In Mexico and the United States of America (USA), a debate was recently opened on the use of some cannabis extracts as anticonvulsive drugs. Given the broad influence that social networks and the media have on the general population, it is worth highlighting the risk to the public of receiving erroneous information and forming opinions that may compromise their health.
In light of this context, taxonomic and biochemical properties, the endocannabinoid system and the historical and legal context of cannabis were reviewed in order to provide a clear overview on the physiological and cultural impacts of cannabis extracts. Subsequently, pre-clinical models, clinical trials and ongoing studies evaluating some phytocannabinoids and synthetic cannabinoids as antiepileptic treatment were assessed.
The obtained information was then subjected to interdisciplinary scrutiny and discussion. Taking into account that plantation and commercialisation of cannabis have been historically illegal in Mexico, the relative lack of evidence regarding its effects in humans and the ideas arising from the aforementioned, it is understandable that the medical community, as well as the general population, have reservations about its suitability as a treatment.
Having developed a clinical neuroethics methodology, an interdisciplinary expert group discussion was coordinated in order to assess the benefits of the medical practice and limit the influence that healthcare professionals’ ethics could have on decision-making. This concluded with three proposals that portray the Hospital General de Mexico Epilepsy Clinic's position, while also contributing important considerations to the mature debate concerning the legalisation of cannabis.
En México y en los Estados Unidos de América (E.U.A.), recientemente se encendió el debate en torno al uso de algunos extractos del cannabis, como antiepilépticos. No obstante debido a la gran influencia que las redes sociales y medios de comunicación representan para la población general, vale la pena destacar el riesgo en el que se encuentran las personas, al recibir información equivocada y formar opiniones que comprometen la salud de ellos o de sus familiares.
A partir de este contexto, se realizó una revisión sobre los aspectos taxonómicos y bioquímicos, del sistema endocannabinoide y del contexto histórico-legal del cannabis, con la finalidad de tener un panorama claro de las implicaciones fisiológicas y culturales del uso de los extractos de la planta. Posteriormente se revisaron los modelos pre-clínicos, ensayos clínicos y estudios actualmente en desarrollo que evalúan algunos fitocannabinoides y cannabinoides sintéticos como tratamiento antiepiléptico.
La información fue después sometida a escrutinio y discusión interdisciplinaria. Tomando en cuenta la ilegalidad del cultivo y comercialización de la planta en México, la relativamente escasa evidencia respecto a sus efectos en seres humanos, así como las ideas que de estos hechos se desprenden, se comprende que tanto la comunidad médica, como la población general tengan prejuicios respecto a su pertinencia como tratamiento.
A partir del desarrollo de una metodología de neuroética clínica se coordinó el diálogo interdisciplinario entre un grupo de expertos, con la finalidad de encontrar el bien en el acto médico, disminuyendo así el efecto que la moral del personal de salud pudiera tener en la toma de decisiones. Esto derivó a la elaboración de tres propuestas que representan la postura de la Clínica de Epilepsia del Hospital General de México, y también brindan consideraciones importantes en el camino al debate bien pensado sobre la legislación del uso del cannabis.
It has been said that “…for farmers it is a source of fibre cultivation; for doctors it remains an enigma; for consumers it generates euphoria; for the police it is a threat; for traffickers it is a lucrative hazard; for convicts and their families it is a chagrin”.1
For thousands of years the cannabis plant has been regarded as a source of fibre, used recreationally due to its psychoactive properties, and has also constituted a therapeutic option for some illnesses. Recently, an important discussion has arisen in Mexico and throughout the world regarding its medicinal application, as the plant is illegal in many countries. However, some believe that this debate is not principally scientific, but rather due to historical, cultural, moral and political implications.2
Specifically, debate has recently been sparked in Mexico and the United States of America (USA) regarding the medicinal use of cannabis as an antiepileptic. The Hospital General de México Epilepsy Clinic is a national and worldwide centre of reference in epilepsy due to its considerable experience in alternative therapies for epilepsy syndromes. It was therefore decided to conduct a review on this subject, followed by an analysis and discussion to provide this Clinic's position and proposals to the medical-scientific community on the use of cannabis derivatives as an anticonvulsant.
This paper will focus on this subject, addressing scientific, historical, legal, medical and bioethical aspects to provide logical conclusions regarding its current use in Mexico.
Taxonomy and biochemical composition of cannabisThe debate surrounding cannabis does not only centre on aspects related to use; its taxonomy and classification have also been subject to scientific discussion. There are currently several botanical studies to try and classify the plant more adequately. The first point that remains unclear is how many species and subspecies exist. According to botanical experts in the field, it is known that cannabis differentiated from Humulus 27.8 million years ago. The plant grows in many places throughout the world, and due to its promiscuity and human intervention to cross plants, there is now confusion in terms of the purity of the species and subspecies.3
There are data to suggest that the plant has been classified since the time of the ancient Greeks, Romans and Arabs. For example, Dioscorides seems to have laid the foundations in the description of two similar plants: Cannabion and Hydrastinan. The German botanist Hieronymus Bock seems to agree with this position in his herbarium of 1539. Subsequently, Leonhart Fuchs also referenced this distinction between the domesticated plant and the wild plant in his book; however, the taxonomist Carl Linnaeus classified it as a single species, C. sativa, with 5 varieties.3,4 Thereafter, Lamarck classified the plant into two species according to its morphology and biochemistry, C. sativa and C. indica (less fibrous with more psychoactive effects). The debate intensifies every time there are different studies with claims that propose a third species, such as C. afghanica or C. ruderalis.3,5
Currently, molecular and genetic techniques are used to classify its morphology through enzyme identification studying the proportion of phytocannabinoid compounds, such as cannabidiol (CBD) and Δ-9-tetrahydrocannabinol (THC).3,5 The latter technique first enabled Fetterman and then Small, Beckstead, Turner and Brenneisen to classify cannabis into a drug-type and fibre-type plant. Hemp is defined as a plant containing 0.2–0.3% THC, while THC levels over this amount are defined as marijuana.4
Finally, Small argued that there is only one species, C. sativa, from which the other subspecies are derived. Although a large part of the scientific community and botanical guidelines agree with his position, the debate continues.3
THC and CBD are the two cannabinoid compounds that have been studied the most; however, the plant contains more than 400 substances and more than 100 are terpenophenolic compounds such as phytocannabinoids.6
Understanding the plant's biochemical properties and providing a solid taxonomy will provide a frame of reference to make decisions regarding its legality and forms of use.3 That is, these differences must be considered and used to delimit, by permitting or prohibiting the use of a plant with such variability in taxonomy, chemistry and resulting effects.3
Endocannabinoid systemMoreover, in addition to the phytocannabinoids produced by the plant, there are also substances considered to be cannabinoids that are physiologically produced by the central nervous system, which are called endocannabinoids; together with their receptors, mechanisms of action and physiological effects, they compose the endocannabinoid system.
The endocannabinoids N-arachidonoyl ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG) are long-chain polyunsaturated fatty acids deriving from membrane phospholipids, specifically arachidonic acid.7,8 Synthesis starts with depolarisation of the postsynaptic membrane or direct activation of metabotropic glutamate receptors. These mechanisms increase intracellular calcium levels, which triggers second messenger cascades that promote endocannabinoid synthesis.9
Anandamide is derived from the precursor N-arachidonoyl-phosphatidylethanolamine (NAPE), synthesised by phospholipase D and degraded by fatty acid amide hydrolase 1 (FAAH1).10 Meanwhile, 2-AG is derived from the hydrolysis of 1,2-diacylglycerol (DAG) by sn-1-selective DAG lipase (DAGLα) and degraded by monoacylglycerol lipase (MAGL).10
Once endocannabinoids are synthesised, they are released into the synaptic cleft. Endocannabinoids and phytocannabinoids act on cannabinoid receptors (CB1R and CB2R) with different affinity. CB1R are abundantly expressed in the brain, particularly in the amygdala, cingulate cortex, prefrontal cortex, nucleus accumbens, ventral tegmental area, lateral hypothalamus, hippocampus, caudate nucleus, putamen, thalamus, substantia nigra and brain stem.11 They are also expressed to a lesser extent in the lungs, liver and kidneys.11 CB2R are mainly expressed in immune system cells, stem cells and, to a lesser degree, in neuronal, glial and endothelial cells.12
CB1R and CB2R receptors are coupled to the α subunit of protein G (Gi/Go). The activation of these receptors inhibits adenylate cyclase (AC), which triggers a second messenger cascade. This signalling cascade induces the inhibition of N- and P/Q-type voltage-gated calcium channels, and moreover stimulates A-type potassium channels.13,14 These mechanisms reduce calcium in the presynaptic membrane, therefore reducing the release of neurotransmitters. CB1R receptors regulate the presynaptic release of neurotransmitters and neuromodulators such as acetylcholine, dopamine, norepinephrine, GABA and glutamate.15
Furthermore, it is important to mention that CBD has low affinity for CB1R and CB2R16; in fact, it acts as an agonist for other types of receptors such as TRP channels (TRPV1, TRPV2, TRPA1), 5-hydroxytryptamine receptors (5-HT1A) and glycine receptors.17–19 It has also been seen to have an antagonist action on TRP melastatin type-8 channels, T-type voltage-gated calcium channels and GPR55-type G protein.20,21 GPR55 was initially characterised as a new cannabinoid receptor coupled to Gα13.20 The inhibition of GPR55 by CBD reduces glutamate release, thus suggesting a potential antiepileptic mechanism; preclinical studies have demonstrated this effect of CBD.22
Several compounds that interact with the cannabinoid system have been proven to have both positive and negative effects in some models of epilepsy.17,19 Preclinical studies are essential to characterise the pharmacokinetic, pharmacodynamic, toxic and therapeutic properties of these substances.
Legal-historical contextThe plant was initially used in Persia and Arabia as an antiseptic and analgesic; it was not until the era of Napoleon when members of his scientific group became interested in its sedative and analgesic effects in soldiers with major burns.23
The first signs of its beneficial use in epilepsy emerged thanks to scientists such as William Brooke O'Shaughnessy. In 1842, this Irish doctor performed research in India, reporting in his studies that he observed a decrease in the frequency and intensity of infant seizures, as well as in hydrophobia (rabies) and risus sardonicus (masseter muscle spasm in tetanus).24 Likewise, in 1861 Dr Russell Reynolds (England)25 provided greater evidence to support this line of research. His work was cited in 1881 by Dr William R. Gowers (England), who affirmed that cannabis may delay paroxysms and mitigate the severity of seizure in some individuals, although he also mentions its capacity to cause delirium and drowsiness.26 Subsequently, in 1949 Davis and Ramsey (USA) reported that THC has greater anti-seizure effectiveness compared to diphenylhydantoin (DPH), adding that due to the weakness of their results, further studies should be conducted.27
Meanwhile, the nascent role of the pharmaceutical industry brought the market debut of new medicines whose objective was to simplify the treatment regimens of various diseases and reduce adverse effects.23 From 1856 to 1937, this prompted a shift in the medicinal image of cannabis, giving it the reputation it is known for today as a drug or poison.23 This idea was reinforced during the literary movement in France, when it gained popularity as a narcotic among the intellectual classes. At the end of this period, in 1937, the Marijuana Tax Act was passed in the USA.23
Recently, due to advances in the pharmaceutical industry and technology, there has been greater interest in some components of the plant to be used in the treatment of epilepsy. These compounds, CBD and CBDV, have never been studied in depth because they do not afford the recreational psychoactive effects that consumers look for.
A few months ago, a heated debate arose due to the diffusion of treatment-refractory epilepsy cases in children that were treated with cannabis extracts.28 These include the case of Charlotte Figi in Colorado, USA, who was diagnosed with Dravet syndrome; and Graciela Elizalde in Monterrey, Mexico, who was diagnosed with Lennox–Gastaut syndrome.28,29 It should be mentioned in the case of Graciela that no results have been published regarding drug response (CBD) since she started her treatment this year in October.
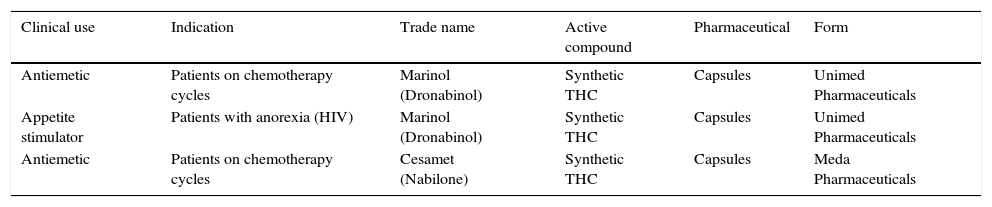
Legality of the medicinal use of cannabisCases such as those mentioned above affecting other neurological, psychiatric and oncological conditions have led to the acceptance of cannabis for medicinal use in several states in the USA and other countries. Just as some medical applications have been approved (Table 1), other clinical uses have not due to a lack of safety or efficacy studies. It must therefore be stressed that the use of cannabis continues to be illegal at the federal level in the USA, although at the state level its legalisation differs according to each state.30
Uses of cannabis approved by the U.S. Food & Drug Administration (FDA).
| Clinical use | Indication | Trade name | Active compound | Pharmaceutical | Form |
|---|---|---|---|---|---|
| Antiemetic | Patients on chemotherapy cycles | Marinol (Dronabinol) | Synthetic THC | Capsules | Unimed Pharmaceuticals |
| Appetite stimulator | Patients with anorexia (HIV) | Marinol (Dronabinol) | Synthetic THC | Capsules | Unimed Pharmaceuticals |
| Antiemetic | Patients on chemotherapy cycles | Cesamet (Nabilone) | Synthetic THC | Capsules | Meda Pharmaceuticals |
These enabling clauses for using the plant are not well known by the population; however, there has been an important migratory effect towards states with complete legalisation (mainly Colorado) in people with epilepsy that is refractory to conventional treatments.30
Today, dozens of companies in the USA (e.g., Healthy Oil, Dose of Nature, Natural Organic Solutions, GW Pharma) have found ways to produce and market CBD. It can be administered topically, through the use of vaporisers or ingested as food or oil; the latter method is used mainly in children with epilepsy and other conditions.32
In Mexico, the Ley General de Salud [General Health Law] allows for the personal use of up to 5g, while articles 235–237 prohibit the planting, harvesting, production or preparation, as well as the consumption, use or medical prescription of psychotropic substances, which are likewise described in article 245 and include the THC contained in C. sativa, indica, americana or marijuana.33
On 4 November 2015, the Supreme Court of Justice granted legal protection to four people so they may consume cannabis and cultivate it for personal use.34 This has led to an increase in the debate and controversy over using the plant in another sense. Subsequently, on 10 November, a congresswoman from the Partido Revolucionario Institucional [Institutional Revolutionary Party] proposed a bill to legalise “the medicinal use of marijuana”.34
Medical and scientific researchPre-clinical studiesAnimal models have helped to understand part of the pathophysiology of epilepsy and to develop new diagnostic and therapeutic methods.35 Despite the limitations of research in animal models, the results obtained can be correlated with the consequent results of clinical studies. The limitations include three important aspects: (1) some animals are genetically modified; (2) the seizures are normally induced and not spontaneous; and (3) the brains studied are phylogenetically less developed than the human brain.35–37
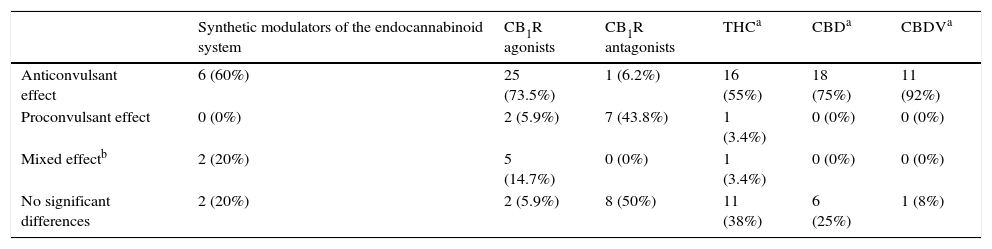
For analysis, Rosenberg has classified into three branches38 the therapeutic properties of the drugs that act on the cannabinoid system tested as monotherapy treatment in animal models (Table 2):
- (1)
Modulators of the endocannabinoid system (MEnS). They are categorised as synthetic compounds (URB597, URB602 and AM404) and phytocannabinoid compounds (CBD and CBDV). Synthetic modulators have been tested in generalised tonic-clonic seizure models using the maximal electroshock seizure (MES) test and the pentylenetetrazol (PTZ) seizure test. Synthetic MEnS have shown favourable results as anticonvulsants, although in some studies proconvulsant and anticonvulsant effects have been observed. Anticonvulsant effects of 100% were shown in the amygdala kindling and status epilepticus focal seizure model with kainic acid (KA). Phytocannabinoid-type modulators have been tested in generalised tonic–clonic seizures (MES, PTZ, 3-mercaptopropionic acid, electroshocks from 6 to 60Hz), genetic seizure models (auditory) and focal seizures (pilocarpine, penicillin, amygdala kindling and cortical implantation of cobalt), showing antiseizure effects. Thus, in general, modulators of the endocannabinoid system presented antiseizure effectiveness, but mainly the phytocannabinoids CBD and CBDV, which presented an effectiveness of 75% and 92%, respectively.
- (2)
CB1R Agonists. These include the synthetic cannabinoids WIN 55,212-2, CP 55,940, arachidonylcyclopropylamide (ACPA), arachidonyl-2′-chloroethylamide (ACEA) and ACEA in combination with phenylmethylsulfonyl fluoride (PMSF); endogenous cannabinoids such as palmitoylethanolamide (PEA), anandamide (AEA) and AEA+PMSF; and one phytocannabinoid, Δ9-THC. These compounds have been tested in generalised tonic–clonic seizure models (MES, PTZ, bicuculline, N-methyl-d-aspartic acid and electroshocks from 6 to 60Hz), genetic seizure models (auditory and absence) and focal seizure models (pilocarpine, penicillin, amygdala kindling, cortical implantation of cobalt and 6Hz psychomotor seizures). The synthetic, endogenous and phytocannabinoid CB1R agonists showed proconvulsant effects in tonic–clonic, genetic and focal seizure models; in others both proconvulsant and anticonvulsant effects were seen, and in others there were no significant differences, such as the case of THC (variable results).
- (3)
Synthetic CB1R Antagonists (SR141716A and AM251). These have been tested in generalised tonic–clonic seizure models (MES and PTZ), genetic seizure models (using auditory stimuli and absence seizures) and focal seizure models (pilocarpine, penicillin, amygdala kindling, cortical implantation of cobalt and traumatic brain injury plus KA), in which no significant differences were found; on the contrary, a proconvulsant effect was seen. Nevertheless, a reduction in seizure susceptibility was seen in the traumatic brain injury plus KA model.
Effects of cannabinoids in animal models of epilepsy.
| Synthetic modulators of the endocannabinoid system | CB1R agonists | CB1R antagonists | THCa | CBDa | CBDVa | |
|---|---|---|---|---|---|---|
| Anticonvulsant effect | 6 (60%) | 25 (73.5%) | 1 (6.2%) | 16 (55%) | 18 (75%) | 11 (92%) |
| Proconvulsant effect | 0 (0%) | 2 (5.9%) | 7 (43.8%) | 1 (3.4%) | 0 (0%) | 0 (0%) |
| Mixed effectb | 2 (20%) | 5 (14.7%) | 0 (0%) | 1 (3.4%) | 0 (0%) | 0 (0%) |
| No significant differences | 2 (20%) | 2 (5.9%) | 8 (50%) | 11 (38%) | 6 (25%) | 1 (8%) |
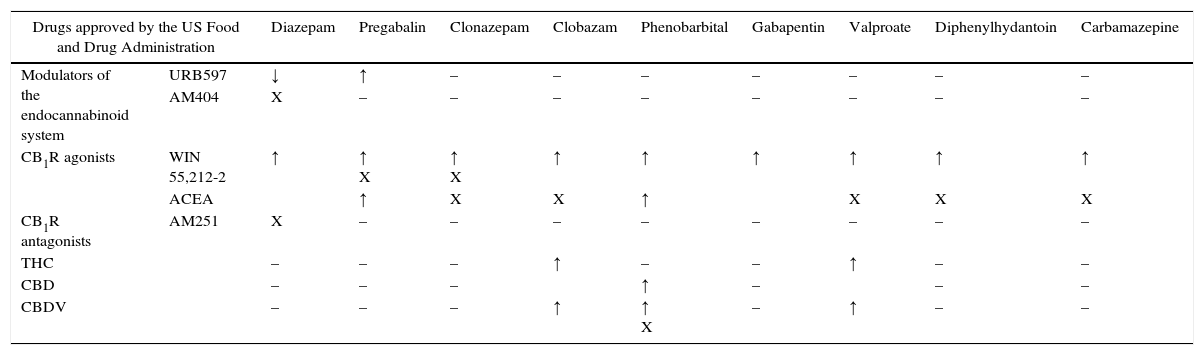
Because more than one drug is commonly used in the treatment of epilepsy to achieve adequate control, the coadjuvant effect of cannabinoids with antiepileptic drugs has been studied.38,39 The combinations studied, together with their effects (increase, decrease or no differences) and percentages are shown in Table 3. Most of the combinations were studied exclusively in MES, except for WIN55,212-2 in combination with clonazepam, valproate and phenobarbital, which have also been tested in the PTZ-induced seizure model and the 6Hz psychomotor seizure model. It should be mentioned that of all the combinations studied, only URB597 with diazepam showed a reduction in antiepileptic effects.
The effects of cannabinoids as coadjuvant antiepileptics.
| Drugs approved by the US Food and Drug Administration | Diazepam | Pregabalin | Clonazepam | Clobazam | Phenobarbital | Gabapentin | Valproate | Diphenylhydantoin | Carbamazepine | |
|---|---|---|---|---|---|---|---|---|---|---|
| Modulators of the endocannabinoid system | URB597 | ↓ | ↑ | – | – | – | – | – | – | – |
| AM404 | X | – | – | – | – | – | – | – | – | |
| CB1R agonists | WIN 55,212-2 | ↑ | ↑ X | ↑ X | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ACEA | ↑ | X | X | ↑ | X | X | X | |||
| CB1R antagonists | AM251 | X | – | – | – | – | – | – | – | – |
| THC | – | – | – | ↑ | – | – | ↑ | – | – | |
| CBD | – | – | – | ↑ | – | – | – | |||
| CBDV | – | – | – | ↑ | ↑ X | – | ↑ | – | – | |
| Drugs approved by the US Food and Drug Administration | Oxcarbazepine | Lacosamide | Topiramate | Ethosuximide | Levetiracetam | Tiagabine | Memantine | Increase in antiepileptic effect (%) | Decrease in antiepileptic effect (%) | No significant differences (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modulators of the endocannabinoid system | URB597 | – | – | – | – | – | – | – | 50 | 50 | 0 |
| AM404 | – | – | – | – | – | – | – | 0 | 0 | 100 | |
| CB1R agonists | WIN 55,212-2 | X | X | ↑ | ↑ | – | – | – | 71 | 0 | 29 |
| ACEA | X | X | X | ↑ | – | – | ↑ | 33.3 | 0 | 66.6 | |
| CB1R antagonists | AM251 | – | – | – | – | – | – | X | 0 | 0 | 100 |
| THC | – | – | – | – | – | – | – | 100 | 0 | 0 | |
| CBD | – | – | – | – | – | – | – | 100 | 0 | 0 | |
| CBDV | – | – | – | ↑ | – | – | – | 80 | 0 | 20 | |
Note. ↑=increase in antiepileptic effect. ↓=decrease in antiepileptic effect. X=no significant difference. –=still not studied.
Regarding the research conducted in humans reviewed to date, there are some studies from the previous century and some that are currently under way; moreover, there is one study in particular that provides some important considerations despite not being a clinical trial.
The Department of Paediatric Neurology at the Children's Hospital Colorado reviewed 75 cases of patients treated with oral cannabinoid extracts in epilepsies that are refractory to conventional treatment and provided questionnaires to the patients’ parents. An improvement of greater than 50% in seizure control was reported in 33% of patients; however, no differences were found in 57%.40
In addition to the anticonvulsant effect, effects were reported for improvement in behaviour/alertness (33%), language (10%) and motor skills (10%).40 The most common adverse effects were an increase in seizures, fatigue and drowsiness. The least common effects reported were developmental regression, abnormal movements, status epilepticus requiring intubation and death.40
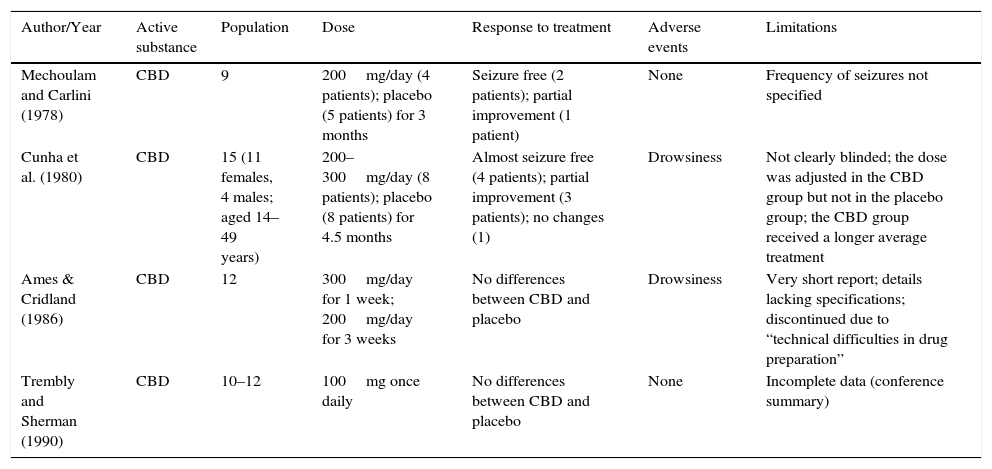
A Cochrane review evaluated four clinical trials in relation to the efficacy of CBD in the control of seizures in epileptic patients with drug resistance.38 The review concluded that a partial anticonvulsant effect was shown in two of these studies, while no significant effect was demonstrated in the remaining two studies (Table 4).38
Clinical trials on CBD in epilepsy and their limitations.
| Author/Year | Active substance | Population | Dose | Response to treatment | Adverse events | Limitations |
|---|---|---|---|---|---|---|
| Mechoulam and Carlini (1978) | CBD | 9 | 200mg/day (4 patients); placebo (5 patients) for 3 months | Seizure free (2 patients); partial improvement (1 patient) | None | Frequency of seizures not specified |
| Cunha et al. (1980) | CBD | 15 (11 females, 4 males; aged 14–49 years) | 200–300mg/day (8 patients); placebo (8 patients) for 4.5 months | Almost seizure free (4 patients); partial improvement (3 patients); no changes (1) | Drowsiness | Not clearly blinded; the dose was adjusted in the CBD group but not in the placebo group; the CBD group received a longer average treatment |
| Ames & Cridland (1986) | CBD | 12 | 300mg/day for 1 week; 200mg/day for 3 weeks | No differences between CBD and placebo | Drowsiness | Very short report; details lacking specifications; discontinued due to “technical difficulties in drug preparation” |
| Trembly and Sherman (1990) | CBD | 10–12 | 100mg once daily | No differences between CBD and placebo | None | Incomplete data (conference summary) |
The limitations of the studies reviewed are: the small population used in the four studies, incomplete published data and ambiguous details in the writing; for example, “partial improvement” is not specified in quantitative terms. The number and frequency of seizures are not specified, nor are measures considered to assess internal and external study validity (randomisation and blinding measures).
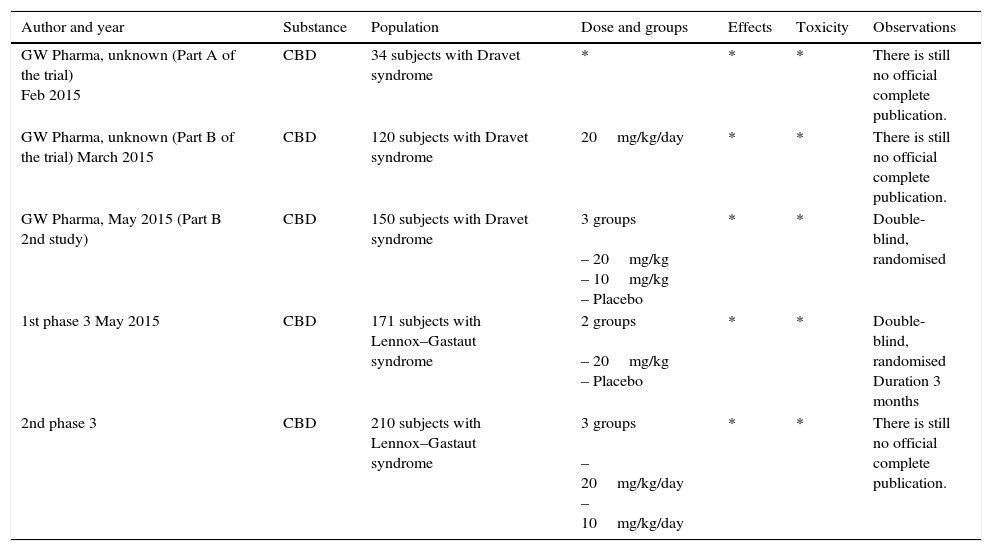
Ongoing trialsOver the last year, many of the current studies on cannabinoids in various conditions have been conducted by GW Pharma. In April of this year, preliminary results were reported by Dr Orrin Devinsky (NY, USA), the principal investigator of part of these drug studies. Nevertheless, the results are not listed in the table because the final official results are not yet available. The report mentions a 54% decrease in 137 of 213 people, throughout the study. At the end of the study, seizures had decreased by 53% in 23 people with Dravet syndrome, and there was a 55% decrease in atonic seizures for 11 people with Lennox–Gastaut syndrome who remained in the study. The adverse effects included diarrhoea (21%), dizziness (17%), tiredness (17%) and decreased appetite (16%).46
The start of a second phase 3 study was announced in June 2015 using CBD in the treatment of Lennox–Gastaut syndrome. Both phase 3 studies compare the drug Epidiolex (CBD) versus placebo, using a double-blind, randomised model lasting 14 weeks. A 2-week drug titration period was used in both studies, followed by a 12-week maintenance period. General information about the methodology used is shown in Table 5. The first results of these trials will probably be available at the beginning of 2016.47
Ongoing clinical trials reported.
| Author and year | Substance | Population | Dose and groups | Effects | Toxicity | Observations |
|---|---|---|---|---|---|---|
| GW Pharma, unknown (Part A of the trial) Feb 2015 | CBD | 34 subjects with Dravet syndrome | * | * | * | There is still no official complete publication. |
| GW Pharma, unknown (Part B of the trial) March 2015 | CBD | 120 subjects with Dravet syndrome | 20mg/kg/day | * | * | There is still no official complete publication. |
| GW Pharma, May 2015 (Part B 2nd study) | CBD | 150 subjects with Dravet syndrome | 3 groups – 20mg/kg – 10mg/kg – Placebo | * | * | Double-blind, randomised |
| 1st phase 3 May 2015 | CBD | 171 subjects with Lennox–Gastaut syndrome | 2 groups – 20mg/kg – Placebo | * | * | Double-blind, randomised Duration 3 months |
| 2nd phase 3 | CBD | 210 subjects with Lennox–Gastaut syndrome | 3 groups – 20mg/kg/day – 10mg/kg/day | * | * | There is still no official complete publication. |
Recently, the pharmaceutical company GW and the government of New South Wales, Australia announced a strategic agreement to continue the study of CBD and CBDV.49 GW anticipates that this will facilitate the first phase 2 study in the world with CBDV, which will probably begin in the first few months of 2016. Aside from this, a small number of children with severely refractory epilepsy, considered to be too serious to take part in a clinical study with CBDV, will be eligible for the “Epidiolex compassionate access programme”. This programme is in phase 3 clinical studies with the FDA for Dravet syndrome and Lennox–Gastaut syndrome.49 Lastly, the agreement will likewise enable the conduct of a phase 3 clinical trial using CBD, and according to the results of said trial, a phase 4 trial.49
Bioethical analysis and discussionThis analysis will be approached using clinical neuroethics, according to personalism and international law. Clinical neuroethics emerged from clinical bioethics and neuroethics as a current demand to identify not only classical bioethical dilemmas in the area of neurology, but also to accompany any area of clinical neuroscience in day-to-day decision-making. Thus, they are specific analyses and general rules that should not be rigidly applied to a given case. This is based on the particularities and complexity of neurological, neurosurgical, neuropsychiatric and neuropsychological patients.
Identification of the dilemma and circumstances (actors, affected parties and environment)First, the bioethical dilemma must be correctly identified. It is not worth the effort unless the problem has been duly identified, or when science or medicine have already provided a clear response to what is correct and right to do. In our group the dilemma arose from the following facts as context (Environment): the lack of a cure and/or effective treatment for some cases of epilepsy in children; the isolated case reports of catastrophic epilepsy in children, successfully treated with CBD; the discussions and opinions released on the matter by several communication media; the questions that have arisen from families and patients in recent months at the Clínica de Epilepsia del Hospital General de México (C.E.H.G.M.) [Epilepsy Clinic of the General Hospital of Mexico] due to information heard through communication media; the expertise and concerns of C.E.H.G.M epileptologists; and lastly, this literature review. According to the list above, the dilemma has been constructed as follows:
Subject: Is it correct to use phytocannabinoid components or synthetic cannabinoids for the treatment of catastrophic epilepsy in children?
As mentioned in the review, only CBD and CBDV can be considered antiepileptics as their mechanisms of action, adverse effects and therapeutic effects have already been studied in preclinical models, and according to some preliminary clinical trial data. Therefore, it is important to emphasise that science and medicine have already delimited the question, ruling out THC due to two reasons taken from the review: its adverse neuropsychological effects,50 and its poor activity as an antiepileptic. Therefore, the question will focus on the use of CBD and CBDV.
Actors (perform the action): Epileptologists at the Epilepsy Clinic of the General Hospital of Mexico
Affected parties: (a) Direct: Patient, (b) Indirect: Family members, Doctors and Society.
Environment (context in which the dilemma is considered): the use of CBD and CBDV is illegal in Mexico because they are compounds derived from cannabis. Furthermore, there are moral stigmas because the most widely known consumption of cannabis (recreational) is associated with social prejudice. The general population of Mexico's image of the plant is partly based on the social violence that results from its illegality. Public opinion is polarised, which can be emotional and poorly founded. Legislators and important decision-makers or people in the media with high-impact opinions are not exempt from this.
Another point to be considered is that decision-making in this case involves the lives of minors, generally young children. This point reveals that parents and doctors may have conflicts of interest; while seeking the greater good for the patient, they may also directly or indirectly seek their own benefit, whether consciously or unconsciously.
Analysis of the action and objectiveObjective: In this case, to provide suitable control of epileptic seizures.
Prescribing any treatment approved as safe is a good action. However, as revealed in the review, there have not been enough suitably conducted and completed studies with CBD and CBDV in humans to demonstrate their complete efficacy as antiepileptics of choice or to corroborate the absence of considerable dangers. Therefore, at this point medicine and science would not be inclined to consider their use until greater scientific evidence is available. It could be said that is against medical ethics to prescribe any patient with a substance that has not been sufficiently studied, as, based on current evidence, the benefits (therapeutic efficacy) of the substances are not widely known and the risks (adverse effects and complications) in some reports are fatal.40
However, we must remember that we are looking to analyse the use in catastrophic cases of epilepsy, assuming they have been properly protocolised and are refractory to all treatments accepted by the expert medical community.
Analysis of the consequencesNext we must assess the risks and benefits for patients, in terms of whether or not to prescribe the substance. It is known that uncontrolled epilepsy patients can develop considerable neuropsychological sequelae that are moreover under-diagnosed.51 Severe sequelae not only affect the life of individuals, but may also lead patients to have a variable degree of dependence, such as the need for a caregiver, and this in turn impacts the family economy and/or dynamics. We must keep in mind the impact on the family, even though this is not the most important consideration or determining factor. Protecting the patient's life and procuring their dignity (considering proportionality) is where medical duty lies.
Therefore, let us summarise as follows: an uncontrolled minor at risk of presenting status epilepticus, which represents a high morbidity and mortality,52 who in the best of cases may live with damaging sequelae, has the option of taking a new treatment that seems to show effectiveness, but the probability of presenting adverse effects, including life-threatening effects, remains unknown.
By performing an analysis in light of human dignity and human life, which are values not only protected by Mexican bioethics,53 but also by the Universal Declaration of Human Rights54 and therefore by article 1 of the Political Constitution of the United Mexican States,55 it would thus be logical to permit the use of any last resource to protect these two values. The use of the substance in the above-mentioned situation would therefore be justified according to bioethics, medical ethics, international law and, theoretically, national law.
Moreover, this decision would also be based on the Declaration of Helsinki (cited countless times in Mexican studies), by the World Medical Association, most recently updated in Brazil, 2013. Paragraph 37 mentions specifically: “In the treatment of an individual patient, where proven interventions do not exist or other known interventions have been ineffective, the physician, after seeking expert advice, with informed consent from the patient or a legally authorised representative, may use an unproven intervention if in the physician's judgement it offers hope of saving life, re-establishing health or alleviating suffering. This intervention should subsequently be made the object of research, designed to evaluate its safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publicly available.”56
It must be considered that bioethics is separate from legality and they will not always concur; nevertheless, judicial aspects are considered to be environmental-contextual elements of the dilemma.
Based on the above, the administration of CBD and CBDV as antiepileptics therefore seems correct; however, all the specifications and particularities of the given case in which the decision will be made must be considered. With an aim to provide clarity to the reader, we shall make three conclusions and proposals as a final result of the discussion among expert epileptologists (neurosurgeons, neurologists, neuropsychologists, neuroscientists) and a bioethics specialist (physician who rotates between clinical bioethics and neuroscience).
Conclusions and positionProposals made by the Hospital General de México Epilepsy ClinicConclusion 1. There are misconceptions among the general public, which includes patients and family members, regarding cannabinoids, their uses, efficacy and risks.
Proposal 1. The relevant authorities (legal, healthcare, etc.) and doctors must make certain in their reports on reforms, laws, permissions and prescriptions or in their explanations regarding the medicinal use of cannabinoids, to project clarity in their messages in order to prevent conflicts in the general population and in the doctor–patient relationship. Legislators are advised to be very specific in terms of the nomenclature of the substances in laws and general announcements. Terms such as “marijuana”, “cannabis” and “cannabis extracts” are considered to be ambiguous.
Conclusion 2. “Marijuana” is not considered in the treatment of epilepsy, but rather its extracts CBD and CBDV. It should be clarified that due to the limited evidence in human research, and because the adverse effects reported in some cases have been serious,40 CBD and CBDV are still not considered to be first choice antiepileptics in any type of epilepsy.
The recent dissemination, through limitless social networks and communication media, regarding the use of “marijuana” in isolated cases, and the opinion of non-experts in the subject, has created confusion for epilepsy patients, family members and the general public. It generates false expectations in patients and affects the patient–doctor relationship.
Proposal II. The competent national authorities, in collaboration with communication media, must disseminate clear and prudent messages based on scientific evidence to the general public with regard to this subject.
Conclusion 3. Clinical neuroethics may justify the use of CBD and CBVD in refractory or catastrophic cases, which have been suitably medically and surgically protocolised. This should be supported by international law that fundamentally protects human life and dignity;
Proposal III. Nevertheless, the decision in terms of prescription relevance must be made exclusively by way of a discussion between 3 or more experts in the field (epileptologists from different disciplines), or by an expert centre, clinic or interdisciplinary group in the field. The interdisciplinary discussion between peers is necessary to prevent conflicts of interest, to decrease the influence of morality in decision-making and to verify the relevance for use in each case. Moreover, these cases must be strictly monitored and scientifically reported, providing knowledge to the medical and scientific community.
As the substances are still widely unknown in terms of efficacy and adverse effects in humans, especially in the long-term, interdisciplinary work is needed to make adequate decisions with regard to prescription, management and patient follow-up. Therefore, discussion among experts shall be relevant if the treatment must be interrupted.
Conclusion 4. Discussion among experts in the area of clinical neuroscience is relevant for decision-making. Many patients in this field have particularities that provide a hindrance, such as chronic pain and suffering, cognitive and affective disorders and state of consciousness disorders that affect patient autonomy. Combined with this, the chronicity of the patient's condition also affects family members and caregivers, which therefore affects their decisions. As a result, dilemmas arise in daily practice.
Proposal IV. Clinical bioethics must be given its place in hospitals to provide relevant guidance for healthcare personnel, patients and family members, especially in the field of clinical neuroscience.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The entire group that collaborated in the Hospital General de México Epilepsy Clinic, especially Georgina Gorian Montealegre, MPSS [Médico pasante en servicio social; social services medical intern]. Dr Antonio Cabrera Cabrera, director of the Bioethics Faculty of the Universidad Anáhuac México Norte, Dr José Damián Carrillo Ruíz, president of the Bioethics Hospital Committee of the Hospital General de México and the Ongoing Medical Education department of the Hospital General de México, for facilitating interdisciplinary work and growth in Clinical Bioethics, Neuroscience and medical academic training in Mexico.