron in the REC. Hepcidin is a 25-amino acid protein that binds to ferroportin, leading to internalisation and subsequent liposome degradation. As ferroportin is known to be an iron exporter, hepcidin traps iron in enterocytes, macrophages and hepatocytes (Fig. 3).6
Hepatic production of hepcidin is regulated by the degree of transferrin saturation and transferrin receptor (TfR) 1 and 2 levels in the liver. Therefore, an increase in the diferric Tf/TfR ratio induces hepcidin expression, which acts by inhibiting ferroportin-1 activity, and with it, basolateral iron transport. However, a decrease in the diferric Tf/TfR ratio halts production of hepcidin in the liver, and iron absorption is restored.
Distribution of ironOnce absorbed, the iron enters the blood stream and binds to Tf for transport. The hepatic synthesis of Tf is regulated by intracellular iron, so that when iron levels decrease, plasma transferrin levels increase. Tf can bind up to 2 iron atoms, and therefore the transferrin saturation index (TSI) is usually around 30–35%. The TSI is involved in regulating erythropoiesis, and this is drastically reduced when the TSI falls below 16%. In contrast, when the TSI increases to over 90%, iron transported by Tf is diverted to the liver, and can cause hepatic haemosiderosis.
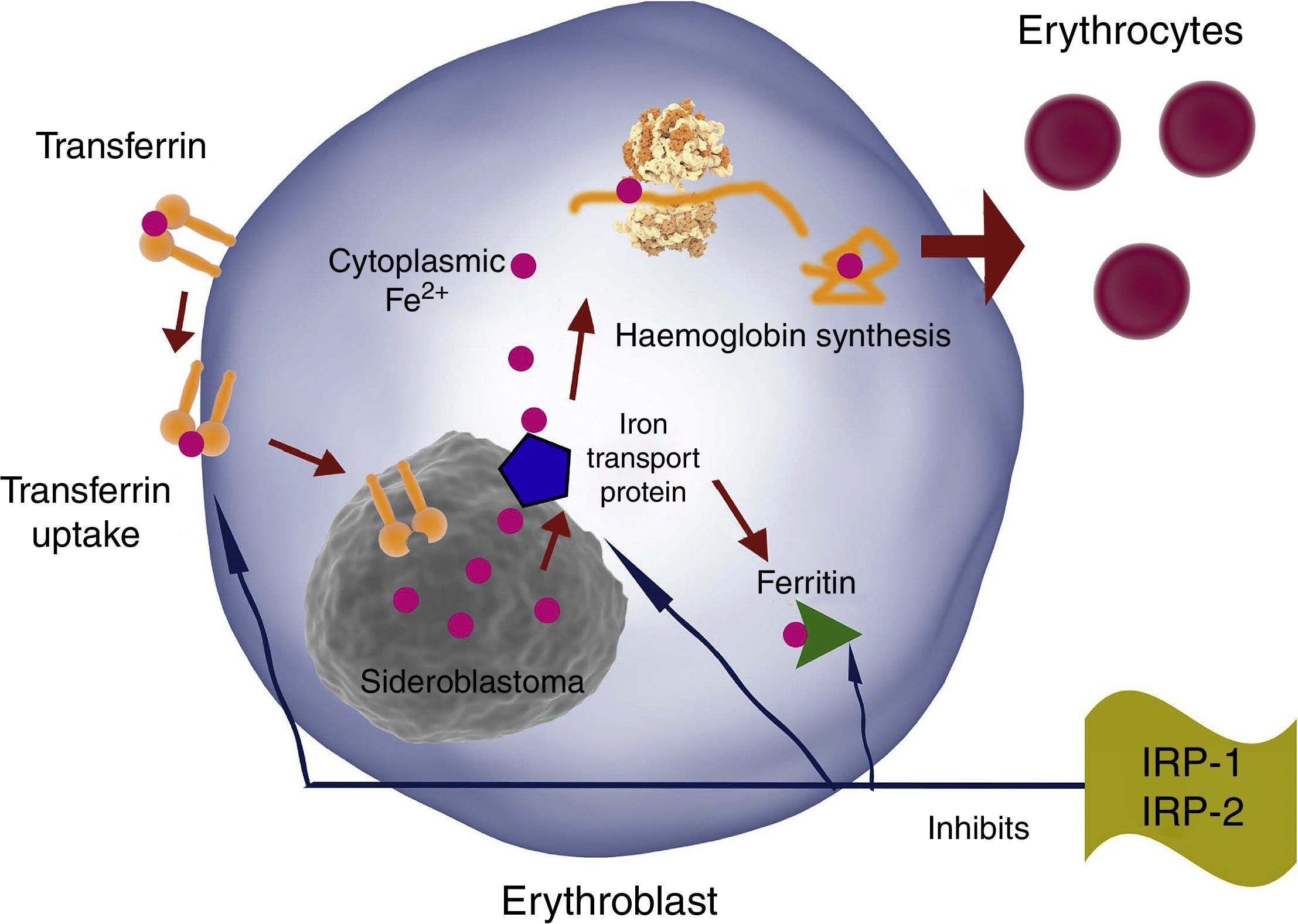
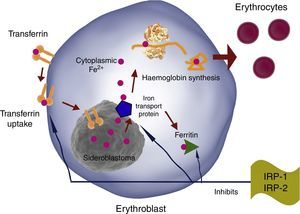
TfR, DMT-1 and ferritin synthesis in erythroblasts is inversely regulated by iron regulatory proteins 1 and 2 (IRP1 and IRP2). Therefore, iron erythroblast uptake is increased by increasing TfR production and reducing ferritin production, and vice versa (Fig. 4).
EPO has been shown to activate IRP-1 during erythropoiesis, causing TfR overexpression in erythroid progenitors. This continues during differentiation, and is mediated by transcriptional and post-transcriptional mechanisms.6,7
Iron storage and recyclingOne hundred and twenty days after entering the circulation, senescent erythrocytes are phagocytised by macrophages in the spleen, liver or bone marrow, where haem oxygenase breaks down the haem group and releases Fe2+, which is transported by Nramp-1 into the cytoplasm. A significant proportion of this iron will be stored in the macrophage as haemosiderosis and ferritin, while the rest is transported through the membrane of the macrophage by ferroportin-1, oxidised by hephaestin to Fe3+, and incorporated into Tf. This Fe recycling pathway is essential, as daily erythron requirements are between 20 and 30mg of iron.
Unlike macrophages and enterocytes, parenchymal cells, particularly hepatic and muscles cells, mainly act as iron receptors. While Fe storage in macrophages is considered innocuous, excess iron in parenchymal cells causes peroxidative damage, which can lead to organ dysfunction.
To prevent this, iron uptake and storage pathways in the liver differ from those found in enterocytes and macrophages. In liver and other parenchymal cells, these processes seem to be affected by HFE, which lowers RTf–Tf affinity and may also reduce DMT-1 expression, while regulating ferritin levels.6–8
Iron storage in cardiomyocytes is of particular interest, as heart failure is the most common cause of death in patients with untreated hereditary haemochromatosis, and also in haemosiderosis secondary to repeated transfusions or ineffective erythropoiesis. Excessive iron in myocytes can cause oxidative stress and myocardial changes caused by hydrogen peroxide-induced DNA damage following the Fenton reaction. Lymphocytes seem to play a major role in these iron storage and oxidative stress processes by releasing pro-inflammatory cytokines.6,7,9,10
Iron loss and excretionIn physiological conditions, between 1 and 2mg of iron is excreted daily. Normal iron loss occurs when epithelial cells are sloughed from the lining of the gastrointestinal tract (main pathway) and from the skin. It is also excreted in the urine, in menstrual blood and breast milk, or lost through perspiration. Normal blood loss during menstruation is around 30ml, although it can be as high as 118ml in some women. Every 100ml of blood contains around 40–50mg iron, and excessive menstrual blood loss is a common cause of iron deficiency anaemia in young women with insufficient dietary iron intake.8
Effects of inflammation on iron metabolismCertain proinflammatory cytokines (TNF, IL-1, IL-6 and interferon gamma), along with the acute-phase proteins (hepcidin and a1-antitrypsin) produced in the liver in response to these cytokines, are known to be involved in the pathogenesis of anaemia of inflammation. This results in: (1) impaired production of erythropoietin (EPO) by peritubular cells in the kidney resulting from loss of red cell mass. (2) Inhibition of the action of EPO on erythroid progenitors. The anitaopotosis action of EPO causes erythroid progenitors to proliferate and differentiate. This action is blocked by proinflammatory cytokines; therefore, anaemia of chronic disease (ACD) leads to a proapoptotic state. (3) Waste of iron due intestinal malabsorption (hepcidin-induced inhibition of lreg-1 and possibly also DMT-1), (4) increased macrophage uptake (DMT-1 stimulation) and storage (increased ferritin levels) of free iron, and inhibition of iron release by macrophages (inhibition of Ireg-1). Tf–TfR binding and internalisation of the Tf–TfR complex is competitively inhibited by a1-antitripsin. Some cytokines regulate ferritin levels through a non-iron-dependent pathway, thus increasing ferritin synthesis in inflammatory states.2,10
Fig. 5 summarises the foregoing description of iron metabolism.
Iron deficiency anaemiaAnaemia is a public health problem that affects both developed and developing countries. It can occur at all stages of life, and is most prevalent in high-risk individuals, such as pregnant women and children between the ages of 2 and 12. Around 50% of all cases of anaemia worldwide are thought to be caused by iron deficiency.
The main risk factors for iron deficiency anaemia are: low iron intake, different levels of chronic blood loss, and malabsorption.11,12 Development of iron deficiency anaemia and the speed of anaemia progression will depend on basal iron body stores. These in turn will depend on the age of the individual, their sex, growth rate, and iron absorption/loss balance.13
Iron requirements are greater during 2 stages of life: during the first 6–18 months of life, and during adolescence (mainly in women, due to menstruation). Low iron intake can significantly delay development of the central nervous system. Iron deficiency in utero or during the first few months of life can also cause structural abnormalities in the brain, because iron is essential for neurogenesis and the differentiation of certain cells and regions of the brain. Iron deficiency appears to affect dopamine and norepinephrine synthesis and catabolism, which causes sleep, learning and memory disorders.14
Women with iron deficiency anaemia are prone to premature birth and low birth weight infants. This is because iron is needed during pregnancy to increase erythrocyte production, which compensates for the relatively hypoxic intrauterine environment, and supplies the foetus with the oxygen it needs for development. Adequate iron transport across the placenta ensures the birth of a full-term, normoweight infant. Iron is necessary for postnatal growth, as it increases red cell volume and builds lean body mass.15,16
EpidemiologyIron deficiency anaemia is a problem at all social and economic levels of society. It is an important factor in the cognitive and physical development of children and in the activity and productivity of adults.16
According to the results of the national health and nutrition survey (ENSANUT 2012), prevalence of anaemia in pre-school infants (1–4 years) in Mexico is 23.3%. The greatest prevalence was observed in infants aged between 12 and 23 months (38%). In children aged under 2 years, anaemia can adversely affect development of the capacity for abstract thought, mathematics, problem solving, and language development. If it is not prevented or treated, the adverse effects of anaemia during this stage of development are irreversible. According to the results of the ENSANUT 2012 survey, prevalence of anaemia in Mexico continues to be a major problem, particularly among children aged under 5 years.17
The national prevalence of anaemia among adolescents aged between 12 and 19 years is 5.6%, predominantly among women. The prevalence of anaemia among women of childbearing age, according to the WHO classification, is 17.9% in pregnant women, and 11.6% in the rest of this population. On average, 1 in 6 pregnant women suffer from iron deficiency anaemia.18,19 A study carried out in 2013 found the prevalence of anaemia during pregnancy to be 4.8% in the first trimester, and 16.32% in the third trimester.20 The prevalence of anaemia in adults aged 60 years and over is 16.5%.18,19
AetiologyThe main cause of iron deficiency anaemia is an imbalance between tissue iron requirements and body iron stores due to diseases involving blood loss. The causes are, in order of prevalence: (1) insufficient iron intake. This occurs in the paediatric population, in children during growth spurts, particularly in premature or low-weight infants, pre-school infants, infants that are weaned early to cow's milk or infant cereals (foods with low iron content), and during growth spurts in adolescence; (2) abnormal iron loss (chronic bleeding). In adolescent and adult women with vaginal bleeding such as dysfunctional uterine bleeding or bleeding secondary to uterine myomatosis. In adults of both sexes, the causes are chronic diseases involving gastrointestinal bleeding, such as erosive gastritis, bowel polyps, intestinal diverticulosis or colon cancer. In Mexico, bleeding caused by hookworm (Necator americanus and Ancylostoma duodenale) infection is common. These are intestinal parasites that feed on human blood, resulting in blood loss of around 0.5ml. Infected patients also present with eosinophilia, a finding that can help diagnose the infection. (3) Increased iron requirement. The main causes of increased demand for iron are pregnancy and breastfeeding. (4) Impaired iron absorption or acidification. Malabsorption can be caused by digestive disorders, usually coeliac disease, inhibited hydrochloric acid secretion (use of proton pump inhibitors); Helicobacter pylori colonisation is also associated with malabsorption of iron and increased iron loss. Another cause is familial iron deficiency, a recessive germline mutation in the TMPRSS6 gene that encodes a type II serine protease in the liver that regulates hepcidin expression. Genetic defects in the DMT-1 iron transport protein have also been associated with impaired absorption and metabolism of iron.13,20,21
Clinical manifestationsIron deficiency symptoms are secondary to anaemia, and include weakness, headaches, irritability, tinnitus, phosphenes, and varying degrees of fatigue and exercise intolerance, commissural cheilitis (fissures in the corner of the mouth), and koilonychia, (spoon nails). Many patients, however, are asymptomatic,1 while others present pica (clay or soil (geophagy) or paper).22 Pagophagia, or ice pica, is considered to be very specific to iron deficiency states.22,23 Iron deficiency is one of the most common causes of restless legs syndrome (RLS), described as intense discomfort in the legs at rest, that is only relieved by walking.24
DiagnosisThe WHO defines anaemia as a haemoglobin (Hb) level of <12g/dL in women and <13g/dL in men, although higher levels have recently been proposed on the basis of sex, age and race.27 A diagnosis of iron deficiency anaemia should be considered in patients with low Hb levels (men <13g/dL, women <12g/dL, pregnant women and children <11g/dL), transferrin saturation (<20%) and ferritin concentration (<15ng/ml).6 In iron deficiency anaemia, it is important to determine Wintrobe indices, in other words, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC).1,25,26 in terms of morphology, iron deficiency anaemia is mainly characterised by microlytic hypochromic erythrocytes, with anisocytosis. These signs, however, are not pathognomonic, as they are also found in thalassemia, chronic diseases and sideroblastic anaemia. The main Wintrobe index is MCH, which is now the most important red cell marker for detecting iron deficiency anaemia. Red cell distribution width (RCDW) is a measure of the range of variation of red blood cell volume. An RCDW higher than 15 suggests iron deficiency anaemia, although this marker is also elevated in megaloblastic anaemia and haemolysis. Another important parameter is the reticulocyte count, where levels below 1% are called aplastic anaemia.27,28 Other findings in iron deficiency anaemia are changes in platelet levels with reactive thrombocytosis, probably due to increased EPO levels, although thrombocytopaenia can also be found.29
Serum iron levels, which show the amount of circulating iron that is bound to transferrin, must be determined in patients with iron deficiency anaemia. The normal range for serum iron is 50–150μg/dL; the normal range for total iron-binding capacity (TIBC) is 300–360μg/dL. Transferrin saturation, which is normally 15–50%, is calculated using the formula serum iron×100÷TIBC. Iron deficiency is associated with saturation levels below 18%. Normal ferritin concentration levels range from 40 to 200ng/ml; a ferritin level of less than 15ng/ml is diagnostic of iron deficiency, with a sensitivity of 59% and a specificity of 99%. Levels of between 15 and 30ng/ml are highly suggestive.21,26 In children, ferritin levels of less than 10ng/ml are suggestive.15 Ferritin, however, is also an acute phase protein, and it is elevated in inflammatory states, infection, liver disease and cancer. In elderly patients or patients with inflammation, iron deficiency might be present even when ferritin levels are between 60 and 100ng/dl.
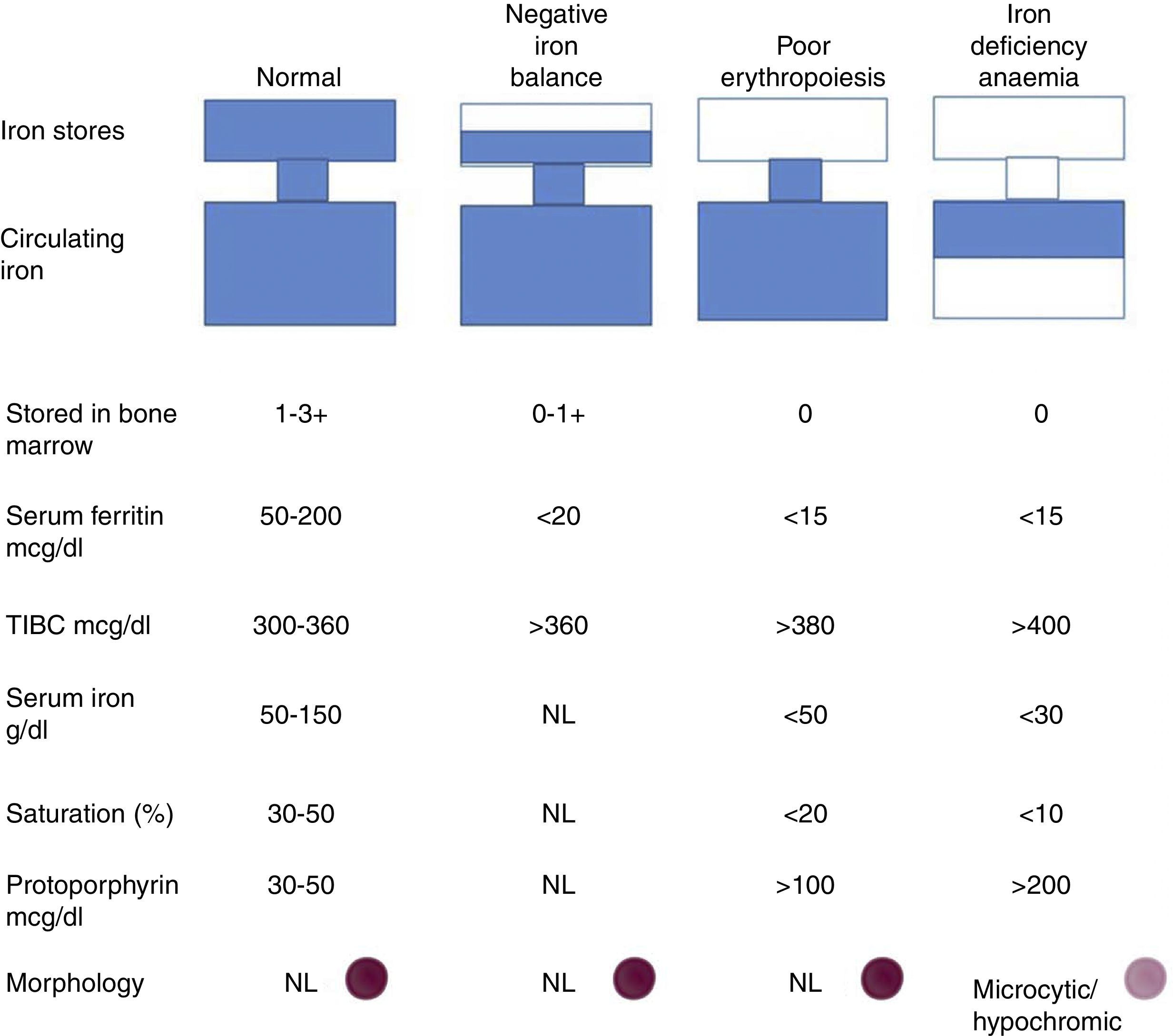
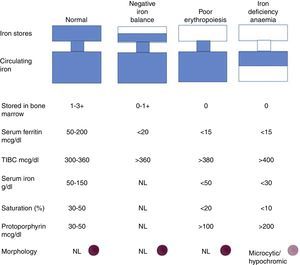
Progression to iron deficiency takes place in 3 stages. The first stage is negative iron balance, in which demand for (or loss of) iron exceeds the body's capacity to absorb dietary iron. This stage is the result of a series of physiological mechanisms that can include blood loss, pregnancy, growth spurts in adolescence, or an iron-poor diet. During this period, body iron stores are shown by serum ferritin levels or bone marrow iron content. While the body stores iron and these stores can be circulated, serum iron levels, TIBC, and red blood cell protoporphyrin levels will remain within normal ranges. At this stage, red blood cell morphology and indexes are normal.
When body iron stores are depleted, serum iron levels start to fall. TIBC and red blood cell protoporphyrin levels gradually increase. By definition bone marrow iron stores are absent when serum ferritin levels fall below <15μg/l. Haemoglobin synthesis is not affected, and serum iron remains within normal ranges despite depletion of body iron stores. When transferrin saturation falls below 15–20%, haemoglobin synthesis deteriorates. This is the iron-deficient erythropoiesis stage. Careful examination of blood smear tests will show the first signs of circulating microlytic cells and hypochromic reticulocytes. Haemoglobin and haematocrit levels start to fall, and this is indicative of iron deficiency anaemia. Transferrin saturation at this point is 10–15%30 (Fig. 6).
Once a diagnosis of iron deficiency anaemia has been established, every effort must be made to determine the pathogenesis of the disease. Celiac disease should be considered in all patients, particularly those with a history of iron deficiency anaemia refractory to oral iron.30
Differential diagnosisAside from iron deficiency, only 3 diseases should be taken into account in the differential diagnosis of hypochromic microlytic anaemia. The first is familial defective globin chain synthesis: thalassemia. The easiest way of differentiating thalassemia from iron deficiency is by studying serum iron levels: in thalassemia, these are characteristically normal. The second disease is anaemia of chronic inflammation, with insufficient transport of iron to red bone marrow. Iron levels usually rule out anaemia of chronic inflammation: ferritin levels are normal or elevated, while TSI and TIBC are typically below normal ranges. Finally, sideroblastic anaemias, particularly familial, should be ruled out as they are characterised by hypochromic red cells and microcytosis.29–31
TreatmentThe aim of therapy is to normalise haemoglobin levels and Wintrobe indices, and replenish iron reserves. A satisfactory therapeutic outcome will depend on correcting the pathways causing loss or malabsorption of iron.
Iron deficiency therapy should start with dietary recommendations (i.e., cereals and fortified bread, red meat, kidney beans and green vegetables). However, when dietary changes alone cannot restore iron stores and haemoglobin to normal levels, or when anaemia is severe, iron supplement therapy should be started.31,32
Oral supplementsOral iron supplements are a cost-effective strategy for restoring iron balance in iron-deficient patients. Early studies suggested that administration of iron combined with vitamin C could benefit iron absorption. However, later research has shown that this dual therapy can cause serious gastrointestinal toxicity.4,32 Iron should be administered either 2h before or 4h after administration of antacids. Iron as a ferrous salt is more easily absorbed. Iron salts should not be taken with food, because the phosphates, phytates and tanates in food bind to the iron and affect absorption. Other factors that can affect absorption of iron salts include antacids, H2 receptor antagonists, proton pump inhibitors, antibiotics (for example, quinolones, tetracyclines), and food and drinks containing calcium. The most economic iron preparation is ferrous sulphate. Each tablet contains 325mg of iron salts, of which 65mg is elemental iron. The adverse effects are felt in the digestive tract, and include abdominal discomfort, nausea/vomiting, diarrhoea and/or constipation. They are directly related to the amount of elemental iron ingested.4,32–34
The recommended dose of oral iron in adults with iron-deficiency anaemia is 100–200mg of elemental iron daily; the recommended dose in children is 3–6mg/kg/day of elemental iron. Multivitamin and mineral supplements should not be used to treat iron deficiency anameia.34 The following is a list of the main oral preparations sold over the counter in Mexico:Ferranina Fol© (Laboratorio Altana Pharma): combines iron polymaltose and folic acid.
Iron polymaltose, the active ingredient in Ferranina Fol©, is a ferritin analogue whose carbohydrate molecule replaces apoferritin in the intestinal iron transport system, leaving it free to be used in haemoglobin synthesis. Ferranina Fol© contains 100mg elemental iron. The recommended dose is 1 tablet every 24h as anaemia prophylaxis, and 2 or 3 tablets every 24h as treatment for iron deficiency aenemia.35
Autrin 600© (Laboratorio Armstrong): This preparation contains ferrous fumarate, folic acid, vitamin B12, C and E. Ferrous iron is more easily absorbed; it is taken up by mucosal cell and in this way is released into the blood stream, where it binds with transferrin. B complex vitamins act alone or as structural components of more complex molecules in catalytic cycles, where they usually act as co-enzymes of carbohydrate, protein or amino acid metabolism, synthesis of DNA and other molecules, red cell maturation, nerve cell function or oxidation–reduction reactions. Vitamin E: This has been shown to prevent the oxidation of essential cell components and the formation of toxic oxidation products, such polyunsaturated fatty acid peroxydation products. Diets that are rich in polyunsaturated fatty acids increase the need for vitamin E. Autrin contains 115mg of elemental iron, and is taken once daily.
Oral therapy follow-upResponse to oral therapy can be poor for the following reasons: (1) Poor iron absorption: concomitant intake of iron absorption inhibitors (for example, tea and coffee) or digestive disorders, such as coeliac disease or irritable bowel syndrome or Helicobacter pylori colonisation; (2) Iron loss or iron requirements in excess of the absorbed dose: gastrointestinal bleeding, dysfunctional uterine bleeding due to disease or familial coagulation disorder such as von Willebrand's disease, kidney failure in response to erythropoietin stimulating agents; (3) comorbid conditions that interfere with bone marrow response: infection, inflammation, cancer, kidney failure, vitamin B12 or folate deficiency, primary bone marrow disease; (4) incorrect myelodysplastic syndrome diagnosis, familial anaemia, endocrine disorders.33,34,36
Intravenous ironIntravenous iron has been shown to elicit the speediest and most sustained erythropoietin response. Indications for iv iron are: intolerance of oral iron or noncompliance with oral iron therapy, malabsorption due to surgery, heavy bleeding, concomitant use of erythropoietin, and anaemia secondary to cancer or chemotherapy.37 The following are the formulations listed in the Mexican and international pharmacopoeia:
Iron dextran: Iron dextran is a colloidal solution of ferric hydroxide in complex with partially hydrolyzed dextran. It contains 50mg of elemental iron, and is suitable for both intramuscular and intravenous administration. The infusion rate should not exceed 50mg/min. When given for the first time, a 25mg IV test dose should be administered over 5min followed by a 15-min observation period. Following this, the remaining dose should be administered within approximately 6h. Iron dextran has been shown to be safe in pregnancy and during the peripartum period. Late onset reactions include hypotension, joint pain, myalgia, general malaise, abdominal pain, nausea, vomiting, and allergic reactions. In 2009, the US Food and Drug Administration (FDA) issued an alert warning of serious anaphylactic reactions following administration of iron dextran. The board recommends that personnel trained in such situations should be present, and that an iv test dose of 0.5ml should be administered over at least 5min, as anaphylaxis is usually evident during this time period, If no reactions occur, the remaining dose can be administered.32–34,36,38
Ferric gluconate complex (Ferrlecit©, Sanofi-Aventis Canada Inc): Ferric gluconate has a molecular weight of approximately 350,000 daltons and is only approved for IV use. Reports from Europe and the US indicate that ferric gluconate in combination with iron dextran can reduce incidence of allergic reaction. Ferric gluconate is effective and safe when administered at a dose of 125mg at a rate of 12.5min–1, or diluted in 100ml isotonic saline solution and injected over 30 to 60min. A test dose is not required.39
Iron sucrose (Venoferrum©, Laboratorio Altana Pharma): Iron sucrose was approved by the FDA in November 2000. It is a water-based iron hydroxide sucrose complex with a molecular weight of 34,000–60,000Da. Iron sucrose is administered intravenously. It has been shown to be safe and effective in chronic kidney disease, inflammatory bowel disease, chemotherapy-induce anaemia, gastric bypass, uterine bleeding, and during the peripartum period. Iron sucrose cannot be administered as a total dose infusion, and doses above 300mg/day are not recommended. The recommended dose for patients with cancer and anaemia receiving erythropoiesis stimulation agents is 200mg injected over 60min and repeated every 2 or 3 weeks. The maximum dose is 600mg per week. The administration rate should not exceed 300mg/min, and rapid administration can cause transitory hypertension, tachycardia and dyspnoea. A test dose is not recommended.34,36,37
Ferric carboxymaltose (Renegy©, Laboratorio Takeda México): Ferric carboxymaltose is a macromolecular ferric hydroxide carbohydrate complex, which allows for controlled delivery of iron within the cells of the reticuloendothelial system, with minimal risk of release of large amounts of ionic iron in the serum. The molecular weight of this complex is approximately 150,000Da. It can be administered at an IV dose of up to 1000mg/week. It is rapidly cleared from the circulation and is distributed to the bone marrow, liver and spleen.40
Total iron deficiency (TID) can be calculated using the Ganzoni formula: TID (mg)=kg (Hb ideal−Hb real) (g/dl)×0.24+iron stores (500mg). This gives the required dosage, and can be used for a single administration or over a course of treatment, depending on the chosen parenteral formulation.
Response criteriaReticulocytosis should be observed within 72h of starting iron supplement therapy (according to some authors this is evident at 7 days). Haemoglobin levels increase by 1–2g/dl every 2 weeks, or 2g/dl every 3 weeks. To replenish iron reserves following normalisation of haemoglobin levels, treatment can be continued in adults for 3–6 months, and in children for 2–3 months.
ConclusionsIron deficiency anaemia is a common disease, and all doctors, both general practitioners and medical or surgical specialists, must be familiar with the underlying physiopathology in order to correctly diagnose and treat this condition. So far, no particular therapy has been shown to be clearly superior, and evidence of effectiveness is scant and controversial. Iron supplements should be chosen taking into consideration general recommendations to start with oral therapy except in certain circumstances (intolerance of oral iron salts, treatment failure, comorbidity) where IV administration is indicated. Poor disease management can be avoided by directing therapy not only at clinical improvement, but also normalisation of blood levels. Even when these have recovered, therapy should continue for a period similar to that required to normalise Hb levels, in other words, around 3 months, to ensure that body iron stores are fully replenished, which is the ultimate goal of iron supplement therapy.
Conflict of interestThe authors declare that they have no conflict of interests.