Skin reactions are the most frequent adverse drug event, and manifest clinically in many ways, ranging from mild, self-limiting reactions, to severe and potentially fatal forms. DRESS syndrome is one of the most severe forms of drug-induced skin reaction, and can be life threatening. However, currently we have no internationally accepted guidelines for the correct characterisation and treatment of some drug-induced skin reactions, DRESS syndrome and other severe drug-induced skin reactions, in particular.
MethodsA retrolective and descriptive study. The sample was selected from the records of patients registered in the Dermatology Department. Twenty-seven cases were selected, and the suspected causative drug, clinical presentation, complications, treatment response and mortality were evaluated.
ResultsThe patients were aged between 17 and 99. DRESS syndrome was the second most common drug-induced skin reaction. Antibiotics were the most commonly associated group of drugs, followed by anticonvulsants. The drug exposure time and the onset of dermatosis were variable. All the patients responded well to treatment with a mortality of 0%.
ConclusionsFor correct diagnosis and intervention it is essential to identify the different clinical patterns of drug-induced skin reactions; DRESS syndrome in particular, is a drug-induced skin reaction that can endanger a patient's life, and therefore it is important that it is correctly identified and managed.
Las reacciones cutáneas son el evento adverso farmacológico más común, manifestándose de manera clínica muy variada, desde leves y autolimitadas hasta formas severas y potencialmente letales. El síndrome DRESS es una de las farmacodermias más graves, pudiendo comprometer la vida. Sin embargo, actualmente aun no contamos con guías internacionalmente aceptadas para la adecuada caracterización y tratamiento de algunas farmacodermias, en particular del síndrome DRESS y otras formas severas.
MétodoEstudio retrolectivo y descriptivo. La muestra fue seleccionada de los expedientes de pacientes del Servicio de Dermatología del Hospital General de México. 27 casos fueron seleccionados, evaluando el fármaco causal, presentación clínica, complicaciones, respuesta a tratamiento y mortalidad.
ResultadosLos pacientes evaluados se encontraron en edades entre 17 a 99 años. El síndrome DRESS fue la segunda farmacodermia en frecuencia. El grupo de fármaco más comúnmente asociado fueron los Antibióticos, seguido por el grupo de anticonvulsivantes. El tiempo de exposición al fármaco y el inicio de la dermatosis fue variable. Todos los pacientes respondieron bien al tratamiento con una mortalidad de 0%.
Conclusionesla identificación de los diferentes patrones clínicos en las farmacodermias es básico para un adecuado diagnóstico e intervención oportuna; particularmente, el síndrome DRESS constituye una farmacodermia que puede poner en riesgo la vida del paciente, de ahí la importancia de su identificación y manejo.
Adverse reactions to drugs are common, they present in 10–20% of hospitalised patients and in approximately 7% of the general population.1 Statistics in the United States indicate that more than 100,000 deaths a year are attributable to adverse drug effects.2,3 Adverse drug reactions can affect any organ, however, muco-cutaneous involvement is the most common.1–4 The basis for diagnosis and appropriate intervention lies in identifying the different clinical patterns of drug-induced skin reactions, which range in severity from mild and self-limiting to potentially lethal. These reactions are considered to be more common in the hospital environment, either as the reason for admission or as an intrahospital complication,2,4 and 2% of drug-induced skin reactions are considered to be severe or even fatal.2,5
Antibiotics are the drugs most commonly used to treat drug-induced skin reactions (41%), chiefly penicillin and sulphonamide derivatives; followed by non-steroid anti-inflammatories (11%) and anticonvulsants (10%).6
DRESS syndrome (Drug Reaction with Eosinophilia and Systemic Symptoms) is a severe form of drug-induced skin reaction because major systemic involvement is associated with it. The term DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms) was proposed by Bocquet and collaborators in 19967–11 in order to remove the ambiguity surrounding the term “drug hypersensitivity syndrome”. The syndrome is also known as drug hypersensitivity syndrome,12,13 delayed drug hypersensitivity syndrome,14 drug-induced delayed multiorgan hypersensitivity syndrome,14 drug induced hypersensitivity syndrome, DIHS,11,15–17 and anticonvulsant hypersensitivity syndrome.18,19 It is rare, and its exact incidence is unknown.20 It is estimated that it occurs in one out of every 1000 to 10,000 patients exposed to drugs.2,8,11,13,19 DRESS syndrome is rare in children and probably underdiagnosed.21
In most cases this syndrome presents between the first and the eighth week after administration of the causative drug,22,23 and some authors even consider that onset can be up to more than 12 weeks after the causative drug has been taken8,19; which implies a much longer latency period than that of other drug-induced skin reactions.24 DRESS syndrome is characterised by the presence of a skin condition, erythema and facial oedema, fever, general malaise, haematological disorders, swollen lymph nodes and involvement of one or more internal organs.2,7,11,25–30
Fever (≥38°C) is usually the first manifestation of this syndrome,11,24,31 and dermatosis appears approximately 24–48h later,24 presenting in 85–90% of patients.8,32 In most cases, the skin condition is a morbilliform drug rash.2,5,8,14,32 It can result in erythroderma in almost 50% of cases.32 In general, in typical DRESS syndrome cases the mucosa is rarely affected; it can manifest as conjunctivitis, cheilitis, ulceration of the oral cavity and genital mucosa.31,32 But it should be remembered that although a morbilliform rash is the most common skin reaction in this syndrome, it may present with skin conditions of varied morphology, such as blisters, pustules, atypical target lesions, purpura, involvement of the mucosa, and can even manifest as Stevens–Johnson syndrome or toxic epidermal necrolysis.33 When the DRESS syndrome skin condition is Stevens–Johnson syndrome or toxic epidermal necrolysis, the condition is defined as DRESS syndrome with severe cutaneous reaction,33,34 and corresponds to approximately 9% of DRESS syndrome cases.33 However the severity of the cutaneous involvement bears no relation to the severity of the involvement of the internal organs.6,32 The most common haematological disorder is eosinophilia (70–80%),6,7,31 and to a lesser extent atypical lymphocytosis can also present,15,35–40 which is even similar to that of mononucleosis.2,24 It is estimated that this syndrome is associated with hepatitis in 50–64% of cases,2,31,35 tubulointerstitial nephritis in 10% of cases,35 and is associated more rarely with interstitial pneumonitis, carditis, colitis, pancreatitis and other conditions.7,10
The manifestations of this syndrome can even persist for several weeks after the causative drug has been withdrawn.7 This pathology has a mortality of 10%.7,10,11,17,19,36–38
The pathogenesis of DRESS syndrome is complex and is still not well understood.2,3,11,14,24,39,40 An imbalance caused by an excess of toxic metabolites arising from detoxification defects (principally those mediated by enzyme detoxification by the hepatic microsomal system)11,14,24 has been put forward as a possible mechanism of this syndrome's pathogenesis. The reactivation of latent viral infections have also been implicated in this syndrome's pathogenesis18; in particular, human herpes virus type 6, Epstein–Barr virus and cytomegalovirus.7,41,42
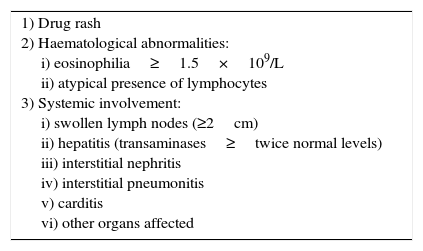
When DRESS was first described, Bocquet and collaborators (1996),8,10 suggested the diagnostic criteria for the syndrome, and these remain the basis for its diagnosis. Three criteria need to be present in order to diagnose DRESS syndrome (see Table 1).
Diagnostic criteria for DRESS syndrome.
| 1) Drug rash 2) Haematological abnormalities: i) eosinophilia≥1.5×109/L ii) atypical presence of lymphocytes 3) Systemic involvement: i) swollen lymph nodes (≥2cm) ii) hepatitis (transaminases≥twice normal levels) iii) interstitial nephritis iv) interstitial pneumonitis v) carditis vi) other organs affected |
The basis of treatment is to establish the causative drug and promptly withdraw it. This is the only area where there is general consensus in the literature on the management of DRESS syndrome,24,29 since many treatments are mentioned in international literature, all with varying results. There are currently no proposed international consensus guidelines for treatment.2,29,43 Recovery starts when the causative drug has been withdrawn, however symptoms can persist for weeks and biochemical changes can last for months until they completely resolve.2,8,24,44 The prognosis depends on the severity of organ involvement.2,17
There are currently no international guidelines on treatment regimens for severe drug-induced skin reactions, particularly for DRESS syndrome. Therefore it is particularly important and necessary that in-depth studies are carried out on this syndrome, principally with regard to its epidemiology, clinical manifestations, and proposals for treatment in terms of efficacy and safety.
Material and methodThis is a retrolective and descriptive study. The sample was selected from the records of patients registered as outpatients and inpatients of the Dermatology Department of the General Hospital of Mexico between January 2007 and May 2010. The records of patients hospitalised with a diagnosis of drug-induced skin reaction between January 2007 and May 2010 were included. Once the records had been selected, the data collection sheets were completed with the findings from these records. DRESS syndrome was diagnosed using Bocquet's diagnostic criteria (see Table 1). We evaluated the frequency of the different types of drug-induced skin reaction as a cause of hospitalisation, the frequency of DRESS syndrome compared to other drug-induced skin reactions, the most frequently associated drugs, the clinical and biochemical characteristics, and the therapeutic response. The analysis of the results is presented as a descriptive analysis using tables, graphs and central tendency and dispersion measures.
ResultsA total of 27 patient records were included in the study. From the data found it was observed that the patients were aged between 17 and 99, with a mean age of 41.29, however, the age group with the greatest number of cases was between 21 and 30. In terms of gender, it was observed that there was a slight predominance of females (59.25%), with a female to male ratio of 1.45:1.
A total of 25.92% of the drug-induced skin reactions studied were DRESS syndrome. According to the distribution by diagnosis, 37.03% of cases were Stevens–Johnson syndrome. DRESS syndrome was the second most frequent, at 25.92% of the cases studied, followed by other drug-induced skin reactions such as erythroderma (11.1%), morbilliform rash (7.4%), polymorphic erythema (7.4%), Stevens–Johnson/toxic epidermal necrolysis superposition syndrome (3.7%), toxic epidermal necrolysis (3.7%) and bullous, disseminated, fixed pigmented erythema (3.7%). With regard to the causative drug, it was found that antibiotics were most associated with drug-induced skin reactions, principally sulphonamides. Anticonvulsants came second in frequency; in this case, all the aromatic anticonvulsants (diphenylhydantoin, carbamazepine and lamotrigine). The NSAIDs were the third most frequent group. There was no distinction here between DRESS syndrome and the other drug-induced skin reactions. The exposure time to the drug and the onset of the drug-induced skin reaction varied, from hours to up to 30 days, and it was observed that the exposure times were longer for anticonvulsants and shorter for NSAIDs.
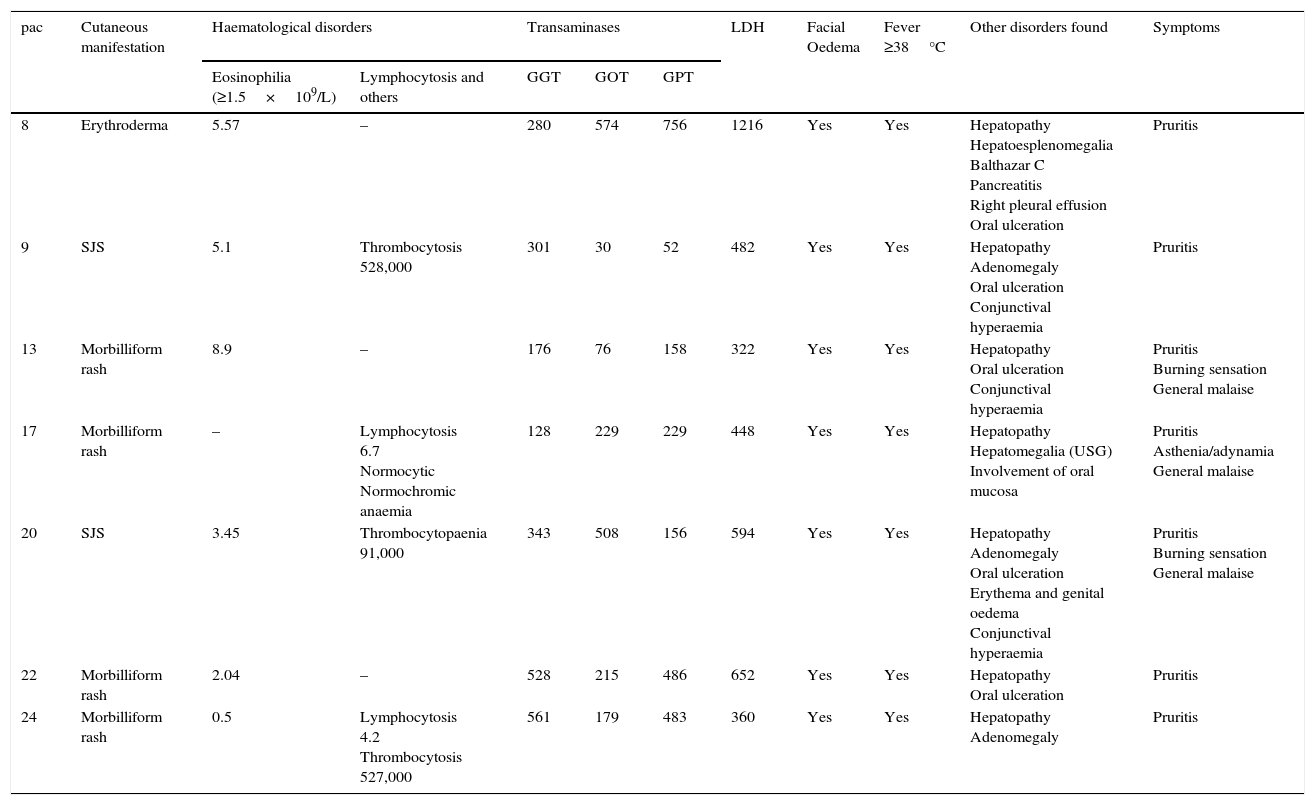
With regard to the clinical presentation of the drug-induced skin reactions that we studied, it was observed that of the 7 patients diagnosed with DRESS syndrome, the presentation of the reaction varied considerably, morbilliform rash predominated in 4 cases (57.14%); followed by Stevens–Johnson syndrome in 2 cases (28.57%), and erythroderma in one case (14.28%). All of the patients with DRESS syndrome had transaminase levels up to twice the normal range or more at the time of diagnosis. Of the 7 patients, 5 had eosinophilia (71.42%), and 2 lymphocytosis (28.57%); further haematological disorders were found: one patient had anaemia, 2 patients had thrombocytosis and one patient thrombocytopaenia. All the patients with DRESS syndrome presented with the characteristic facial oedema and fever ≥38°C at the onset of the disorder (Tables 2 and 3).
Clinical and laboratory findings in DRESS syndrome patients.
| pac | Cutaneous manifestation | Haematological disorders | Transaminases | LDH | Facial Oedema | Fever ≥38°C | Other disorders found | Symptoms | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eosinophilia (≥1.5×109/L) | Lymphocytosis and others | GGT | GOT | GPT | |||||||
| 8 | Erythroderma | 5.57 | – | 280 | 574 | 756 | 1216 | Yes | Yes | Hepatopathy Hepatoesplenomegalia Balthazar C Pancreatitis Right pleural effusion Oral ulceration | Pruritis |
| 9 | SJS | 5.1 | Thrombocytosis 528,000 | 301 | 30 | 52 | 482 | Yes | Yes | Hepatopathy Adenomegaly Oral ulceration Conjunctival hyperaemia | Pruritis |
| 13 | Morbilliform rash | 8.9 | – | 176 | 76 | 158 | 322 | Yes | Yes | Hepatopathy Oral ulceration Conjunctival hyperaemia | Pruritis Burning sensation General malaise |
| 17 | Morbilliform rash | – | Lymphocytosis 6.7 Normocytic Normochromic anaemia | 128 | 229 | 229 | 448 | Yes | Yes | Hepatopathy Hepatomegalia (USG) Involvement of oral mucosa | Pruritis Asthenia/adynamia General malaise |
| 20 | SJS | 3.45 | Thrombocytopaenia 91,000 | 343 | 508 | 156 | 594 | Yes | Yes | Hepatopathy Adenomegaly Oral ulceration Erythema and genital oedema Conjunctival hyperaemia | Pruritis Burning sensation General malaise |
| 22 | Morbilliform rash | 2.04 | – | 528 | 215 | 486 | 652 | Yes | Yes | Hepatopathy Oral ulceration | Pruritis |
| 24 | Morbilliform rash | 0.5 | Lymphocytosis 4.2 Thrombocytosis 527,000 | 561 | 179 | 483 | 360 | Yes | Yes | Hepatopathy Adenomegaly | Pruritis |
Laboratory levels that are considered normal: eosinophils 0.1–0.3, lymphocytes 1.0–3.0, platelets 150,000–450,000, GGT 7–50IU, GOT 15–41IU, GPT 14–45IU, LDH 98–192IU. Only the maximum alterations found in each of the patients are shown in the Table.
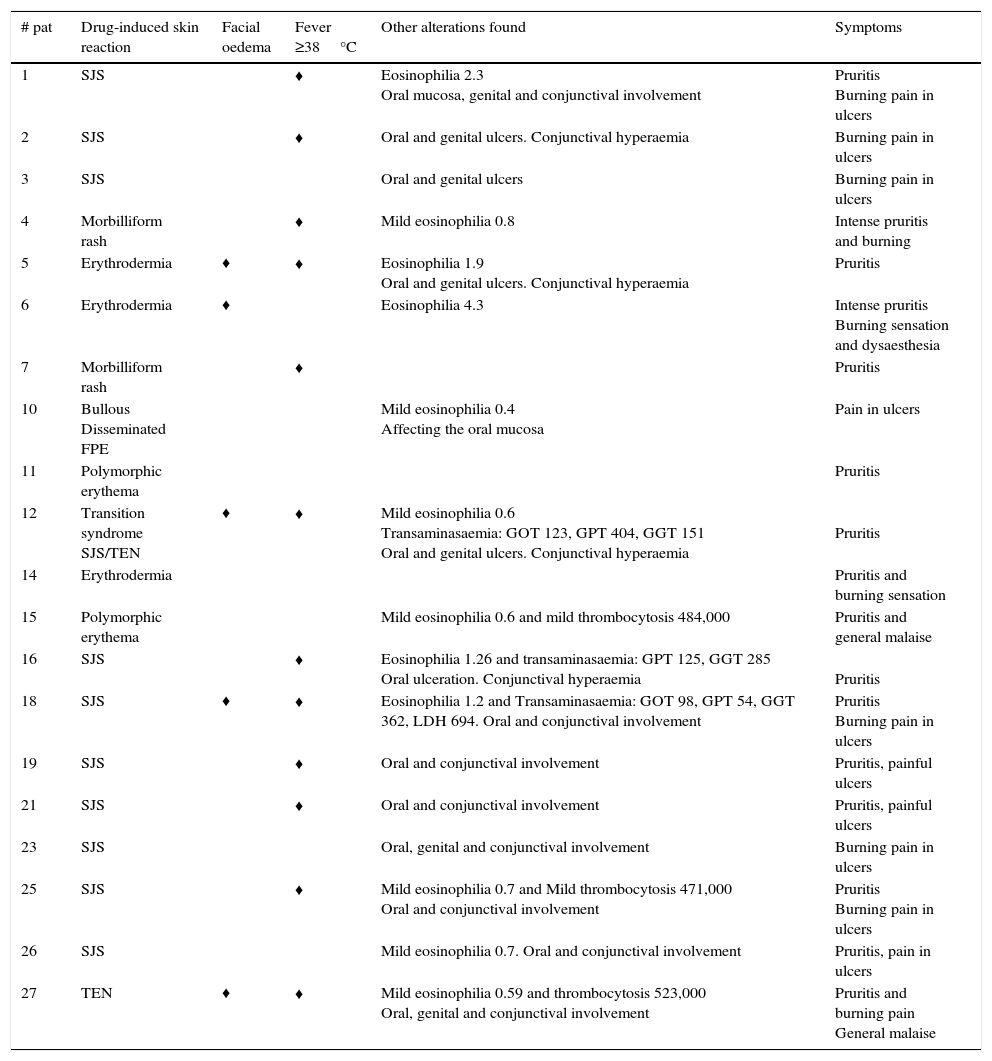
Clinical and laboratory findings in the patients with drug-induced skin reactions other than DRESS syndrome.
| # pat | Drug-induced skin reaction | Facial oedema | Fever ≥38°C | Other alterations found | Symptoms |
|---|---|---|---|---|---|
| 1 | SJS | ♦ | Eosinophilia 2.3 Oral mucosa, genital and conjunctival involvement | Pruritis Burning pain in ulcers | |
| 2 | SJS | ♦ | Oral and genital ulcers. Conjunctival hyperaemia | Burning pain in ulcers | |
| 3 | SJS | Oral and genital ulcers | Burning pain in ulcers | ||
| 4 | Morbilliform rash | ♦ | Mild eosinophilia 0.8 | Intense pruritis and burning | |
| 5 | Erythrodermia | ♦ | ♦ | Eosinophilia 1.9 Oral and genital ulcers. Conjunctival hyperaemia | Pruritis |
| 6 | Erythrodermia | ♦ | Eosinophilia 4.3 | Intense pruritis Burning sensation and dysaesthesia | |
| 7 | Morbilliform rash | ♦ | Pruritis | ||
| 10 | Bullous Disseminated FPE | Mild eosinophilia 0.4 Affecting the oral mucosa | Pain in ulcers | ||
| 11 | Polymorphic erythema | Pruritis | |||
| 12 | Transition syndrome SJS/TEN | ♦ | ♦ | Mild eosinophilia 0.6 Transaminasaemia: GOT 123, GPT 404, GGT 151 Oral and genital ulcers. Conjunctival hyperaemia | Pruritis |
| 14 | Erythrodermia | Pruritis and burning sensation | |||
| 15 | Polymorphic erythema | Mild eosinophilia 0.6 and mild thrombocytosis 484,000 | Pruritis and general malaise | ||
| 16 | SJS | ♦ | Eosinophilia 1.26 and transaminasaemia: GPT 125, GGT 285 Oral ulceration. Conjunctival hyperaemia | Pruritis | |
| 18 | SJS | ♦ | ♦ | Eosinophilia 1.2 and Transaminasaemia: GOT 98, GPT 54, GGT 362, LDH 694. Oral and conjunctival involvement | Pruritis Burning pain in ulcers |
| 19 | SJS | ♦ | Oral and conjunctival involvement | Pruritis, painful ulcers | |
| 21 | SJS | ♦ | Oral and conjunctival involvement | Pruritis, painful ulcers | |
| 23 | SJS | Oral, genital and conjunctival involvement | Burning pain in ulcers | ||
| 25 | SJS | ♦ | Mild eosinophilia 0.7 and Mild thrombocytosis 471,000 Oral and conjunctival involvement | Pruritis Burning pain in ulcers | |
| 26 | SJS | Mild eosinophilia 0.7. Oral and conjunctival involvement | Pruritis, pain in ulcers | ||
| 27 | TEN | ♦ | ♦ | Mild eosinophilia 0.59 and thrombocytosis 523,000 Oral, genital and conjunctival involvement | Pruritis and burning pain General malaise |
Levels considered normal: eosinophils 0.1–0.3, lymphocytes 1.0–3.0, platelets 150,000–450,000, GGT 7–50IU, TGO 15–41IU, TGP 14–45IU, only the maximum alterations found in each of the patients are shown in the Table.
The patients were managed by strictly monitoring their vital signs and electrolyte balance and ensuring general skin care. They were also given antihistamines, antipyretic treatment and analgesics when necessary. Only one (14.28%) of the 7 patients with DRESS syndrome was managed with continuous i.v. infusion of pentoxifylline, 1200mg over 24hours, as monotherapy; it was then given orally until the treatment was stopped. The 6 remaining patients (85.71%) were treated with systemic corticosteroids at a dose of 0.5–1mg/kg/day, and then with a reduction regime; of these, only one was managed using systemic steroids as monotherapy, and the other 5 were given systematic corticosteroids combined with pentoxifylline 1200mg/kg/day for 5–14 days, then orally until treatment was gradually stopped. Seventeen (85%) of the 20 patients who had drug-induced skin reactions other than DRESS syndrome, were managed with pentoxifylline 1200mg given by continuous i.v. infusion over 24h for 5–14 days and then orally, or were given the drug orally from the onset. Thirteen (65%) of these 17 patients treated with pentoxifylline were managed with monotherapy and 4 (20%) received combined therapy with systemic corticosteroids. While the 3 (15%) remaining patients were managed with systemic steroids as monotherapy; two of the cases were diagnosed with erythroderma and one with Stevens–Johnson.
Out of the total number of 27 patients, 3 (11.11%) had to be treated in the intensive care unit, 2 of whom (66.66%) were diagnosed with DRESS syndrome and one (33.33%) with toxic epidermal necrolysis. The remaining patients were managed as inpatients in the Dermatology Department. There was no record of the death of any of the patients (Table 4).
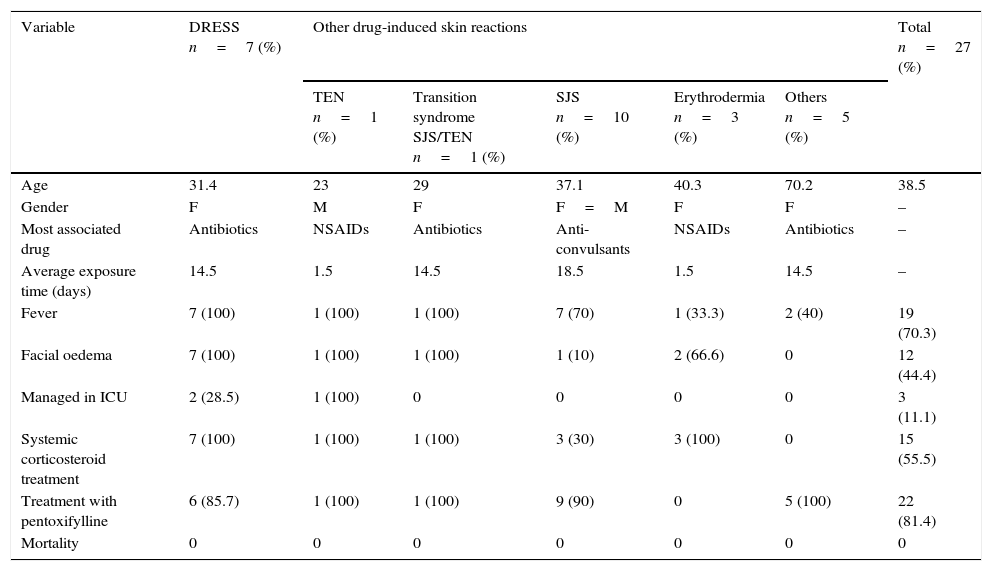
Most significant variables between DRESS syndrome and other drug-induced skin reactions evaluated.
| Variable | DRESS n=7 (%) | Other drug-induced skin reactions | Total n=27 (%) | ||||
|---|---|---|---|---|---|---|---|
| TEN n=1 (%) | Transition syndrome SJS/TEN n=1 (%) | SJS n=10 (%) | Erythrodermia n=3 (%) | Others n=5 (%) | |||
| Age | 31.4 | 23 | 29 | 37.1 | 40.3 | 70.2 | 38.5 |
| Gender | F | M | F | F=M | F | F | – |
| Most associated drug | Antibiotics | NSAIDs | Antibiotics | Anti-convulsants | NSAIDs | Antibiotics | – |
| Average exposure time (days) | 14.5 | 1.5 | 14.5 | 18.5 | 1.5 | 14.5 | – |
| Fever | 7 (100) | 1 (100) | 1 (100) | 7 (70) | 1 (33.3) | 2 (40) | 19 (70.3) |
| Facial oedema | 7 (100) | 1 (100) | 1 (100) | 1 (10) | 2 (66.6) | 0 | 12 (44.4) |
| Managed in ICU | 2 (28.5) | 1 (100) | 0 | 0 | 0 | 0 | 3 (11.1) |
| Systemic corticosteroid treatment | 7 (100) | 1 (100) | 1 (100) | 3 (30) | 3 (100) | 0 | 15 (55.5) |
| Treatment with pentoxifylline | 6 (85.7) | 1 (100) | 1 (100) | 9 (90) | 0 | 5 (100) | 22 (81.4) |
| Mortality | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations used: DRESS=Drug Reaction with Eosinophilia and Systemic Symptoms, ICU=Intensive Care Unit, TEN=toxic epidermal necrolysis, SJS=Stevens–Johnson Syndrome, FPE=fixed pigmented erythema, n=number, Tx=treatment, GOT=glutamic oxaloacetic transaminase, GPT=glutamate pyruvate transaminase, GGT=gamma-glutamyl-transpeptidase, LDH=lactate dehydrogenase, IU=international units, USG=ultrasonography, NSAIDs=nonsteriodal anti-inflammatory drugs.
This study addressed severe forms of drug-induced skin reactions which required hospitalisation, and 27 records were included which met the selection criteria. The most frequently associated causative drugs were, firstly, antibiotics, chiefly from the sulphonamide and penicillin groups, this is in line with the literature consulted,6 followed in our case by anticonvulsants and non-steroid anti-inflammatories. It is also worth mentioning that this causative drug frequency was the same for both DRESS syndrome patients and patients with other drug-induced skin reactions. And although this DRESS syndrome was initially described in the literature with a greater number of cases relating to anticonvulsants,13,18 numerous studies and case presentations have shown that this group of drugs is not the only group which can cause the syndrome. This is another reason why the syndrome should not be categorised as “anticonvulsant hypersensitivity syndrome”.
We observed that the exposure time to the drug varies greatly. In the patient records that we checked it ranged from one to 30 days, which in practical terms translates to exposure times from one to 4 weeks, which is consistent with the literature reviewed.7,44
Stevens–Johnson syndrome was the most frequently observed clinical presentation of drug-induced skin reaction in the cases reviewed. This might be because only patients who needed to be hospitalised due to the severity of the skin reaction were reviewed, and the most frequently reported reaction in the literature is a morbilliform rash,3 yet this drug-induced skin reaction rarely requires hospitalisation and can be managed on an outpatient basis.
It should be noted that the liver is the internal organ which is most frequently affected in this syndrome2,8,24; and that liver involvement can range from a mild increase in transaminases, enlarged liver and acute toxic hepatitis to massive fulminant hepatic necrosis.2,8,24,42,45 Practically any organ can be affected, and it is precisely multiorgan involvement that characterises and differentiates this drug-induced skin reaction.46
Furthermore, there is currently no consensus with regard to therapeutic regimens for DRESS syndrome,29,43 however, most of the literature agrees that drug management of this disorder should include systemic corticosteroids as first-line treatment.2,22 In our review, 6 of the 7 patients diagnosed with DRESS syndrome were treated with systemic corticosteroids, only one case received them as monotherapy, the rest were given systemic corticosteroids in combination with pentoxifylline. And only one of the 7 patients reviewed was treated using pentoxifylline as monotherapy. Pentoxifylline was used because it is a drug with an anti-inflammatory and immunomodulating action, principally due to its tumour necrosis factor-alpha action.47,48
It is very important to mention that all of the patients responded satisfactorily to treatment, both clinically and biochemically.
Finally, it is worth highlighting that identifying the different drug-induced skin reaction patterns is the basis for diagnosis and appropriate intervention; this is particularly important with drug-induced skin reactions which might have an impact on the patient's life such as DRESS syndrome. Fortunately it is this syndrome that is reported most frequently in scientific literature; we consider that this is because it is being more widely diagnosed.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestNone.