Camptocormia is a major disabling abnormality characterized by severe forward flexion of the thoracolumbar spine. We report here on the effectiveness of deep brain stimulation (DBS) for the management of a case of untreatable idiopathic camptocormia. The patient, a 51-year-old male, with an 11-year-long history of radicular pain. Camptocormia symptomatology initiated 4 years ago. Preoperative muscle electrodiagnostic testing was within normal limits. Myopathy was ruled out. In the standing position myokymic discharges were recorded. Under local anesthesia and stereotactic control, electrodes for DBS were placed bilaterally in the globus pallidus internus. Patient's symptoms disappeared immediately following DBS. This response cannot be attributed to the surgical procedure itself. When stimulators were turned “off” accidentally, the patient returned immediately to his pre-surgery condition. Erect posture and walking were restored when stimulators were back “on”.
La Camptocormia es un trastorno incapacitante caracterizado por flexión anterior severa del tronco. Aquí presentamos un caso de camptocormia idiopática intratable donde la estimulación cerebral profunda (ECP) fue altamente eficaz. El paciente, un hombre de 51 años de edad, con historia de dolor radicular de 11 años y sintomatología de camptocormia de 4 años de evolución. Se descartó la presencia de miopatías. Las pruebas musculares preoperatorias mediante electrodiagnóstico resultaron normales, aunque en la posición de pie se registraron descargas mioquímicas. Para proveer la ECP se colocaron electrodos en el globo pálido interno bilateralmente, bajo anestesia local y control estereotáctico. Los síntomas de camptocormia desaparecieron inmediatamente después de la ECP. Esta respuesta no es atribuible a la intervención quirúrgica en sí, pues cuando el estimulador se apagó accidentalmente, el paciente regresó a su condición pretratamiento y la postura erecta se restauró al restablecer la estimulación.
Sir Benjamin Collins Brodie (1783–1862) described in 1818 a disease in which the low back pain and abnormal curvature of the spine were caused by a vertebral destructive process, perhaps he never named this entity.1–3
The term camptocormia was first used by Souques and Rosanoff-Saloff (cyphose hystérique, 1915) to describe bent spine in military personnel (“bent back of soldiers” or “war hysteria”) who had been sent to the front lines in the First World War.1,2 Originally considered as a psychogenic disorder, the expression has been adapted to describe severely flexed postures observed in Parkinson disease (Unified Parkinson's Disease Rating Scale and the Hoehn and Yahr scale) and other etiologies (drug induced, muscular disease, paraneoplastic and etcetera); is a heterogeneous disorder, even described as idiopathic in some cases.3–6
Camptocormia is more of a syndrome rather than a sign; it can be attributed to the progressive weakness of the antigravity muscles linked to trunk extension,5 as well as weakness of the gluteus maximus.4
Camptocormia, also known as bent spine syndrome (BSS), is a major disabling acquired abnormality characterized by severe forward flexion of the thoracolumbar spine (angle over 45°) with total correction to the supine position. This abnormal posture is involuntary and is only evident when the patient is standing or walking. At severe status, normal activities are difficult or impossible to perform.1–7
We report here on the neurological recovery of a patient diagnosed with idiopathic camptocormia after functional neurosurgery treatment.
Clinical caseA 51-year-old man, business consultant, with a history of spine events for radicular pain without myelopathy: a lumbar disk surgery (L5-S1) eleven years ago, and two years ago a cervical C4-C5 and C5-C6 discectomy and anterior fusion.
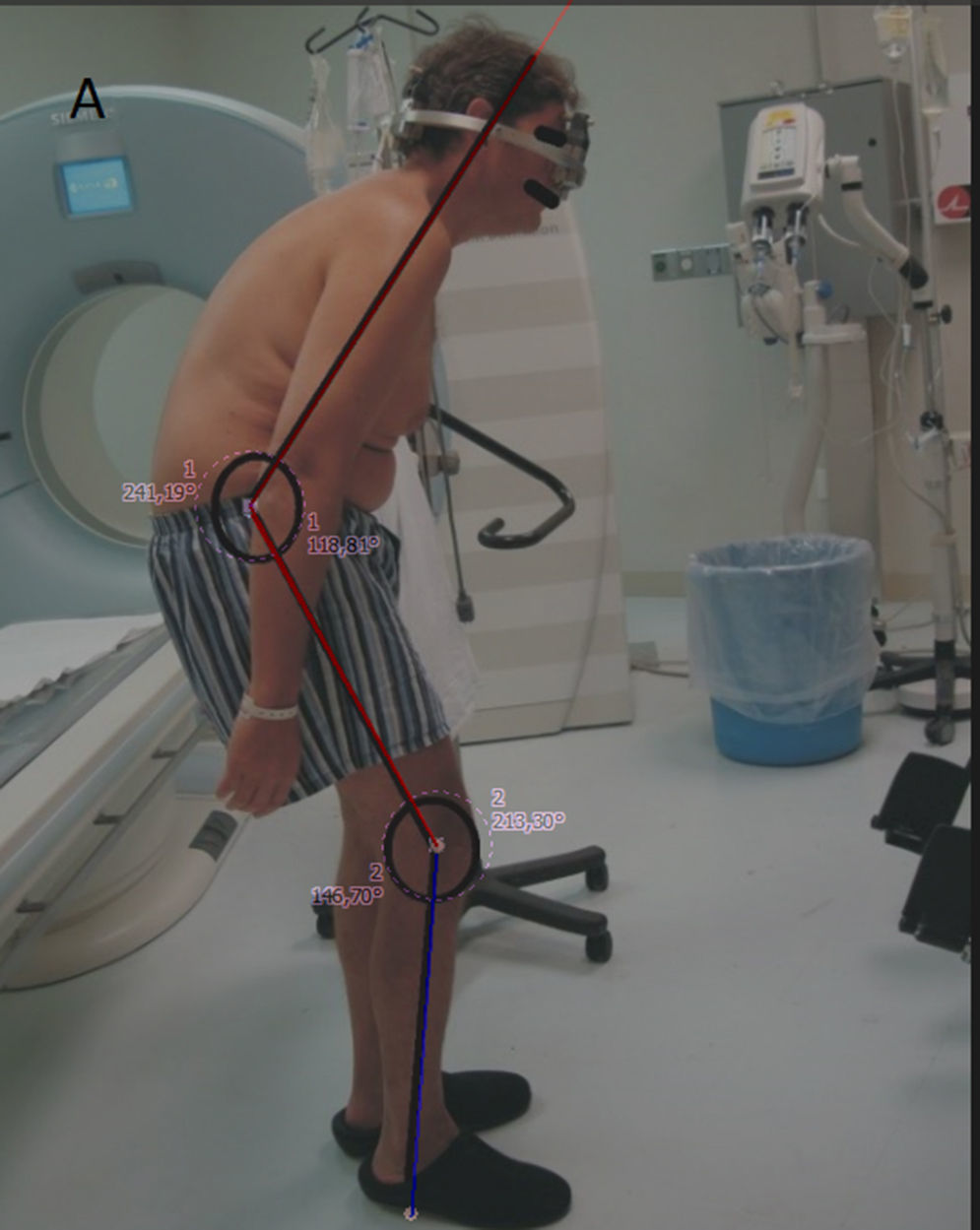
Four years ago he initiated his camptocormia clinical presentation with the sensation of anterior traction of his body, resulting in uncontrollable trunk flexion that was progressive, and became permanent, severely impairing his daily activities and the quality of his life (Fig. 1).
Eight months before being referred to us he was diagnosed with idiopathic camptocormia, and was started unsuccessfully treatment on levodopa. Later, on two occasions, he received botulinum toxin type A injections into the rectus abdominus, it caused discrete clinical benefit for 3 months after the first dose; the second dose produced no effect. In our hands, electromyography (EMG) and imaging studies (plain X-rays and MRI) did not reveal any abnormality associated with camptocormia.
During the physical exploration, he was found in good general condition (vital signs: blood pressure 128/85, 72 BPR, 18 BPM, 36.6°C; height 1.81m, weight 86kg), suffering weakness for the truncal extension and pronounced back flexion with an approximate angle of 120° (chess over knees); compensatory hyperextension of the neck and half-bent knees (210°). Also, the position of the arms hanging and swinging like an ape walking.
Preoperatively, motor and sensory nerve conduction velocity were within normal limits in upper and lower extremities. Median, ulnar, peroneal, and tibial nerves were studied. With the patient lying in the supine position, F waves and H reflexes were also found to be within normal limits.
Simultaneous EMG four-channel recordings of abdominal muscles (right-left, upper-lower) were obtained preoperatively in the operating room. Insertional activity was normal when the patient was lying on his back. Spontaneous EMG recordings did not show fibrillation nor fasciculation. During voluntary contraction, motor unit action potentials (MUAPs) showed normal parameters, and recruitment and interference patterns were within normal limits. When the patient was in the sitting position, a spontaneous mild contraction of the lower and upper rectus abdominus was felt through palpation. EMG recordings showed muscle activity with discharges in doublets, morphology was polyphasic and amplitude varied from 0.5 to 3mV and the firing rate was 10–20Hz. This pattern was continuous with no apparent variations. As soon as the patient assumed the standing position, he instantly showed moderate trunk flexion, and the EMG recordings revealed a significant increase in frequency and number of discharges, screening doublets or multiplets with a firing rate of 20–50Hz. The recording was typical of myokymic discharges. It must emphasized that EMG recordings did not display a denervation pattern. Thus, myopathy was ruled out. EMG digital recordings were analyzed by a one-way analysis of variance; there were no significant differences of the mean areas (mV/msec) among muscle activities from all four channels.
Due to the patient's condition was refractory to medication, functional neurosurgery became the best therapeutic option. On January 22, 2009 at the “Hospital Ángeles del Pedregal” in Mexico City, the patient was submitted to bilateral GPi placement of electrodes for DBS (Soletra, Medtronic) under local anesthesia and stereotactic control. Parameters for the stimulators were set as follows: left, amplitude 2.5V, width 90ms, frequency 145Hz, C+1-, impedance 816Ohm; right, amplitude 2.5V, width 90ms, frequency 145Hz, C+1-, impedance 1086Ohm. During the surgical procedure, a continuous free EMG was recorded, in which no MUAPs were detected and only occasional isolated fibrillations were identified. Also, no positive sharp waves or fasciculations were identified.
Immediately after surgery the patient showed notable improvement in his posture with the stimulators in the “on” position. At this time, during the sitting and standing positions, EMG recordings showed only occasional and transient discharges. Amplitude varied from 0.5 to 1.5mV. Discharge frequency was less than 10Hz.
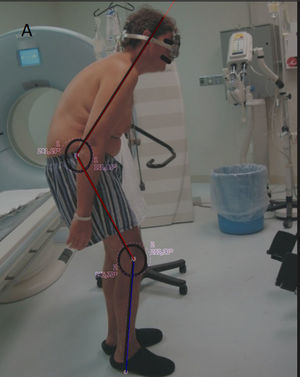
The clinical progress was remarkable and his quality of life improved noticeably, he returned to the work with no side effects nor complications (Fig. 2).
On November 24, 2009, he suddenly reverted to his preoperative condition after going through a security arch that turned “off” the stimulators. Once turning them back “on” again, he immediately returned to his improved postoperative posture, which he has maintained to date without requiring any further adjustments to his stimulators. After a year of follow-up, the patient has shown no adverse effects due to DBS.
DiscussionDespite the absence of concrete data on the pathogenesis of the disease, there are two theories that attempt to explain the clinical onset of the syndrome.
The first theory suggest that camptocormia has a central origin in which there exists disorders at striatum and their projections on reticulospinal tract, or the thalamus, where the trunk is strongly represented. The another theory is related with peripheral mechanisms, including the antigravity muscles myopathy associated with trunk extension.2,7
For these reasons, the response of the camptocormia to systemic and local therapies has been variable.6
Randomized studies have shown that treatment with high-frequency electrical stimulation (deep-brain stimulation, DBS), which involves the surgical implantation of a device, is superior to medical therapy for improving motor function and quality of life for patients. As well, according to the current literature DBS is only indicated in the cases of drug-resistant or severe motor fluctuations unmanageable by pharmacological treatment.8–12
There are few reports in the literature regarding the treatment of camptocormia. In 2005, Micheli et al. reported a patient underwent to bilateral DBS in Gpi with recovery of trunk flexion.13
We report here on the neurological recovery of a patient diagnosed with idiopathic camptocormia after functional neurosurgery treatment. Here we confirm these findings and further show that the neurological benefit obtained from DBS is not related to surgical manipulations per se but to the electrical stimulation in this brain region: when the stimulators were accidentally shutdown the patient suddenly returned to his preoperative condition. Once the stimulators were turned back “on” again, his recovery was immediate.
The globus pallidal interna (GPi) and the subthalamic nucleus (SN) are both accepted targets for DBS, however stimulation of the GPi has been shown superior efficacy to improve motor symptoms in patient with idiopathic camptocormia diagnosis.8,9
Follett and company9 compared the bilateral stimulation of GPi versus the SN and they reported similar data in both study group, but this trial revealed higher efficacy about long-term durability of the effect, decrease over time about improving motor function and stable response to pallidal stimulation over three years of follow up.
Additionally, an extra benefit described is the successful conversion to subthalamic stimulation as salvaged therapy after pallidal stimulation failed. For the moment is not known the possibility the conversion to pallidal stimulation in patients whom the long-term subthalamic stimulation failed.9
The precise mechanism(s) underlying the improvement seen here induced by bilateral GPi-DBS to maintain the erect posture and bipedal walking is not completely understood. GPi stimulation in humans has been associated with a suppression of neuronal activity in the thalamus.11,12,14 Sakas and his group6 have postulated that specific patterns of oscillatory activity in the GPi may be vital for the favorable response. Observations made in the GPi of parkinsonian patients during surgery suggest that stimulation may release neurotransmitters such as GABA that might be involved in the therapeutic effects of GPi-DBS.
Although DBS has been effective in the treatment of movement disorders and is rapidly being explored for the treatment of other neurologic disorders, the scientific understanding of its mechanism(s) of action remains unclear. Four general hypotheses have been developed to explain the mechanism(s) of DBS: depolarization blockade, synaptic inhibition, synaptic depression, and stimulation-induced modulation of pathologic network activity, the latter representing the most likely mechanism for DBS.15–17
In our case, immediately following pallidal DBS the patient regained his normal posture and was able to return to a normal life-style. The impact of GPi electrical brain stimulation (and not of the surgical intervention, as such) became evident when the patient abruptly returned to his preoperative condition after crossing a security arch which shutdown his stimulators. After resumption of brain stimulation his recovery was immediate. The GPi placement of the stimulators rests on the notion that in some cases the pathological mechanism underlying camptocormia has been attributed to a diseased striatum and its projections to the reticulospinal tract or the thalamus.1,3
After extensive electrodiagnostic testing, our patient's BSS was found to be unrelated to any apparent neurological or neuromuscular disease and was therefore diagnosed as idiopathic camptocormia. The major preoperative finding was the myokymic discharges. This type of discharge is usually associated with tetany. It should be pointed out that myokymic discharges, as observed in this case, are different than neuromyotonic discharges. The main difference between these abnormal muscle activities is the firing rate. Myokymic discharges show a firing rate<150Hz, whereas neuromyotonic discharges show a firing rate>150Hz. As mentioned herein, EMG recordings in this patient showed a firing rate<150Hz in all cases.
In summary, GPi-DBS resulted in an immediate and almost complete recovery of the patient's bent spine. The sudden loss of brain stimulation after crossing a security arch, which shut down the stimulators, immediately resulted in the patient's return to his pre-operative state. Recovery of neurological function was also immediate after stimulators were turned back “on”. This experience supports our contention that patient's posture correction is, in fact, a response to pallidal DBS.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interests.