Cancer testis antigens (CTA) are expressed in several types of cancer but not in normal adult tissue, with the exception of testicular germ cells, and for this reason are considered targets for immunotherapy.
ObjectiveThe aim of this study was to determine the frequency and clinical significance of CTA expression in cells from patients with chronic myeloid leukaemia (CML).
Materials and methodsWe analysed 8 genes of the CTA family (MAGE-A3, MAGE-A4, MAGE-B2 MAGE-C1, BAGE-1, GAGE-2, LAGE-1 and NY-ESO-1) in 10 peripheral blood samples from healthy donors and 65 bone marrow samples from CML patients using reverse transcriptase polymerase chain reaction (RT-PCR). Eleven samples from hematopoietic progenitor stem cells from patients receiving conventional treatment (hydroxyurea and INFα) and 21 samples from patients receiving tyrosine kinase inhibitors (Imatinib, Nilotinib, Dasatinib) were analysed.
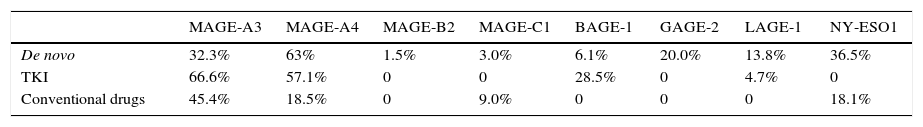
ResultsFrequency of CTA genes in CML patients was 32.3%, 63.0%, 1.5%, 3.0%, 6.1%, 20.0%, 13.8% and 36.9% for MAGE-A3, MAGE-A4, MAGE-B2, MAGE C1, BAGE, GAGE, LAGE and NY-ESO, respectively. The correlation between expression and clinical parameters showed statistical significance in the MAGE-A3 genes in sex (p=0.018), MAGE-C1 in leukocytes (p=0.014) in platelets (p=0.002), and finally LAGE-1, which was associated with platelets (p=0.001). The hematopoietic progenitor stem cells from patients in treatment showed a higher frequency of expression than de novo patients. In patients receiving conventional drugs (CD), frequency of MAGE-A3 expression was 50%, and 69.2% with TKI, MAGE-A4 frequency was 20% with CD and 53.8% with TKI. Finally, frequency of NY-ESO-1 was 20% with CD and 0% with TKI. The frequency of MAGE-A4 expression was highest, followed by MAGE-A3, NY-ESO-1, GAGE-2, LAGE-1, BAGE-1, MAGE-B2 and MAGE-C1.
ConclusionDetection of CTA genes by molecular biology techniques is important due to their use as biomarkers in LMC, in the monitoring of disease progression and in patients who are resistant to chemotherapy. The frequency of CTA expression in treated and de novo differs significantly.
Los antígenos testiculares de cáncer (ATC) están expresados en diversos cánceres, pero no en tejido adulto normal, con excepción de células germinales de testículo, por esta característica son considerados como blancos para inmunoterapia.
ObjetivoEl objetivo de este trabajo fue determinar la frecuencia de expresión de los ATC y su impacto clínico en células de pacientes con leucemia mieloide crónica (LMC).
Material y métodosSe analizaron a nivel molecular 8 genes pertenecientes a los ATC (MAGE-A3, MAGE-A4, MAGE-B2 MAGE-C1, BAGE-1, GAGE-2, LAGE-1 y NY-ESO-1) en 10 muestras de sangre periférica (SP) de individuos sanos y 65 muestras de médula ósea de pacientes con LMC, mediante reacción en cadena de la polimerasa por transcriptasa reversa (RT-PCR). Además, se analizaron 11 muestras de células progenitoras hematopoyéticas de pacientes que estaban en tratamiento con fármacos convencionales (Hidroxiurea e interferon alfa) y 21 pacientes con inhibidores de tirosina cinasa (Imatinib, Nilotinib, Dasatinib).
ResultadosLa frecuencia de los genes ATC en pacientes con LMC fue de 32.3%, 63.0%, 1.5%, 3.0%, 6.1%, 20.0%, 13.8% y 36.9% para MAGE-A3, MAGE-A4, MAGE-B2, MAGE-C1, BAGE, GAGE, LAGE y NY-ESO respectivamente. Se encontró correlación entre la expresión de los genes MAGE-A3 en sexo (p=0.018), MAGE-C1 leucocitos (p=0.014) y plaquetas (p=0.002) y finalmente LAGE-1 el cual se asocia con plaquetas (p=0.001). Las células progenitoras hematopoyéticas de pacientes en tratamiento presentaron una mayor frecuencia de expresión que los pacientes no tratados, en los pacientes con tratamientos convencionales la frecuencia de los genes MAGE-A3 fue del 50% y con ITK de 69.2%, para MAGE-A4 fue de 20% en FC y 53.8% en ITK, por último el gen NY-ESO-1 en FC fue del 20% y en ITK no se encontró expresión. La frecuencia de expresión del gen MAGE-A4 fue mayor comparada con los otros ATC.
ConclusiónLa detección de los genes ATC por medio de técnicas de biología molecular es de gran relevancia ya que pueden ser utilizados como marcadores tumorales en LMC. Existe una diferencia significativa entre la frecuencia de expresión de los genes ATC entre los pacientes con y sin tratamiento.
Chronic myeloid leukaemia (CML), clinically the most important of all chronic proliferative disorders due to its prevalence and prognosis, is a clonal proliferation of a pluripotent stem cell common to all 3 haematopoietic cell lines.15,24 It is characterised by the presence of the Philadelphia chromosome (Ph), an abnormality giving rise to a truncated chromosome 22 due to translocation with chromosomes 9, designated t(9;22)(q34;q11)22 9, t(9;22)(q34;q11). This gives rise to the BCR-ABL oncogene, which allows the leukaemic clone to survive.11 In addition to the BCR-ABL oncogene, CLM cells are also characterised by (epigenetic) changes4,12,19 in methylation patterns,5,13 which turn on genes such as cancer testis antigens (CTA), which are involved in the deregulation of proliferation and apoptotic pathways.14 CTAs include various families of genes found on both sexual and somatic chromosomes, one of the most important being MAGE (melanoma-associated antigens) genes.6–8,11 Prevalence of MAGE-A3 gene expression in metastatic melanomas1,2 has been reported to be as high as 73.0%, while expression in oesophageal cancers is 47.0%, in brain and throat cancers 49.0%, and in bladder cancer 36.0%.23 Expression of the NY-ESO-1 gene has been found in other malignant diseases, including cancer of the lung,9,20 breast, prostate, and oesophageal cancer, from which it derives its name.10,16,17 Unlike peripheral blood cells, malignant stem cells have specific characteristics that make them resistant to cancer drugs. These include expression of drug-resistance (ABC family) genes, arrest of the cell cycle in the G0 phase as a survival strategy, low oxygen levels, etc.21 Because of this, expression of some CTA genes can be heterogeneous, thus giving malignant stem cells an advantage in terms of evolution and survival.25 Tumour expression of these genes is associated with a higher degree of malignancy, such as in lymphomas and myelomas and also in cancer of the skin and lungs.3,22 Isolation of CTA genes is important, as they can be used as tumour markers18 in CML, as markers of disease progression, and in monitoring patients that do not respond to chemotherapy. Consequently, expression of CTA genes from bone marrow samples quantified by means of reverse transcription polymerase chain reaction (RT-PCR) was analysed in 2 patient populations: de novo patients (blasts), and patients admitted to the haematology ward of the General Hospital of Mexico (CD34+Lin− stem cells).
Materials and methodsType of studyObservation, retrospective, cross-sectional, descriptive.
Sample collectionThe samples analysed in this study were obtained from patients treated in the Dr. Eduardo Liceaga General Hospital of Mexico, after having obtained their informed consent.
De novo patients. Bone marrow samples obtained between 2011 and 2013 from patients with CML. The samples were obtained from routine studies (morphology, immunophenotyping, karyotype, etc.), and therefore do not involve any additional risk. All patients were of legal age, had not received treatment, and had complete medical records. In total, 18 samples were obtained, of which 16 did not fulfil one or more of the criteria for analysis and were excluded. This left 65 suitable samples.
Treatment patients. Thirty two samples of haematopoietic stem cells (HSC) from treatment patients were included. None of the 65 de novo patients were included in this patient group, as a greater number of cells are needed to isolate HSCs in the case of patients receiving treatment. Patients treated with tyrosine kinase inhibitors (TKI) (12 with imatinib, 6 with nilotinib, 3 with dasatinib), and with conventional drugs (CD), (3 with hydroxyurea, and 8 with INFα), were included.
Controls. Negative control: we used 10 blood samples from health individuals obtained from the blood bank of the Hospital General of Mexico. Positive control: normal testicular tissue obtained from biopsy. After analysis in the pathology laboratory, the remaining tissue was donated to the molecular biology laboratory.
Isolation of mononuclear and haematopoietic stem cellsWe used the Lymphoprep (Nycomed Pharma AS, Oslo, Norway) medium to isolate mononuclear cells from bone marrow samples taken from patients and from the peripheral blood of healthy individuals. CD34+Lin− HSCs from CML patients under treatment were isolated by means of the StemSep® (Stem Cell Technologies) negative isolation kit.
Preparation of testicular tissueTissue was broken down using Polytron homogenisers by addition of the Trizol® (Invitrogen, Carlsbad, Calif.) reagent, after which RNA was isolated following the manufacturer's instructions.
Isolation of RNA and cDNA synthesisTotal RNA was isolated from the samples taken from patients, from HSCs, from healthy individuals, and from testicular tissue using the Trizol® (Invitrogen, Carlsbad, Calif.) reagent. Complementary DNA was synthesised from 1μg of RNA by means of the oligo (dT) 15 primer (Promega) and M-MLV reverse transcriptase (Promega).
Reverse transcription polymerase chain reaction (RT-PCR)RT-PCR was performed using the GoTaq® DNA polymerase amplification protocol (Promega corporation). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to verify cDNA integrity. The primers used for the MAGE-A3 and MAGE-B2 genes were the same ones previously used by Martínez et al. in 2007. The primers for BAGE-1, GAGE-2, LAGE-1 and NY-ESO1 were designed in the molecular biology laboratory (Table 1).
Sequence of primers used in the isolation of cancer testis antigens.
| Gene | Sequence | Product size (bp) |
|---|---|---|
| MAGE-A3 | 5′-TGGAGCACCAGAGGCCCCC-3′ (F) 5′-GGACGATTATCAGGAGGCCTGC-3′ (R) | 725 |
| MAGE-A4 | 5′-GAGCAGACAGGGCCAACCG -3′ (F) 5′-AAGGACTCTGCGTCAGGC-3′ (R) | 445 |
| MAGE-B2 | 5′-CTGACTTCCGCTTTGGAGGC -3′ (F) 5′-GCACCCCCAGAAACAGAAGAGGAACA-3′ (R) | 230 |
| MAGE-C1 | 5′-GACGAGGGATCGTCTCAGGTCAGC-3′ (F) 5′-ACATCCTCACCCTCAGGAGGG-3′ (R) | 631 |
| BAGE-1 | 5′-TGGCTCGTCTCACTCTGG -3′ (F) 5′-TCCTGTTGAGCTGCCGTCT-3′ (R) | 247 |
| GAGE-2 | 5′-TATGCGGCCCGAGCAGTT-3′ (F) 5′-CCTGCCCATCAGGACCATC-3′ (R) | 201 |
| LAGE-1 | 5′-CTGCGCAGGATGGAAGGTGCCCC-3′ (F) 5′-GCGCCTCTGCCCTGAGGGAGC-3′ (R) | 332/561 |
| NY-ESO1 | 5′-GCTTCAGGGCTGAATGGAT-3′ (F) 5′-AAAACAGGGCAGAAAGC-3′ (R) | 307 |
Statistical analysis was performed using the chi square (X2) and Fisher's exact tests to associate isolation of CTAs with each patient's clinical records. Two-sample t-tests (2t) were used to analyse the frequency of gene expression in de novo and treatment patients. The data were analysed using SPSS statistics 19.0 (IBM SPSS Statistics).
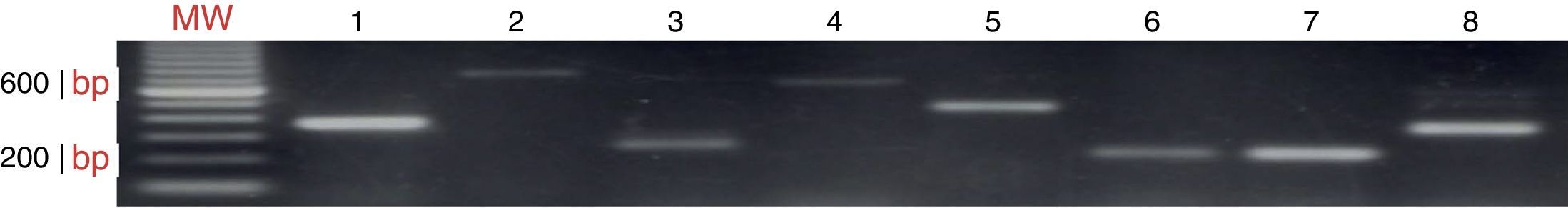
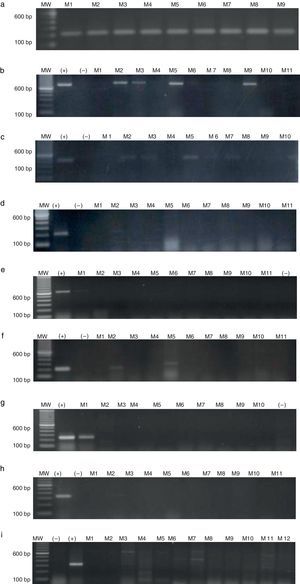
ResultsAmplification of CTAs in testicular tissue and healthy individualsEach gene isolated in testicular tissue was standardised (Fig. 1) and used as the positive control. The RT-PCR products were sequenced to verify that the genes amplified in testicular tissue were in fact the genes of interest. The positive confirmation rate was over 99%.
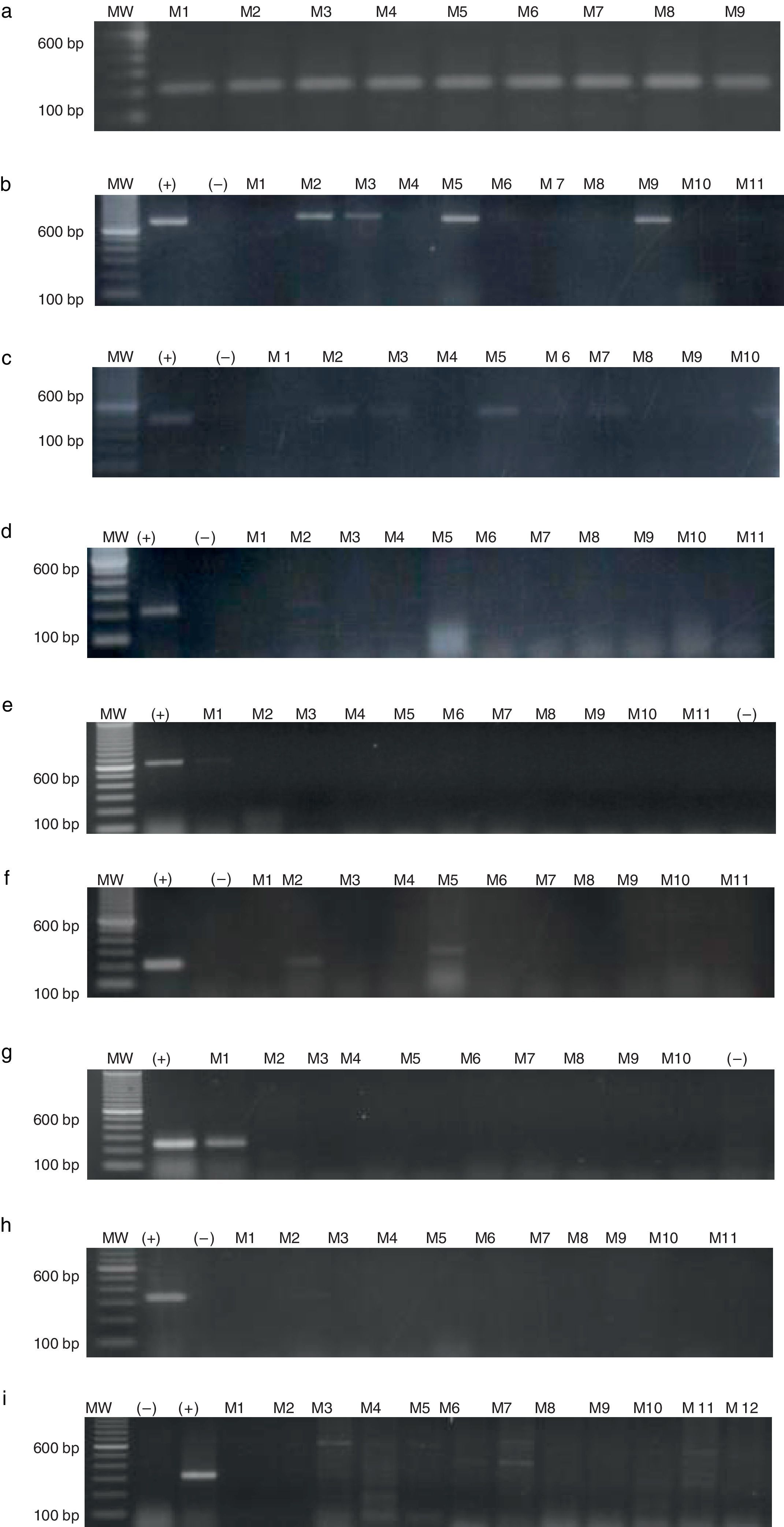
CTAs in de novo patientsSamples from a total of 65 de novo CML patients were analysed, 37 men and 28 women, with an age range of 18–70 years. The chronic phase was the most prevalent (96.9%), followed by the accelerated phase (1.5%). As shown in Table 2, the most prevalent genes were MAGE-A4, NY-ESO1 and MAGE-A3, with a frequency of 63.0%, 36.9% and 32.4%, respectively. Expression of MAGE-B2 and MAGE-C1 genes was less frequent (3.07% and 1.50%). Fig. 2 shows the amplification of CTA genes from CML patients on 2% agarose gel, using testicular tissue as positive control and healthy individuals as controls.
Agarose gel electrophoresis showing RT-PCR quantification of MAGE gene expression in CML patients. (a) GAPDH (217bp), (b) MAGE-A3 (725bp), (c) MAGE-A4 (445bp), (d) MAGE-B2 (230bp), (e) MAGE-C1 (631bp), (f) BAGE-1 (247bp), (g) GAGE-2 (210bp), (h) LAGE-1 (332/561bp), (i) NY-ESO1 (307bp). MW, molecular weight; (+) positive control, testicular tissue; (–)=negative control, healthy individual; M=patients.
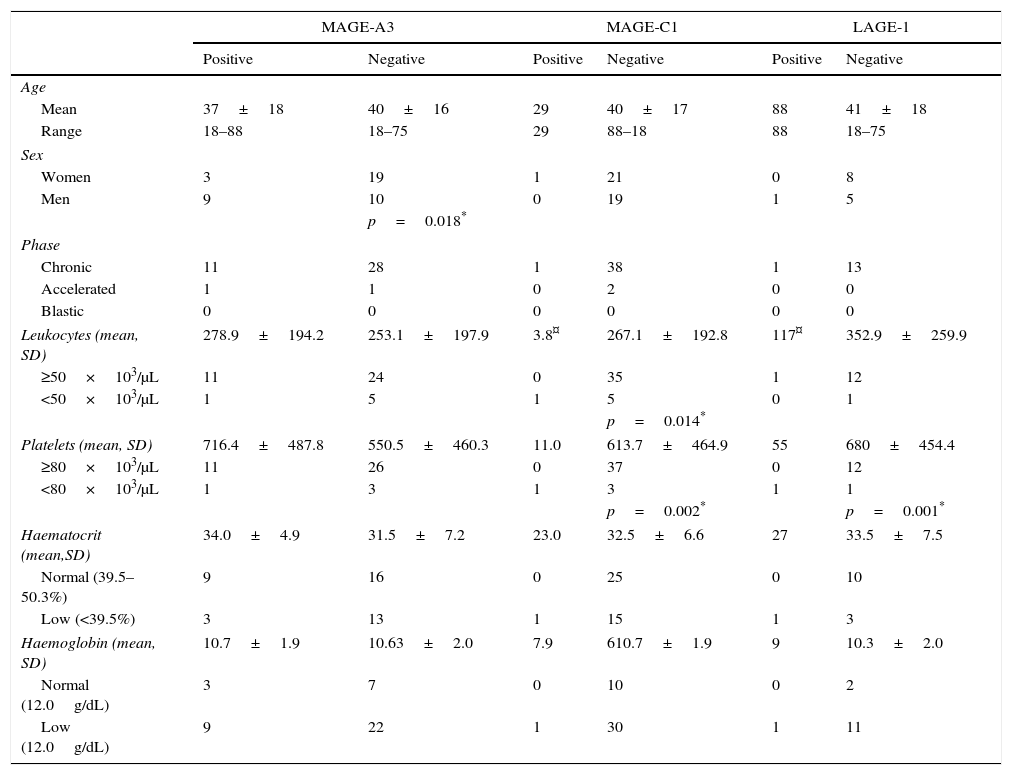
A clinical correlation was established between gene expression and the patients’ clinical parameters, including age, sex, leukocytes, haemoglobin, platelets and haematocrit. These results are shown in Table 3. Only statistically significant genes (MAGE-A3, MAGE-C1 and LAGE1) are shown. On the chi-squared test, MAGE-A3 expression correlated significantly (p≤0.05) with sex (p=0.018), showing that this gene is less prevalent in women. MAGE-C1 expression correlated significantly with leucocyte (p=0.014) and platelet (p=0.002) counts, in other words, above-normal levels are associated with absence of this gene. Finally, LAGE-1 had a significance of p=0.001 for platelets, indicating that platelet counts were normal in patients negative for this gene.
Clinical characteristics of patients and correlation with CTA expression.
| MAGE-A3 | MAGE-C1 | LAGE-1 | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Age | ||||||
| Mean | 37±18 | 40±16 | 29 | 40±17 | 88 | 41±18 |
| Range | 18–88 | 18–75 | 29 | 88–18 | 88 | 18–75 |
| Sex | ||||||
| Women | 3 | 19 | 1 | 21 | 0 | 8 |
| Men | 9 | 10 | 0 | 19 | 1 | 5 |
| p=0.018* | ||||||
| Phase | ||||||
| Chronic | 11 | 28 | 1 | 38 | 1 | 13 |
| Accelerated | 1 | 1 | 0 | 2 | 0 | 0 |
| Blastic | 0 | 0 | 0 | 0 | 0 | 0 |
| Leukocytes (mean, SD) | 278.9±194.2 | 253.1±197.9 | 3.8¤ | 267.1±192.8 | 117¤ | 352.9±259.9 |
| ≥50×103/μL | 11 | 24 | 0 | 35 | 1 | 12 |
| <50×103/μL | 1 | 5 | 1 | 5 | 0 | 1 |
| p=0.014* | ||||||
| Platelets (mean, SD) | 716.4±487.8 | 550.5±460.3 | 11.0 | 613.7±464.9 | 55 | 680±454.4 |
| ≥80×103/μL | 11 | 26 | 0 | 37 | 0 | 12 |
| <80×103/μL | 1 | 3 | 1 | 3 | 1 | 1 |
| p=0.002* | p=0.001* | |||||
| Haematocrit (mean,SD) | 34.0±4.9 | 31.5±7.2 | 23.0 | 32.5±6.6 | 27 | 33.5±7.5 |
| Normal (39.5–50.3%) | 9 | 16 | 0 | 25 | 0 | 10 |
| Low (<39.5%) | 3 | 13 | 1 | 15 | 1 | 3 |
| Haemoglobin (mean, SD) | 10.7±1.9 | 10.63±2.0 | 7.9 | 610.7±1.9 | 9 | 10.3±2.0 |
| Normal (12.0g/dL) | 3 | 7 | 0 | 10 | 0 | 2 |
| Low (12.0g/dL) | 9 | 22 | 1 | 30 | 1 | 11 |
Sixty five CML samples correlated with CTA genes. The p value shows significance of the correlation between genes and clinical and pathological characteristics.
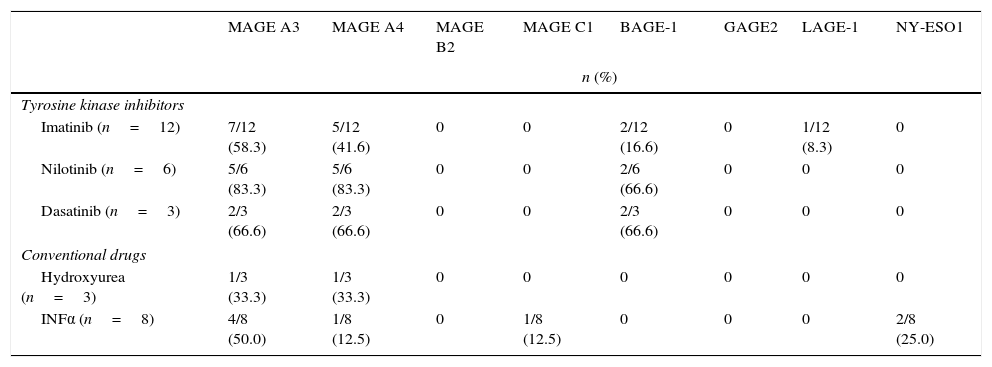
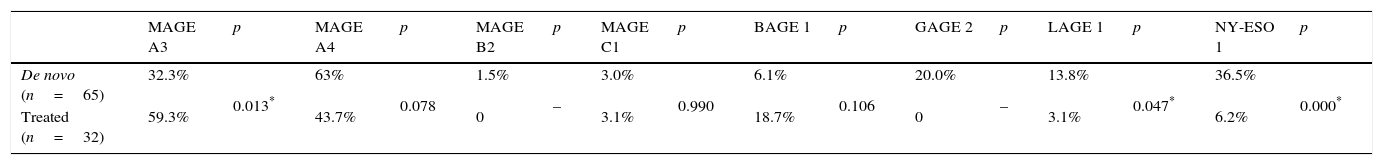
The most prevalent genes isolated in HSCs of treatment patients (both TKI and CDs) were MAGE-A3 and MAGE-A4 (Table 4). Expression of MAGE genes was more frequent in patients treated with TKI than in those receiving CDs. The HSCs of patients treated with TKI showed expression of MAGE-A3 (66.6%), MAGE-A4 (57.1%), BAGE-1 (28.5%) and LAGE-1 (4.7%), and in patients receiving CDs MAGE-A3 (45.4%), MAGE-A4 (18.5%), MAGE-C1 (9.0%) and NY-ESO-1 (18.1%) were isolated (Table 5). Analysis of the frequency of expression between de novo patients and the HSCs of treatment patients (Table 6) showed that MAGE-A3 and MAGE-A4 genes are the most prevalent in all 3 groups. HSC expression of MAGE-A3 was higher in treatment patients, while the opposite was true of MAGE-A4 expression, which was higher in the de novo group. All eight CTAs included in the gene panel were isolated in de novo patients, albeit in different proportions, while only a few were found in the HSCs of treatment patients. The difference between de novo and treatment patients was not statistically significant (p=0.093).
Frequency of cancer testis antigen expression in HSCs of patients treated with tyrosine kinase inhibitors and conventional drugs. n=32.
| MAGE A3 | MAGE A4 | MAGE B2 | MAGE C1 | BAGE-1 | GAGE2 | LAGE-1 | NY-ESO1 | |
|---|---|---|---|---|---|---|---|---|
| n (%) | ||||||||
| Tyrosine kinase inhibitors | ||||||||
| Imatinib (n=12) | 7/12 (58.3) | 5/12 (41.6) | 0 | 0 | 2/12 (16.6) | 0 | 1/12 (8.3) | 0 |
| Nilotinib (n=6) | 5/6 (83.3) | 5/6 (83.3) | 0 | 0 | 2/6 (66.6) | 0 | 0 | 0 |
| Dasatinib (n=3) | 2/3 (66.6) | 2/3 (66.6) | 0 | 0 | 2/3 (66.6) | 0 | 0 | 0 |
| Conventional drugs | ||||||||
| Hydroxyurea (n=3) | 1/3 (33.3) | 1/3 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| INFα (n=8) | 4/8 (50.0) | 1/8 (12.5) | 0 | 1/8 (12.5) | 0 | 0 | 0 | 2/8 (25.0) |
Frequency of CTA expression in de novo patients and in haematopoietic stem cells of patients treated with tyrosine kinase inhibitor and conventional drugs.
| MAGE-A3 | MAGE-A4 | MAGE-B2 | MAGE-C1 | BAGE-1 | GAGE-2 | LAGE-1 | NY-ESO1 | |
|---|---|---|---|---|---|---|---|---|
| De novo | 32.3% | 63% | 1.5% | 3.0% | 6.1% | 20.0% | 13.8% | 36.5% |
| TKI | 66.6% | 57.1% | 0 | 0 | 28.5% | 0 | 4.7% | 0 |
| Conventional drugs | 45.4% | 18.5% | 0 | 9.0% | 0 | 0 | 0 | 18.1% |
Frequency of CTA expression in de novo patients and in haematopoietic stem cells of treated patients.
| MAGE A3 | p | MAGE A4 | p | MAGE B2 | p | MAGE C1 | p | BAGE 1 | p | GAGE 2 | p | LAGE 1 | p | NY-ESO 1 | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De novo (n=65) | 32.3% | 0.013* | 63% | 0.078 | 1.5% | – | 3.0% | 0.990 | 6.1% | 0.106 | 20.0% | – | 13.8% | 0.047* | 36.5% | 0.000* |
| Treated (n=32) | 59.3% | 43.7% | 0 | 3.1% | 18.7% | 0 | 3.1% | 6.2% |
Comparison of CTA expression frequency in treated and de novo patients. The two-sample t test (2t) was used, with a significance level of p<0.05
Cancer testis antigens (CTA) are recognised by cytotoxic T cells, making them both clinically and immunologically important as targets for immunotherapy. This is particularly relevant because CTAs are only expressed in testicular and placental tissue, and presence of these genes indicates a neoplastic transformation. CTAs act by regulating proliferation and apoptosis pathways in tumour cells, and are therefore ideal tumour cell markers. Current understanding of CTAs now suggests that these genes could be useful in haematological disorders apart from leukaemia, and also in lymphomas and other myeloproliferative neoplasms. Analysis of the frequency of CTA expression showed that MAGE-A4 was the most prevalent (63%) at diagnosis, followed by NY-ESO1 and MAGE-A3. In a previous study by our group in 2007 analysing different types of leukaemia in 115 patients with acute leukaemia (76 ALL and 34 AML) and 5 with CML, frequency of MAGE-A3 in AML was 41.2% (14/34), in ALL 30.2% (23/76), and in CML 20.0% (1/5). Other differences could be due to the type of population studied. In this study of 65 samples, prevalence of MAGE-A3 expression is 32.3%, a finding consist with that reported by Martínez et al. in 2007. Andrade et al. in a 2008 study in multiple myeloma showed that CTA expression is higher in leukaemia than in other types of cancer. This group reported a frequency of MAGE-A3, MAGE-A4, GAGE-2, LAGE-1, BAGE-1 and NY-ESO-1 expression of 36%, 0%, 33%, 49%, 28% and 33%, respectively. We, in contrast, found even higher levels for MAGE-A3 (32.3%), MAGE-A4 (63.0%), BAGE-1 (6.1%), GAGE-2 (20.0%) and NY-ESO-1 (36.6%). These differences could be due differing degrees of malignancy, since progression is faster in acute leukaemia.
The clinical correlation in de novo patients showed that absence of CTAs is associated with various clinical parameters, such as sex, and leucocyte and platelets counts. Although expression of the MAGE-A4 gene was most frequent, differences between patients were not statistically significant. In contrast to other studies, we analysed diffuse large B-cell lymphoma in terms of MAGE expression, showing that these genes are associated with the clinical phase of the disease.26 Leutkens, in a 2010 study in CML, performed a clinical correlation with CTA and found an association between the PRAME gene and imatinib therapy, but no correlation between MAGE and other clinical parameters. In our study, however, MAGE was correlated with sex, leucocyte and platelet count. These differences could be attributed to the characteristics of the study population and the clinical characteristics of each patient. All 8 CTA genes analysed were isolated in de novo patients, showing that ATC expression is more frequent at onset of CML. Once TKI or conventional drug therapy had started, only a few of the 8 CTAs included in the panel were isolated in HSCs. Interestingly, MAGE-A3 and MAGE-A4 were found in both populations, and the frequency of some genes (MAGE-A3, MAGE-C1, BAGE-1) remained unchanged or even increased. This difference could be due to the type of cell analysed: in the case of treatment patients, haematopoietic stem cells were analysed, which express different markers from those found in mature cells. According to Colmene et al. in 2009, leukaemic stem cells create bone marrow niches that allow them to evade the action of cancer drugs by expressing genes that perpetuate the leukaemic clone. Under normal circumstances, as explained by Costa et al., CTAs are turned on during embryonic development, and then turned off after cell differentiation. We found CTA expression in cells treated with TKI, which could be explained as a pathway independent from the BCR-ABL oncogene. Comparing CTAs and the three TKIs studied showed a higher expression of CTAs in the HSCs of patients treated with Imatinib, but not with Dasatinib and Nilotinib. Patients treated with conventional drugs also showed CTA expression, albeit to a lesser extent. Analysing both treatment approaches suggests that conventional drugs could act on a different pathway from that targeted by TKIs, and this could in turn affect the expression of some CTAs. RT-PCR analysis shows that CTAs, mainly MAGE genes, are expressed in CML patients at onset of the disease and also after the start of therapy. De novo patients expressed all 8 CTAs included in the study, in contrast to treatment patients, who only expressed some of these genes. Comparing the frequency of expressing in de novo and treatment patients showed significant differences in levels of expression of CTA genes in HSCs at onset of disease and after the start of treatment. This could suggest a potential role for CTAs as markers of minimal residual disease and the likelihood of progression to other more aggressive phases of chronic myeloid leukaemia.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interests.
This project was sponsored by CONACyT, under ID numbers 80085 and 162269 and by Research Office of the General Hospital of Mexico with ID numbers DIC/09/204/03/131 and DIC/08/204/04/017.