Parkinson's disease is a chronic, debilitating, progressive neurological disorder of multifactorial origin. It affects between 0.3% and 2% of the over-65 population worldwide, with a predilection for men, and is characterised by bradykinesia, muscular rigidity, resting tremor and postural instability. Parkinson's is caused by decreased dopamine levels due to the loss of dopaminergic neurons in the substantia nigra. Because dopamine is a highly oxidisable molecule, precursors such as levodopa, together with catechol-O-methyltransferase and monoamine oxidase inhibitors to prevent degradation, are used in the treatment of this disease. These therapies, however, are not without their adverse effects. Surgical treatments for Parkinson's include pallidotomy, therapy deep brain stimulation, and stem cells. A more recent development involves a titanium dioxide micro-implant containing nanopores that stabilise the dopamine for continuous release. When inserted into the caudate nucleus, this micro-implant was found to counteract 85% of symptoms in hemiparkinsonian rats, and is a promising therapy for patients with Parkinson's disease.
La enfermedad de Parkinson es un trastorno neurológico con una prevalencia del 0.3 a 2% de la población mundial mayor de 65 años de edad, es crónica, debilitante y progresiva, de origen multifactorial. Afecta mayormente a los hombres que las mujeres en una proporción de 2:1. Se caracteriza por la bradicinesia, la rigidez muscular, temblor de reposo e inestabilidad postural. Esta enfermedad es causada por una pérdida de neuronas dopaminérgicas en la sustancia nigra y la consecuente disminución de la dopamina en el cuerpo estriado. La dopamina es una molécula altamente oxidable, es por ello que se utiliza en los tratamientos precursores de la misma, como la levodopa, también se emplean inhibidores de la catecol-O-metiltransferasa y de la monoamino oxidasa, para evitar su degradación. El uso de estos tratamientos farmacológicos tienen efectos colaterales. Entre los tratamientos quirúrgicos utilizados se encuentran la palidotomía, la terapia de estimulación profunda del cerebro, la aplicación de células madre y recientemente se ha desarrollado un microimplante de dióxido de titanio con nanoporos que contienen a la dopamina estable y misma que se libera de forma continua. Este microimplante insertado en núcleo caudado de ratas hemiparkinsonianas, contrarresta los síntomas en un 85%. Este microimplante es una terapia muy prometedora para los pacientes con Parkinson.
Parkinson's disease has been known for over 4000 years. Descriptions of people afflicted with trembling of the head and hands, who have difficulty eating, swallowing saliva, and who lose their power of concentration have been found in documents dating from the time of the Hindu Vedas, Ancient Egypt and Chine (2000–800 BC).1,2
The Renaissance genius Leonardo da Vinci (1452–1519) was one of the first to directly characterise Parkinson's, describing it as “the movements of paralytics whose head and members move without control of the soul; the soul, with all its force, cannot stop these extremities from trembling”.2
In 1810, British sailors exposed to mercury showed signs of “trembling, paralysis and sialorrhea, followed later by more changes”, an example of Parkinson's due to mercury poisoning.2 Modern research has shown that certain pesticides, herbicides and chemical can also cause Parkinson's.3 Despite these descriptions, it was not until 1817 that British doctor James Parkinson described the disease in his “Essay on Shaking Palsy”. Based on his observation of 6 patients, Parkinson described “shaking palsy” as “Involuntary tremulous motion with lessened muscular power in parts not in action and even when supported; with a propensity to bend the trunk forward and to pass from a walking to a running pace. These patients at times become immobilised, as if frozen, although their hands show a slight tremor”.4
Parkinson's disease around the worldParkinson's disease (PD) is a progressive, degenerative disorder of the nervous system. It is usually brought on by a combination of genetic and environmental factors associated, in some cases, with ageing, although the disease has also been described in young patients. Parkinson's is a progressive disease with a mean duration of between 10 and 13 years, affecting more men than women.5
Worldwide, the disease is estimated to affect between 4.1 and 4.6 million individuals over the age of 50 years, with a prevalence of between 0.3% and 2% in the over-60 age group.6
The disease is found in all regions of the world, and among all ethnic groups. It is most prevalent among Caucasians aged between 60 and 84 years, with 260 cases per 100,000 inhabitants, and less so among black, Hispanic and Asian people. Studies from China, Taiwan, Japan and Singapore have reported a higher prevalence in individuals aged between 69 and 79 years.5
A study of 588 patients with PD diagnosed between 1994 and 1995 by members of the Kaiser Permanente Medical Care Programme of Northern California reported an increased incidence in individuals over the age of 60 years, with only 4% of cases being under the age of 50 years. The age- and gender-adjusted rate per 100,000 inhabitants was highest among Hispanics (16.6, 95% CI: 12.0, 21.3), followed by non-Hispanic whites (13.6, 95% CI: 11.5, 15.7), Asians (11.3, 95% CI: 7.2, 15.3), and blacks (10.2, 95% CI: 6.4, 14.0). These data suggest that incidence of Parkinson's disease varies by race and ethnicity (Table 1).7
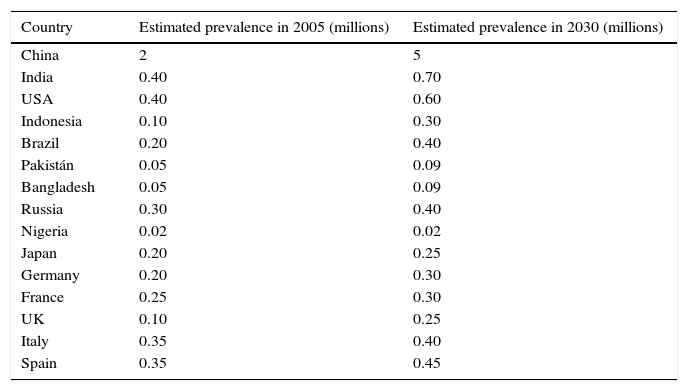
Estimated prevalence of PD (2005–2030).
| Country | Estimated prevalence in 2005 (millions) | Estimated prevalence in 2030 (millions) |
|---|---|---|
| China | 2 | 5 |
| India | 0.40 | 0.70 |
| USA | 0.40 | 0.60 |
| Indonesia | 0.10 | 0.30 |
| Brazil | 0.20 | 0.40 |
| Pakistán | 0.05 | 0.09 |
| Bangladesh | 0.05 | 0.09 |
| Russia | 0.30 | 0.40 |
| Nigeria | 0.02 | 0.02 |
| Japan | 0.20 | 0.25 |
| Germany | 0.20 | 0.30 |
| France | 0.25 | 0.30 |
| UK | 0.10 | 0.25 |
| Italy | 0.35 | 0.40 |
| Spain | 0.35 | 0.45 |
The estimated increase in prevalence of PD in the over-50 age group in the most densely population countries in Western Europe and the world from 2005 to 2030 Some countries are not shown due to lack of precise estimations for the percentage of population with PD 8
No official epidemiological reports on the prevalence and incidence of PD in Mexico have yet been published, and too few studies have been conducted to draw up a reliable demographic and clinical profile of PD patients. The only data available are estimates of the number of individuals diagnosed with PH nationwide, which suggest a prevalence of between 40 and 50 cases per 100,000 inhabitants/year. The Mexican Health Ministry's National Institute of Neurology and Neurosurgery “Manuel Velasco Suárez” estimates PD to be the fourth cause for consultation. A retrospective chart review of 402 records identified 212 patients diagnosed with Parkinson's. Mean age was 63.1±11.4 years, 93% had mild to moderate disease, 70% were receiving levodopa, and 95% presented at least 1 non-motor symptom. Age at onset was approximately 3 years lower than that reported in other series, with a prevalence of men over women (54.5 vs. 59.9 years).6,9 It is important to bear in mind that many patients with early PD must wait a long time receiving the definitive diagnosis because the early symptoms of this disease can be confused with those of other nervous system disorders.
PD usually presents in the elderly; due to recent increases in life expectancy, estimates suggest that the number of PD patients will double by 2030, turning the disease into a public health problem.6,8
Parkinson's diseaseParkinson's disease is a neurological disorder that affects around 1% of the population aged over 60 years. It is a chronic, debilitating and progressive disease characterised by multiple motor and non-motor symptoms. The range of symptoms associated with PD affect a patient's ability to function normally, and therefore undermine their quality of life.10,11
PD is caused by a loss of nerve cells in the pars compacta of the substantia nigra, which is part of the basal ganglia system known as the caudate nucleus and putamen. These changes in the basal ganglia system affect the patient's ability to maintain an upright position, perform spontaneous fine and gross movements and automatic movements of the extremities.12 Post-mortem findings in some PD patients have described the presence of Lewy bodies in neurons and an accumulation of alpha-synuclein proteins (α-syn). Lewy bodies, however, are not exclusive to PD, and can also be found in other neurological disorders, such as dementia.13,14
Clinical manifestationsClinically, PD is characterised by neurological alterations, such as bradykinesia, resting tremor, rigidity and postural instability.13 Although these 4 motor symptoms are typical of PD, other non-motor symptoms may also be observed, such as sleep disturbances, depression, constipation, orthostatic hypotension, swallowing problems, and also memory problems or dementia in more advanced stages of the disease.13
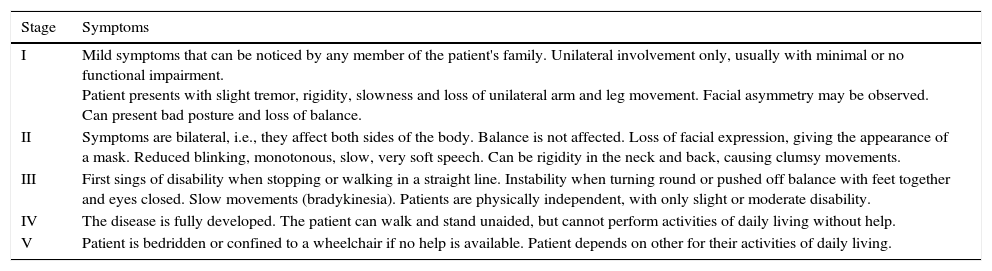
The Hoehn and Yahr scale, developed after studying the medical histories of 802 patients from the Vanderbilt Clinic of the Columbia-Presbyterian Medical Centre, USA, between 1949 and 1964, classifies severity of Parkinson's symptoms into 5 stages of progression. Mean age of the patients studied was 55.3 years, and onset occurred between the ages of 50 and 69 years (Table 2).15
Stages of Parkinson's disease according to Hoehn and Yahr.
| Stage | Symptoms |
|---|---|
| I | Mild symptoms that can be noticed by any member of the patient's family. Unilateral involvement only, usually with minimal or no functional impairment. Patient presents with slight tremor, rigidity, slowness and loss of unilateral arm and leg movement. Facial asymmetry may be observed. Can present bad posture and loss of balance. |
| II | Symptoms are bilateral, i.e., they affect both sides of the body. Balance is not affected. Loss of facial expression, giving the appearance of a mask. Reduced blinking, monotonous, slow, very soft speech. Can be rigidity in the neck and back, causing clumsy movements. |
| III | First sings of disability when stopping or walking in a straight line. Instability when turning round or pushed off balance with feet together and eyes closed. Slow movements (bradykinesia). Patients are physically independent, with only slight or moderate disability. |
| IV | The disease is fully developed. The patient can walk and stand unaided, but cannot perform activities of daily living without help. |
| V | Patient is bedridden or confined to a wheelchair if no help is available. Patient depends on other for their activities of daily living. |
Summary of the 5 stages of Parkinson's disease according to Hoehn and Yahr.15
Non-motor symptoms of PD may also be observed in the early stages of the disease. In 50–80% of Parkinson's patients, these include:
Sensory symptoms: Pain and hyposmia are found in 90% of patients in asymptomatic stages of the disease.
Autonomic dysfunction: orthostatic hypotension, bladder problems, erectile dysfunction and constipation are present throughout the disease.
Neuropsychiatric symptoms: anhedonia, apathy, anxiety, depression, behavioural alterations, dementia and psychosis.
Sleep disturbances: sleep fragmentation, poor sleep efficiency, reduction of slow wave and rapid eye movement sleep, excessive daytime somnolence, and restless legs syndrome, among others.11,16,17
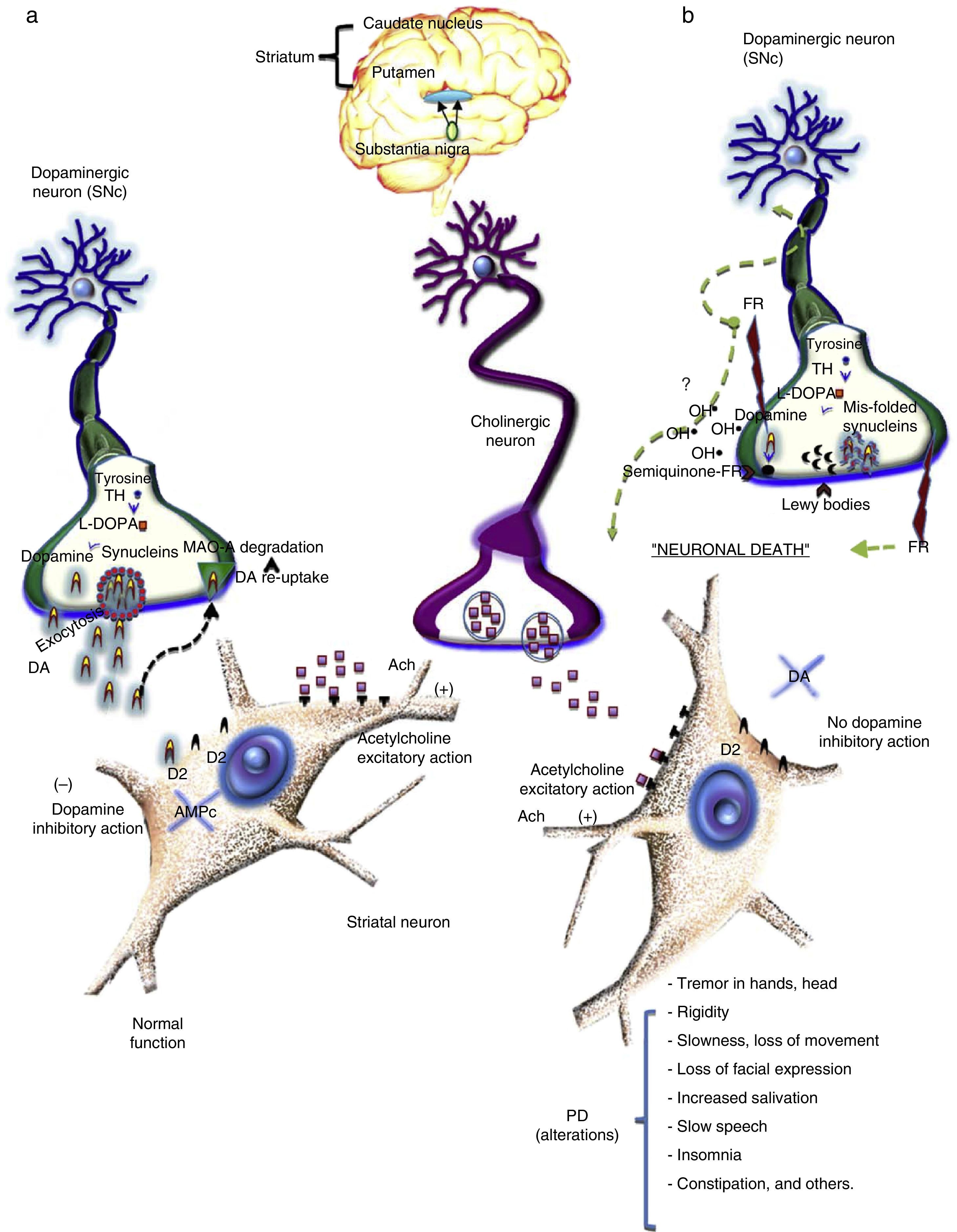
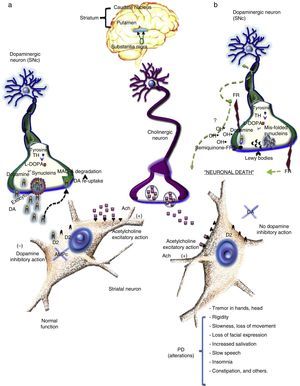
The destruction of dopaminergic neurons in the substantia nigra pars compacta (SNc) reduces levels of dopamine (DA) in the striatum. Dopamine is a chemical messenger that is responsible for transmitting signals between the substantia nigra and the next “relay station” in the brain, the striatum. This enables the body to perform delicate, voluntary movements.18 DA is a catecholamine formed of a benzene ring with two hydroxyl side groups together with a chain formed by ethylamine or an ethylamine derivative.18 Dopamine is released from nerve endings which contain high levels of tyrosine hydroxylase (the rate-limiting enzyme in dopamine synthesis) and l-DOPA, the principal enzymes involved in the de novo synthesis of DA (Fig. 1). As a chemical messenger, DA is an important neurotransmitter in mammals, and is involved in a variety of functions, including locomotor activity, reward-motivated behaviour, emotions (falling in love, motivation, attention), memory, learning, neuroendocrine regulation, and intake of water and food. In the peripheral nervous system, dopamine modulates heart and liver function, vascular tone, and gastrointestinal motility.19 DA is released from nerve endings when dopaminergic neurons are stimulated by an action potential, voltage-gated calcium channels open, and dopamine is released by exocytosis into the synaptic cleft. Once released, DA binds to its postsynaptic D2 receptors. Under normal conditions, the modulating effects of DA and acetylcholine (Ach) on striatum neurons balance the brain's electrophysiological activity, allowing the individual to maintain their balance and muscle tone and enabling them to successfully perform both fine and gross motor movements and other functions (Fig. 1a).
(a) Dopamine is a catecholamine derived from the amino acid tyrosine, which is hydroxylised by the enzyme tyrosine hydroxylase (TH) and decarboxilised by l-DOPA. The resulting DA is then carried in small vesicles by proteins such as alpha-synuclein and released by exocytosis into the synaptic cleft. DA is activated when it binds to its D2 receptors in the postsynaptic membrane of the striatum. Any DA that does not bind to receptors is recaptured (DAT) and degraded by a series of chemical reactions, starting with MAO-A. (b) In this process, DA synthesis described in (a) takes place. According to one hypothesis, loss of DA in Parkinson's disease is related to an accumulation of free radicals that can damage DNA and RNA, causing proteins such as alpha-synuclein to mis-fold and preventing the formation of DA carrier vesicles. Consequently, DA is not released into the neurons, and the mis-folded synuclein proteins accumulate in the terminal, thus forming Lewy bodies. Meanwhile, oxidation of DA produces hydroxyl radicals, which can damage cellular membranes, DNA, RNA and cellular organelles, thus damaging dopaminergic neurons.
Any DA that does not bind to receptors is taken up by dopamine transporters (DAT), degraded into dihydroxyphenylacetic acid (DOPAC) by a series of chemical reactions catalysed by monoamine-oxidase A (MAO-A, B) and catechol-O-methyltransferase (COMT). DOPAC is released from the neuron and degraded to form homovanillic acid (HVA).19
Dopaminergic receptors are coupled to G, and have been grouped into 2 families, depending on their effect on an enzyme called adenylyl cyclase (AC): the D1 family includes subtypes D1 and D5, while the D2 family includes subtypes D2, D3 and D4. D1 family receptors are coupled to Gs proteins which activate AC, thus increasing AMPc levels, while D2 receptors coupled to Gi proteins inhibit AC and reduce AMPc levels. D1 receptors are less sensitive to dopamine than D2 receptors. Both receptors are found in dopaminergic projection areas, although they may be located on different neurons. In normal striatum activity, activation of dopaminergic receptors reduces the activity of the nigrostriatal pathway.19
In the healthy brain, dopamine (inhibitory neurotransmitter) and acetylcholine (excitatory neurotransmitter) levels are balanced. However, the loss of dopaminergic neurons reduces dopamine levels and with it their inhibitory effect on acetylcholines, causing them to bombard the base ganglia, which are responsible for motor planning and some cognitive functions, with excitatory signals. This will only occur when striatum dopamine levels are reduced by approximately 80%.
DA release and binding to post-synaptic receptors determines, in turn, the release of γ-aminobutyric acid (GABA). This inhibitory neurotransmitter coordinates movement regulated by DA and Ach. In PD, dopamine levels are diminished and cholinergic activity is increased in the striatum. This means that the GABA needed to coordinate movement is not released, giving rise to the symptoms of Parkinson (rigidity, resting tremor and hypokinesia. To overcome this, dopaminergic activity must be increased and cholinergic and glutamatergic activity in the striatum reduced, since excess excitatory stimulation causes the symptoms of PD.
Depletion of striatal DA levels leads to hyperactivity in the striatum-external globus pallidus (GPe) pathway, increasing GABAergic inhibition of the globus pallidus and reducing the inhibitory effect of GABA on the subthalamic nucleus (STN). This, in turn, becomes hyperactive and sends excitatory signals to the internal segment of the GP (GPi) and the SNc. These latter structures send inhibitory projections to the thalamus, thus reducing excitatory projections in the motor cortex, and causing Parkinson's.
Aetiology of PDSeveral theories have been proposed to explain the aetiology of PD, a disease that is known to involve both genetic and environmental factors. Genetic factors involved in PD involve mutations and polymorphisms on more than 10 genes. There is strong evidence that 6 of these (PARK1, PARK2, PARK5, PARK6, PARK7 and PARK8) are causal or associated genes involved in PD. PARK3 has only been identified in 3 German families. The candidate gene is PARK3, which has been shown to have a sporadic or familial association with PD. PARK4 is no longer considered an independent locus, as it has been shown to be an expansion of the PARK1 gene (α-synuclein). Mutations found on PARK1, PARK2, PARK5, PARK6 and PARK7 explain up to 5% of all PD cases. PARK8 or LRRK2 explain between 2% and 7% of all cases of PD worldwide.20
Environmental factors involved in PD include gender (it affects men more than women, at a ratio of 2:1), age (1% prevalence among the over-60s, and more than 4% among the over-85s). Neuroinflammation is known to cause an imbalance between neurotrophins and pro- and anti-inflammatory cytokines. This is usually the result of injury (brain trauma) or an accumulation of toxic proteins (such as exposure to herbicides, pesticides, welding fumes and contaminated well water) that cause the oxidative stress to which dopaminergic neurons are particularly susceptible.21
The most widely accepted hypothesis for the loss of dopaminergic neurons involves oxidative stress. According to this theory, an uncontrolled increase of free radicals in turn increases oxidative stress. This results in oxidative reactions that could cause direct damage to membrane lipids, cytoplasmic proteins, and even DNA and RNA (Fig. 1b). This latter effect would lead to mutations in proteins, such as synuclein misfolding that allows DA to escape from the vesicle. Over time, the chain reaction caused by these oxidised macromolecules lead to neuronal death.22 Excessive levels of free radicals, meanwhile, can accelerate dopamine oxidation. Dopamine autoxidation, enhanced by oxygen and iron, produces a semiquinone, and these reactions can give rise to hydroxyl radicals (OH), which are highly reactive radicals and can damage biological molecules, thus causing neuronal damage and ultimately cellular death (Fig. 1b).23
Clinical treatmentA wide variety of drugs are used to treat Parkinson's, among them dopamine precursors (levodopa). Levodopa (l-DOPA) is still highly effective in the treatment of PD, although other agents have also been developed, including antivirals (amantadine), dopamine agonists, ergoline derivatives (bromocriptine), non-ergoline dopamine agonists (apomorphine), monoamine oxidase B inhibitors (MAO-B) (selegiline), catechol-O-methyltransferase (COMT) (entacapone) and anticholinergics (methixene) (Table 3).23
Drugs used in PD and their adverse effects.
| Category | Drugs | Indications | Adverse effects |
|---|---|---|---|
| Antiviral | Amantadine | Administered at the first sign of mild symptoms. Reduces involuntary movements. | Insomnia, blotchy skin, oedema, agitation or hallucinations. |
| Dopamine precursors | Levodopa carbidopa | Levodopa is the most effective symptomatic therapy in Parkinson's (above all for slow movements and rigidity, tremor, balance problems and postural changes). | Nausea, vomiting, hypotension, arrhythmia, somnolence, sleep disturbances, nightmares, hallucinations, psychosis |
| Dopamine agonists ergolines | Bromocriptine, lisuride pergolide, cabergoline | Prescribed in patients intolerant of or unresponsive to dopamine agonists. | Nausea, vomiting, hypotension, sudden somnolence, hallucinations. |
| Dopamine agonists non-ergolines | Apomorphine, pramipexole ropinirole, rotigotine | Administered as monotherapy in the early stages of the disease to delay start of levodopa. If used in combination with levodopa, can mitigate the severity of levodopa adverse effects. | Apomorphine causes somnolence, nausea and vomiting. Confusion or hallucinations. Can cause granulomas at the injection site that may ulcerate or become painful or pruritic. |
| Monoamine oxidase inhibitors (MAO-B) | Selegiline rosagiline | MAO-B inhibitors in combination with levodopa prevent degradation of dopamine and increase availability in the brain by blocking the MAO-B enzyme that degrades levodopa and dopamine. | Abnormal movements, together with nausea, vomiting, yellow skin and eyes, dizziness, persistent diarrhoea or constipation. |
| Catechol-O-methyltransferase inhibitors (COMT) | Entacapone tolcapone | COMT inhibitors in combination with levodopa prevent degradation of dopamine and increase availability in the brain by blocking the catechol-O-methyltransferase enzyme that degrades levodopa and dopamine. | Abnormal movement, sleep disturbances, arterial hypotension, nausea, vomiting, yellow skin and eyes, dizziness, persistent diarrhoea or constipation, red-brown urine (this effect is innocuous). |
| Anticholinergics | Trihexyphenidyl benztropine biperiden ethopropazine methixene procyclidine | Improve tremor and rigidity. Less effective on slow movements. Reduce production of saliva. Preferably administered in patients over 70 years. | Dryness of the mouth, constipation, blurred vision, loss of memory, urine retention, hallucinations and confusion. Somnolence and dizziness (can affect the ability to drive). |
Summary of the principal drug therapies used in Parkinson's disease and their adverse effects over time.25–31
Dopamine agonists act by stimulating different dopamine receptors, and dopamine inhibitors act by blocking dopamine pathways.24
Long-term L-DOPA therapy is known to potentially facilitate the loss of dopaminergic neurons, as neuronal autoxidation produces reactive oxygen species (ROS), and accumulation of these free radicals can ultimately damage dopaminergic neurons (Table 3).23
Surgical treatment of PD- (1)
Pallidotomy
In pallidotomy, a region on the brain involved in the control of movement is destroyed in order to reduce gross and fine motor activity. Pallidotomy can be either uni- or bi-lateral. Negative effects can include haemorrhage, weakness, visual and speech disturbances, and confusion (Table 4).32
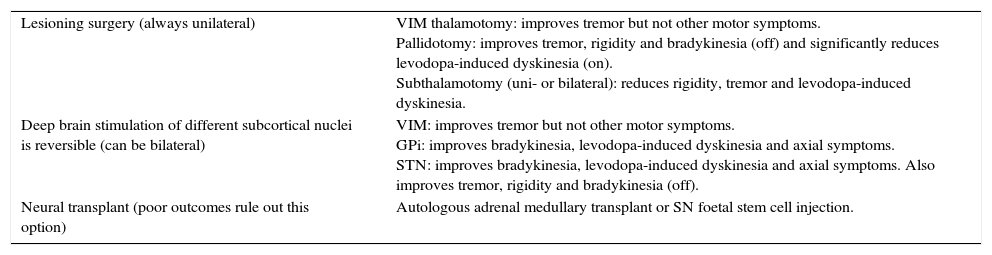
Table 4.Surgical techniques.
Lesioning surgery (always unilateral) VIM thalamotomy: improves tremor but not other motor symptoms.
Pallidotomy: improves tremor, rigidity and bradykinesia (off) and significantly reduces levodopa-induced dyskinesia (on).
Subthalamotomy (uni- or bilateral): reduces rigidity, tremor and levodopa-induced dyskinesia.Deep brain stimulation of different subcortical nuclei is reversible (can be bilateral) VIM: improves tremor but not other motor symptoms.
GPi: improves bradykinesia, levodopa-induced dyskinesia and axial symptoms.
STN: improves bradykinesia, levodopa-induced dyskinesia and axial symptoms. Also improves tremor, rigidity and bradykinesia (off).Neural transplant (poor outcomes rule out this option) Autologous adrenal medullary transplant or SN foetal stem cell injection. Summary of surgical techniques used in Parkinson's disease. Taken from Chap 49 Enfermedad de Parkinson.36
- (2)
Deep brain stimulation therapy for PD
Deep brain stimulation (DBS) is a surgical procedure used to treat the symptoms of PD, such as tremor, rigidity, slow movements and walking problems. It is only used in patients whose symptoms cannot be controlled by drug therapy (Table 4).25–31
The DBS system consists of 3 components: the electrode, the extension, and the implantable pulse generator (IPG). During surgery, the electrode is inserted through a small opening in the skull and implanted in the brain. The tip of the electrode is positioned within a specific area of the brain, usually the thalamus, subthalamic nucleus, the pedunculopontine nucleus, the zona incerta, the prelemniscal radiations or the globus pallidus. The extension is an insulated wire that is passed under the skin of the head, neck, and shoulder, connecting the lead to the IPG, which is implanted under the skin near the collarbone. Electrical impulses are sent from the IPG up along the extension wire and the lead and into the brain. DBS delivers electrical impulses to specific areas of the brain that control movement. These impulses block abnormal electrical signals that cause PD motor symptoms. There is a small chance that placement of the stimulator may cause bleeding or infection in the brain.33,34
- (3)
Stem cell therapy for PD
Neural stem cell therapy (NSC) consists in transplanting non-differentiated foetal brain neural stem cells into the brain of Parkinson's patients so that they can differentiate into dopaminergic neurons and thus replace neuronal losses in the substantia nigra. Although clinical trials are currently underway, it is still unclear whether this stem cell therapy will produce adverse effects such as tumour formation and immune reactions. Only when these effects have been ruled out will the therapy be more widely available (Table 4).35,36
Microimplantation of titanium dioxide using recently developed nanotechnology reduces symptoms in hemiparkinsonian rat models.
In Mexico's Centro de Investigación en Ciencia Aplicada y Tecnología Avanzada (CICATA) Legaria-IPN scientists have synthesised a titanium dioxide (TiO2) matrix using the Sol–Gel technique in which complexes were implanted, creating the TiO2-DA complex. During the synthesis process, tetrabutyl titanate (Ti(OC4H9)4) and ethanol (EtOH) were mixed to obtain the TiO2 precursor solution, to which a small amount of deionised water and ethanol solution was added. Triethylene glycol (TEG) was also added. The solution was then set aside to allow it to hydrolyse, and an amorphous TiO2 solution was obtained which was added to the dopamine. The final solution is a crystalline orange-coloured TiO2DA complex. The stoichiometric composition of the final product is: Ti:NH(C2H4OH)2:EtOH:DIH2O:TEG:DA=1:1:14.1:1:1.028:0.0274.37,38
The newly synthesised complex was characterised using infrared spectroscopy, X-ray diffraction, optical spectroscopy and transmission electron microscopy. The TiO2DA complexes encapsulate the dopamine and ensure its stability (it does not oxidise) for around 2000h under environmental conditions. The inserted complex is able to release dopamine by continual, long-term diffusion.37 Researchers at the Faculty of Medicine of the National Autonomous University of Mexico (UNAM) used stereotactic surgery to implant the dopamine complex in the caudate nucleus of 6-hydroxydopamine (6-OHDA)-lesioned hemiparkinsonian rat models. The group found that after insertion of the microimplant in the caudate nucleus of hemiparkinsonian rat models with gross and fine motor alterations, the animals recovered between 85% and 90% of their motor activity almost immediately after awakening from surgery. In this study, the gross motor (open field movement) and apomorphine-induced rotation behaviours and fine motor activity (taking and holding food in the front extremities) were analysed pre- and post-dopaminergic implantation. Both motor activities improved following the surgical procedure: rotational behaviour and periods of immobility were reduced, the animal's area of activity was increased, and it was able to successfully hold food and eat food. These findings correlated with those of microPET studies, which showed higher levels of the 11C-(+) DTBZ marker in both the left and right striatum at different post-implantation measuring points (1, 21, 90, 180 and 360 days). This confirms the continuous release of dopamine from the complex implanted in hemiparkinsonian rat models. The animals were maintained alive, with the implant, for around 26 months.38
Future outlookThere is still no definitive cure or effective treatment that can guarantee Parkinson's patients a better quality of life. The disease is gradually spreading, and now presents in younger patients. Although modern drug therapies used to treat PD are initially effective, the therapeutic response diminishes over time and adverse effects can appear. Surgery should be performed when medication is no longer effective, and among the surgical procedures available, deep brain stimulation is the best option. Nevertheless, surgery is costly to the public health system and beyond the reach of most patients. Even after surgery, the patient will still need medication and regular check-ups to adjust the strength of the electric impulses and to change the batteries every 2–5 years, depending on use.
In view of the current situation, it is imperative to find new and improved therapies to control the symptoms of PD and improve the quality of life of patients around the world. The results obtained from research into continuous dopamine release microimplants in hemiparkinsonian rat models are promising. This nanotechnology could be used in the future to restore depleted dopamine levels in certain areas of the brain, and might be the ideal therapy for PD patients. This treatment has many advantages: it is a low-cost product that can be rapidly and easily manufactured; due to its microscopic size it can be implanted using minimally invasive techniques, and it has an immediate beneficial effect on the symptoms of the disease. These benefits could make it accessible to a larger number of patients.
Conflict of interestThe authors declare that they have no conflicts of interest in the research.
The authors acknowledge the financial support of the Consejo Nacional de Ciencia y Tecnología251151, Red de Nanociencias y Micro-Nanotecnologías (SIP)20150030 and Programa de Apoyo a Proyectos de Investigación e Innovación TecnológicaIT200813.