The identification and analysis of ecological guilds have been fundamental to understand the processes that determine the structure and organization of communities. However, reviewing studies that have tried to categorize species into trophic guilds we found many different criteria on which such categorizations are based; consequently, a single species may have several guild designations, limiting its accuracy and applicability. In this paper we propose a classification scheme for trophic guilds as a first step to establish a common terminology. For this purpose we considered 1502 species of mainland birds and mammals from North America (Mexico, USA, and Canada). This classification takes into account 3 main criteria to identify each guild: main food type, foraging substrate and activity period. To determine the trophic guilds and assign species to them, we performed a cluster analysis to classify species according to their similarities in feeding patterns. The resulting hierarchical classification distinguishes 6 main levels of organization, which may occur in different combinations among taxonomic groups and sites: 1) taxon (e. g., birds or mammal), 2) diet (e. g. granivore, insectivore), 3) foraging habitat (e. g., terrestrial, arboreal), 4) substrate used for foraging (e. g., ground, foliage), 5) foraging behavior (e. g., gleaner, hunter), and 6) activity period (e. g., nocturnal, diurnal). We identified 22 guilds for birds and 27 for mammals. This approach aims to group together species that use similar resources in a similar way, and extend the usefulness of this approach to studies intend to analyze the organization of biotic communities.
La identificación y el análisis de gremios ecológicos han sido fundamentales para entender los procesos que determinan la estructura y organización de las comunidades. Sin embargo, revisando los estudios que han clasificado las especies en gremios, encontramos que tales clasificaciones están basadas en diferentes criterios; como consecuencia, una especie puede tener varias designaciones gremiales, limitando su precisión y aplicabilidad. En este trabajo proponemos un esquema de clasificación en gremios tróficos como primer paso para establecer una terminología común. Para ello, se consideraron 1 502 especies de aves y mamíferos distribuidos en América del Norte (México, EUA y Canadá). Esta clasificación tiene en cuenta 3 criterios: la dieta principal, el sustrato de forrajeo y el período de actividad. Para determinar los gremios tróficos se realizó un análisis de conglomerados que nos permitió clasificar las especies en función de similitudes y diferencias en sus patrones de alimentación. Esta clasificación es jerárquica y distingue 6 principales niveles de organización que pueden presentarse en diversas combinaciones entre grupos taxonómicos y lugares: 1) taxon (e. g., aves, mamíferos); 2) dieta (e. g., granívoro, insectívoro); 3) hábitat de forrajeo (e. g., terrestre, arbóreo); 4) sustrato donde obtiene su alimento (e. g., suelo, follaje); 5) técnica de forrajeo (e. g., cazador, colector), y 6) periodo de actividad (e. g., nocturno, diurno). Se identificaron 22 gremios de aves y 27 de mamíferos. Este enfoque tiene como objetivo agrupar a las especies que utilizan los mismos recursos de una manera similar y destacar la utilidad de los gremios tróficos en estudios que analicen la forma en que están organizadas las comunidades bióticas.
The term “guild” was originally proposed and defined by Root (1967) as a group of species that exploit the same class of environmental resources in a similar way. The way Root applied the concept in his own work clarifies the importance he gave to functional relationships in a guild. For instance, Root described a “foliage-gleaning guild” containing 5 species that overlapped in their foraging maneuver, use of substrate and diets. The term thus groups together species, without regard to taxonomic position, that overlap significantly in their niche requirements. Moreover, the concept focuses attention on all sympatric species involved in a competitive interaction, regardless of their taxonomic relationship (Root, 1967; Wiens, 1989a). Consequently, we can expect that each species fulfills an ecological role according to its use of resources within a community (Ricklefs, 2010).
Since Root (1967) proposed the term “guild”, there has been a steady rise in the use of the concept in 3 major contexts in the ecological literature (Terborgh and Robinson, 1986; Blondel, 2003): 1) studies aiming to determine how species belonging to the same guild partition the resources (e. g., M’Closkey, 1978; Browers and Brown, 1982; Wiens, 1989b); 2) studies of single communities to identify the resources that determine the community structure (e. g., Diamond, 1975; Landres and MacMahon, 1980; Corcuera, 2001), and 3) comparisons of different communities in similar or contrasting environments (e. g., Karr, 1980; Gómez de Silva and Medellín, 2002; Mouillot et al., 2006; Adams, 2007). Therefore, biologists can use the guild concept to show how different taxa interrelate and how habitat change influences community dynamics and not just individual species.
However, despite the debates around the guild concept and its relevance in community ecology, it has been used with little attention on its theoretical basis, to the point that the term has been losing precision and acquiring a variety of meanings (Jaksić, 1981; Gitay and Noble, 1997). Moreover, other terms have been proposed as a means to provide more precision to the concept; for instance: structural guild, referred to as a group of species using the same resource, but not necessarily in the same manner (Szaro, 1986); management guild, a group of species with similar responses to changes in their environment (Verner, 1984); or functional group, defined as a group of species that respond similarly to environmental factors (Friedel et al., 1988). Accordingly some authors have used different terms more or less synonymously to “guild” and “functional group” (see MacMahon et al., 1981). Recently, Blondel (2003) provided a comprehensive review of the differences between these 2 concepts.
Some studies have proposed different types of grouping species, according to various concepts. On one hand, Gitay and Noble (1997) distinguished between groups based on resource use by species (structural guild and functional guild) and groups based on the response of species to environmental changes (response group and functional group). On the other hand, Wilson (1999) suggested to apply the term “alpha guilds” to groups of species that used the same resource, and “beta guilds” to groups of species facing similar environmental conditions. Both proposals distinguish between resource used (i.e., guilds) and environmental conditions to assign species into a guild. The variety of terms is wide, and a detailed review of these concepts is beyond the scope of this work.
In addition to the proliferation of connotations to the term “guild”, many approaches have been taken to assign species to a guild, and comparisons between different studies have been difficult because of differences in terminology. For instance, Root (1967) defined the “foliage-gleaning guild,” in which Polioptila caerulea was included, but in subsequent works this species was classified as: “foliage and bark gleaning,” by Wagner (1981); “insectivore,” by Emlen (1981); “upper foliage and branch gleaner,” by De Graaf and Wentworth (1986); “canopy insectivore,” by Hutto (1989) and Greenberg et al. (1997); “twig insectivore,” by Greenberg et al. (2000); and “forest gleaner,” by Corcuera (2001). The lack of consensus on a common terminology results in many different ways of grouping species into guilds, limiting its accuracy and generalization (De Graaf et al., 1985; Hawkins and MacMahon, 1989; Simberloff and Dayan, 1991).
Most studies have binned species into guilds using food resource sharing as the sole criterion (e. g., herbivores, carnivores, insectivores), regardless of the way they exploit the resource (e. g., Cagnolo et al., 2002; Feeley, 2003; Aragón et al., 2009). A problem with using such coarse categories is that species overlap on the resource used; hiding the ecological role they play at using similar resources in different ways. Root (1967) gave us a clear example when he divided insectivore birds in foliage gleaning insectivores, and flycatching insectivores. He considered that including the way in which species exploit resources was more informative about how species fulfill the niche space according to their ecological role.
Other approaches have classified species using as criteria a mix of food resources with other variables, such as nesting site, habitat type (e. g., Connell et al., 2000; French and Picozzi, 2002), morphological characteristics –e. g., quadruped, biped, flying, body size– (e. g., Fox and Brown, 1993; Adams, 2007) or their response to environmental conditions (e. g., Landres, 1983; Szaro, 1986; Croonquist and Brooks, 1991; Mac Nally et al., 2008). Although these classifications have proved valuable for the study of communities, they lack universality since are restricted to each particular study. Certainly, Root (1967) mentioned that it is possible to categorize species into guilds based on several types of resources. For example, he assigned Parus inornatus to the foliage-gleaning guild with regard to its foraging habits, but also it belongs to the hole-nesting guild according to its nest-site requirements. In any case, it is necessary to explicitly inform about the type of guild is being analyzed (i.e., foraging, nesting, habitat, or reproductive guild).
Another significant problem to obtain a uniform classification of guilds is to establish what criteria should be considered to classify species into guilds. Jaksić (1981) recognized 2 general approaches to characterize guilds: a priori and a posteriori. The first one is based on predefined guild categories and then fit species into them. The second approach is based on field surveys and statistical evaluation of variables describing foraging strategies, which reduces subjectivity.
Therefore, multivariate statistical techniques have been proposed to make the process of guild delineation more objective (e. g., principal components analysis, cluster analysis, canonical correlation; Voigt et al.,2007); however, since these techniques are mainly based on the proportion of items, for example, the food type contained in the diet (Jaksić and Medel, 1990; Marti et al., 1993), they require too much information thus are limited to few species (Holmes and Recher, 1986; Sarrías et al., 1996; Muñoz and Ojeda, 1997; Rau and Jaksić, 2004; Zapata et al., 2007). Furthermore, these classifications consider only the shared resources and neglect the way in which species use them, an important aspect in guild assignment.
The overall result is that each study using guilds must be evaluated on its own merits. Beside one must be cautious when comparing guild analysis between studies because it frequently occurs that classifications do not match and species are designated to different guilds. Undoubtedly, guilds can be combined in different ways for different purposes; however, unified criteria are important to reach a common classification and terminology.
In this study we attempted to recover the original definition of “guild” proposed by Root (1967), avoiding some of the problems mentioned above (Jaksić, 1981; Hawkins and MacMahon, 1989; Simberloff and Dayan, 1991; Gitay and Noble, 1997; Wilson, 1999). Here we propose a hierarchical classification scheme for trophic guilds applied to North American birds and mammals using 2 main characteristics. First, we considered 3 main classification variables: main food item, foraging substrate and activity period. These 3 components were selected because they are the basic information available for most species. Second, the combination of these variables produce exclusive guilds, i.e., every species belongs to a single guild. These 2 attributes make this proposal widely applicable for most species of birds and mammals and allow unequivocal designations of species into guilds that facilitates intra- and inter-community analyses.
Materials and methodsList of speciesTo classify birds and mammals into guilds, we first obtained the list of North American (Mexico, USA and Canada) species, excluding those mostly associated with marine and coastal environments, and non-native species. The final list consisted of 1 502 species, of which 858 were birds and 644 mammals. For both groups, their taxonomy was reviewed and updated, eliminating problems of synonymy and taxonomic changes. For mammals, we followed the criteria described by Hall (1981), Ramírez-Pulido et al. (2005), and Wilson and Reader (2005). For birds, the corresponding authorities were the American Ornithologists’ Union (AOU, 1998) and supplements 42 to 50 (AOU, 2000; Banks et al., 2002, 2003, 2004, 2005, 2006, 2007, 2008; Chesser et al., 2009).
Main food itemsTo classify species based on diet, we considered the main food resource used by birds and mammals as follows: vertebrates (birds, mammals, reptiles, amphibians and fish), aquatic and terrestrial invertebrates, carrion, nectar, fruits, seeds, other plant material (e. g., stems, buds, leaves, etc.) and grass. Additionally, we included vertebrate blood as a resource for vampire bats. A key issue was how to assign the main food item to each species. To solve this, we took into account the food type representing the highest percentage in the diet of each species (when the relevant information existed), using only data for adult individuals and considering reproductive season for migratory birds. If quantitative information was not available, we considered the main food type reported in the literature. In cases where even this information was not available, we made a decision based on the information for the genus of the target species.
Foraging substrate and techniqueThe foraging substrate refers to the place where organisms obtain their food. In this component we considered 4 main divisions: ground, air, trees, and freshwater. However, given the differences between birds and mammals, these categories have subdivisions exclusive to each group (Table 1). Additionally, we considered for each species its foraging behavior or technique used to obtain its food. This information indicates the way they use the resource. For birds, we considered: gleaner, excavator, hawker, aerial chaser, and scavenger. For mammals we considered: hunter, hawker, excavator, browser, grazer and scavenger.
Type of foraging substrates used to classify guilds
| For birds |
| Ground: species that take food or capture their prey at ground level. |
| Arboreal: species that get their food in trees. In turn, this category can be divided into bark excavator or bark gleaner. |
| Foliage gleaner: species that collect their prey in the foliage of plants. In turn, this category can be divided into undergrowth, lower canopy and upper canopy. |
| Air (under canopy): species that catch their food on the fly below the tops of the trees. |
| Air (above canopy): species that catch their food on the fly above the trees. |
| Freshwater: species that feed on organisms in lakes and rivers. |
| For mammals |
| Ground: species that take food or capture their prey at ground level. |
| Arboreal: species that get their food in trees. |
| Air: species that catch their food in the air. |
| Freshwater: species that feed on plants or other organisms in lakes and rivers. |
| Fossorial: species that get their food underground. |
Elton (1933) divided species into diurnal and nocturnal as a means to understand community structure, but this feature has been largely ignored for analyzing resource exploitation by species in guild classifications. Notable exceptions are the works of Schoener (1974), Marti et al. (1993) and more recently Kronfeld-Schor and Dayan (2003), who integrated this aspect into the analysis of community structure. In this study we considered 2 classes: 1) diurnal, if the activity period started mainly in the morning and continued during the day; and 2) nocturnal, when activity starts in the late hours of the afternoon and continues throughout the night.
Assigning species into a guildInformation about diet, foraging substrate and activity period was obtained from a review of published specialized literature and information available online (Appendix 1, Electronic Supplementary Material: http://www.revistas.unam.mx/index.php/bio/issue/archive). With this information in hand, we built a binary matrix of species traits for a cluster analysis. The similarity matrix was obtained using the Jaccard’s Similarity Coefficient (Krebs, 1989; Manly, 1994) and the dendrogram was constructed with the single linkage algorithm (Hammer et al., 2001). To determine the level of similarity that defines the groups in the dendrogram, we considered 2 criteria simultaneously: 1) the average similarity between all pairs of species (Crisci and López-Armengol, 1983), and 2) the largest increase of dissimilarity between successive clusters in the dendrogram (a measure of the variability of error; Hair 1995). When both criteria were not coincident, priority was given to larger similarity (Gauch, 1982; Crisci and López-Armengol, 1983).
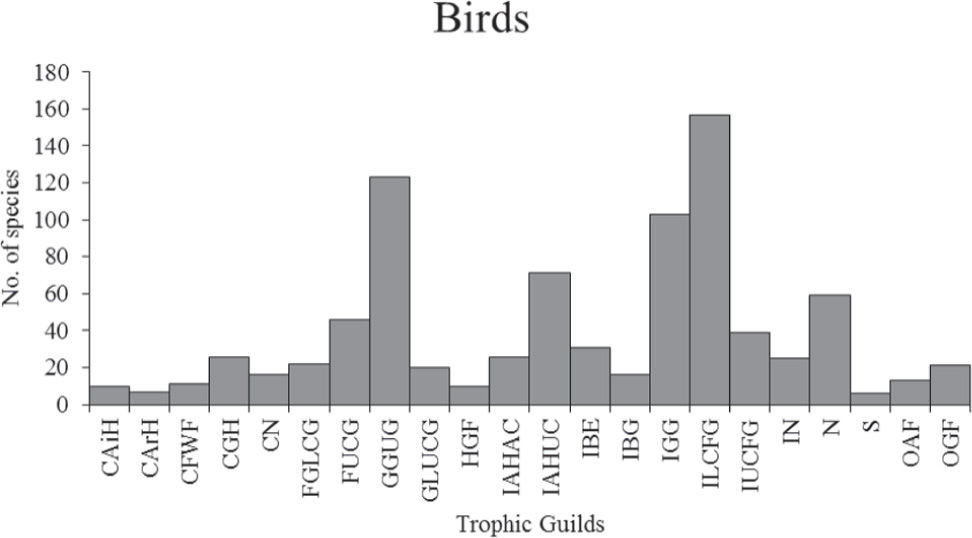
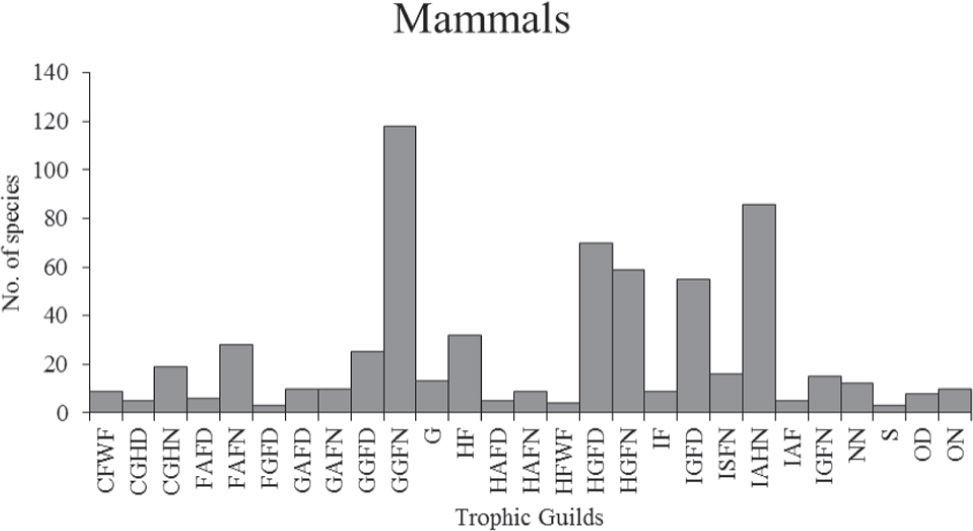
ResultsGuild classificationThe cluster analysis summarizes the relationships among species based on their feeding patterns (Appendix 2, Electronic Supplementary Material: http://www.revistas.unam.mx/index.php/bio/issue/archive). determining 22 bird and 27 mammal guilds (Figs. 1 and 2, respectively). In addition, the cluster analysis also helped to identify particular features associated to each of these guilds. Below we provide a brief description of the guilds identified for birds and mammals; the full list of species with their associated guild is available in Electronic Supplementary Material (Tables S1, S2; http://www.revistas.unam.mx/index.php/bio/issue/archive).
Number of bird species by guild. Carnivore: air-hawker (CAiH), carnivore: arboreal-hawker (CArH), carnivore: ground-hawker (CGH), carnivore: nocturnal (CN), carnivore: freshwater-forager (CFWF), frugivore: ground to lower canopy gleaner (FGLCG), frugivore: upper-canopy gleaner (FUCG), granivore: ground to undergrowth gleaner (GGUG), granivore: lower to upper canopy gleaner (GLUCG), herbivore: ground forager (HGF), insectivore: air hawker above canopy (IAHAC), insectivore: air hawker under canopy (IAHUC), insectivore: bark excavator (IBE), insectivore: bark gleaner (IBG), insectivore: ground gleaner (IGG), insectivore: lower canopy foliage gleaner (ILCFG), insectivore: upper canopy foliage gleaner (IUCFG), insectivore-nocturnal (IN), nectarivore (N), scavengers (S), omnivore: arboreal forager (OAF), omnivore: ground forager (OGF).
Number of mammal species by guild. Carnivore: ground hunter-diurnal (CGHD), carnivore: ground hunter-nocturnal (CGHN), carnivore: freshwater forager (CFWF), frugivores: arboreal forager-diurnal (FAFD), frugivores: arboreal forager-nocturnal (FAFN), frugivores: ground forager-diurnal (FGFD), granivore: arboreal forager-diurnal (GAFD), granivore: arboreal forager-nocturnal (GAFN), granivore: ground forager-diurnal (GGFD), granivore: ground forager-nocturnal (GGFN), herbivore: arboreal forager-diurnal (HAFD), herbivore: arboreal forager-nocturnal (HAFN), herbivore fossorial (HF), herbivore: ground forager-diurnal (HGFD), herbivore: ground forager-nocturnal (HGFN), herbivore: freshwater forager (HFWF), grazers (G), insectivore: aerial hawker-nocturnal (IAHN), insectivore: arboreal forager (IAF), insectivore fossorial: (IF), insectivore ground forager-diurnal (IGFD), insectivore: ground forager-nocturnal (IGFN), insectivore forager-nocturnal (IFN), nectarivore-nocturnal (NN), sanguinivore (S), omnivore-diurnal (OD), omnivore-nocturnal (ON).
The 22 guilds obtained for birds can be grouped into 8 broader groups based on diet, as follows: carnivores (5 guilds), frugivores (2 guilds), granivores (2 guilds), herbivores (1 guild), insectivores (8 guilds), nectarivores (1 guild), scavengers (1 guild), and omnivores (2 guilds). In the following paragraphs we describe each guild, providing examples of species belonging to them.
1. Carnivores. Air-hawker: species that specialize in hunting prey in the air; their main food item is other birds and bats. Representative species of this guild are falcons, e. g., Falco columbarius (merlin), F. peregrinus (peregrine falcon). Arboreal-hawker: represented by some eagles specialize in hunting prey in the canopy, such as monkeys, reptiles or birds; for example, Harpia harpyja (harpy eagle) and Geranospiza caerulescens (crane hawk). Ground-hawker: includes birds of prey that feed on a wide variety of vertebrates (birds, mammals, reptiles) that they catch on the ground, e. g., Buteo jamaicensis (red-tailed hawk), Circus cyaneus (northern harrier). Nocturnal: species active mainly at night; including mostly owls that hunt several species of vertebrates, e. g., Bubo virginianus (great horned owl). Freshwater-forager: species that feed mainly on fish and a large number of aquatic invertebrates caught in rivers or lakes, e. g., Pandion haliaetus (osprey), Megaceryle torquata (ringed kingfisher), Cinclus mexicanus (american dipper).
2. Frugivores. Ground to lower canopy gleaner: species that forage on the ground and in the lower parts of trees or shrubs, e. g., Tinamus major (great tinamou), Crax rubra (great curassow). Upper-canopy gleaner: birds foraging on fruits mainly in the upper parts of trees, e. g., Ortalis vetula (plain chachalaca), Aratinga holochlora (green parakeet).
3. Granivores. Ground to undergrowth gleaner: these birds glean seeds principally on the ground and shrubs and rarely forage in trees, e. g., Callipepla squamata (scaled quail), Junco hyemalis (dark-eyed junco). Lower to upper canopy gleaner: these species get their food on any of the tree strata, e. g., Loxia curvirostra (red crossbill), Acanthis flammea (common redpoll).
4. Herbivores. Ground forager: represented mainly by species distributed in northern United States and Canada. These birds eat different parts of plants mostly on the ground, e. g., Lagopus lagopus (willow ptarmigan), Dendragapus obscurus (dusky grouse).
5. Insectivores. Air hawker above canopy: species feeding mainly on insects caught in the air above the tree canopy, e. g., Cypseloides storeri (white-fronted swift), Elanoides forficatus (swallow-tailed kite), Ptiliogonys cinereus (gray silky-flycatcher). Air hawker under canopy: birds feeding mainly on insects caught in the air mostly under the canopy, e. g., Elaenia martinica (caribbean elaenia), Momotus momota (blue-crowned motmot), Contopus pertinax (greater pewee). Bark excavator: species that feeds on insects caught on the internal side of tree bark, e. g., Melanerpes chrysogenys (golden-cheeked woodpecker), Picoides scalaris (ladder-backed woodpecker), P. villosus (hairy woodpecker). Bark gleaner: these species feed on insects caught on the surface of barks, e. g., Sittasomus griseicapillus (olivaceous woodcreeper), Sitta canadensis (red-breasted nuthatch), Mniotilta varia (black-and-white warbler). Ground gleaner: includes species that feed primarily on insects caught on the ground, e. g., Campylorhynchus brunneicapillus (cactus wren), Turdus migratorius (american robin), Automolus ochrolaemus (buff-throated foliage-gleaner). Lower canopy foliage gleaner: species that feed on insects caught on the foliage, foraging from the lower to middle parts of trees, e. g., Thamnophilus doliatus (barred antshrike), Piaya cayana (squirrel cuckoo), Vireo huttoni (Hutton’s vireo). Upper canopy foliage gleaner: Includes species that feed on insects caught on the foliage, foraging from the middle to high parts of trees, e. g., Leptodon cayanensis (gray-headed kite), Coccyzus americanus (yellow-billed cuckoo), Piranga ludoviciana (western tanager). Nocturnal: species feeding on insects at night, e. g., Megascops cooperi (pacific screech-owl), Chordeiles acutipennis (lesser nighthawk), Nyctibius jamaicensis (northern potoo).
6. Nectarivores. Nectarivore: species which main food item is nectar from flowers, e. g., Campylopterus curvipennis (wedge-tailed sabrewing), Amazilia candida (white-bellied emerald), Lampornis amethystinus (amethyst-throated hummingbird).
7. Scavengers. Scavenger: species that feed on carrion, e. g., Coragyps atratus (black vulture), Cathartes burrovianus (lesser yellow-headed vulture).
8. Omnivores. This group included species that cannot be differentiated by any type of food, yet they can be distinguished by their foraging habits. Arboreal forager: species that feed on a wide variety of foods (insects, vertebrates, seeds, fruits, parts of plants) obtained from the canopy of trees, e. g., Cyanocorax morio (brown jay), Cacicus melanicterus (yellow-winged cacique). Ground forager: includes species that forage on several types of food, including carrion, mainly on the ground, e. g., Corvus corax (common raven), Pica hudsonia (black-billed magpie), Quiscalus mexicanus (great-tailed grackle).
Mammal guildsFor mammals, we obtained 27 guilds grouped into 8 feeding groups, namely carnivores (3 guilds), frugivores (3 guilds), granivores (4 guilds), herbivores (7 guilds), insectivores (6 guilds), nectarivores (1 guild), sanguinivores (1 guild), and omnivores (2 guilds).
1. Carnivores. Ground hunter-diurnal: mammals feeding mainly on vertebrates hunted on the ground during the day, e. g., Puma yagouaroundi (jaguarundi), Mustela frenata (long-tailed weasel). Ground hunter-nocturnal: species that hunt on the ground at night, e. g., Lynx rufus (bobcat), Panthera onca (jaguar), Gulo gulo (wolverine). Freshwater forager: mammals that feed mainly on aquatic vertebrates in rivers or lakes, e. g., Noctilio leporinus (greater bulldog bat), but can also eat aquatic invertebrates, e. g., Lontra canadensis (North American river otter), Rheomys mexicanus (Mexican water mouse).
2. Frugivores. Arboreal forager-diurnal: includes mammals that eat fruits collected on trees during the day, e. g., Alouatta pigra (Mexican black howler monkey), Sciurus deppei (Deppe’s squirrel). Arboreal forager-nocturnal: includes mammals that eat fruits collected on trees during the night, e. g., Artibeus jamaicensis (Jamaican fruit-eating bat), Potos flavus (kinkajou). Ground forager-diurnal: mammals which primary food is fruit collected on the ground, e. g., Dasyprocta mexicana (Mexican agouti).
3. Granivores. Arboreal forager-diurnal: species that eat seeds and forage primarily in the canopy during the day, e. g., Sciurus griseus (western gray squirrel), Tamiasciurus mearnsi (Mearns’s squirrel). Arboreal forager-nocturnal: species that eat seeds in the canopy at night, e. g., Glaucomys sabrinus (northern flying squirrel), Ochrotomys nuttalli (golden mouse). Ground forager-diurnal: includes animals that feed mainly on seeds collected on the ground, e. g., Tamias merriami (Merriam’s chipmunk), Ammospermophilus harrisii (Harris’s antelope squirrel). Ground forager-nocturnal: mammals that forage seeds on the ground mostly at night, e. g., Dipodomys merriami (Merriam’s kangaroo rat), Peromyscus maniculatus (deer mouse), Zapus trinotatus (Pacific jumping mouse).
4. Herbivores. Arboreal forager-diurnal: mammals that feed on several plant parts, including fruit, seeds, and leaves, mainly in the canopy and during the day, e. g., Sciurus arizonensis (Arizona gray squirrel), Sciurus colliaei (Collie’s squirrel). Arboreal forager-nocturnal: mammals that feed in the canopy on a large variety of plant parts, including fruit, seeds, leaves; mainly at night, e. g., Arborimus pomo (Sonoma tree vole), Tylomys bullaris (Chiapan climbing rat). Fossorial: mammals adapted to live and carry out most of their activities (eating, resting and reproducing) underground, feeding mainly on stems, roots and bulbs, e. g., Geomys arenarius (desert pocket gopher), Thomomys mazama (western pocket gopher). Ground forager-diurnal: mammals that feed on a great variety of plant parts mainly on the ground, foraging mainly during the day, e. g., Tayassu pecari (white-lipped peccary), Cynomys mexicanus (Mexican prairie dog). Ground forager-nocturnal: mammals that feed on a great variety of plant parts mainly on the ground and foraging mainly at night, e. g., Lepus californicus (black-tailed jackrabbit), Cuniculus paca (lowland paca). Freshwater forager: mammals that feed mainly on aquatic vegetation, e. g., Ondatra zibethicus (muskrat), Castor canadensis (American beaver). Grazers: species that eat mainly on grass and leaves, e. g., Bison bison (American bison), Odocoileus virginianus (white-tailed deer).
5. Insectivores. Aerial hawker-nocturnal: includes bat species that feed mainly on insects caught in the air at night, e. g., Balantiopteryx plicata (gray sac-winged bat), Pteronotus personatus (Wagner’s mustached bat), Myotis albescens (silver-tipped myotis). Arboreal forager: includes species that feed mainly on insects in the trees, e. g., Cyclopes didactylus (silky anteater), Marmosa mexicana (Mexican mouse opossum). Fossorial: species of fossorial habits that feed mainly on insects caught underground, e. g., Scapanus latimanus (broad-footed mole), Blarina carolinensis (southern short-tailed shrew). Ground forager-diurnal: mammals that feed on insects caught on the ground, during the day, e. g., Cryptotis nelsoni (Nelson’s small-eared shrew), Sorex arizonae (Arizona shrew), Sorex ventralis (chestnut-bellied shrew). Ground forager-nocturnal: mammals that feed on insects caught on the ground at night, e. g., Spilogale pygmaea(pygmy spotted skunk), Onychomys leucogaster (northern grasshopper mouse). Forager-nocturnal: bats feeding primarily on insects, but that rarely catch their prey on the fly; however, they do not have preference for any type of substrate to forage, so they can get their food on the ground or in trees; e. g., Mimon crenulatum (striped hairy-nosed bat), Tonatia saurophila (stripe-headed round-eared bat).
6. Nectarivores. Nectarivore-nocturnal: bat species that feed primarily on nectar, e. g., Glossophaga leachii (gray long-tongued bat), Leptonycteris curasoae (southern long-nosed bat).
7. Sanguinivores. Represented by only 3 species of bats that feed on vertebrate blood, e. g., Diaemus youngi (white-winged vampire bat).
8. Omnivores. Like birds, these are mammals that cannot be distinguished by their type of food or foraging substrate yet can be classified by their activity period. Omnivorediurnal: mammals that eat a wide variety of food items. They get their food from different substrates, mainly during the day, e. g., Nasua narica (white-nosed coati), Martes americana (American marten). Omnivore-nocturnal: mammals that eat a wide variety of food. They get their food from different substrates, mainly at night, e. g., Canis latrans (coyote), Bassariscus astutus (ringtail).
DiscussionSince Root (1967) proposed the guild concept, a number of authors have discussed guild terminology, definitions and its applications in ecological studies (see Jaksić, 1981; Terborgh and Robinson, 1986; Hawkins and MacMahon, 1989; Wiens, 1989a; Simberloff and Dayan, 1991). Consequently, new terms of guilds have been suggested (e. g., Gitay and Noble, 1997; Wilson, 1999), and many approaches have been undertaken to assign species to a guild (e. g., Jaksić and Medel, 1990; Lešo and Kropil, 2007). Because of this, very few studies have sought to establish the firm basis for a common terminology in ecological guilds (e. g., De Graaf et al., 1985).
Considering the lack of a unified classification for ecological guilds, we reviewed the available information about guild classifications for birds and mammals, and found 2 contrasting situations for these groups. Root’s guild concept has been used in a large number of studies of birds, and they may involve a great number of species and several guilds (e. g., Cody, 1983; Case et al., 1983; Pearman, 2002; Adamík et al., 2003; Korňan et al., 2013), but only one work has attempted to provide a guild classification for a great number of North American birds (De Graaf et al., 1985); yet, they used different terminologies, making it of little use for comparisons. For mammals, most studies regarding guild classifications were focused only on a few species within particular feeding guilds, such as granivores or insectivores, or a few species belonging to a taxonomic group, such as Rodentia or Carnivora (e. g., Fox and Brown, 1993; Zapata et al., 2007; Aragón et al., 2009). Also in some cases, morphological traits were used to classify guilds –e. g., quadruped, biped, flying, body size– (e. g., Fox and Brown, 1993; Adams, 2007).
Although, these classifications have provided valuable results in the study of communities, these do not strictly follow the original concept of guild (Root, 1967), and are restricted to specific studies. Moreover, until now, there is not a study that has attempted to provide a guild classification for mammals in the sense established by Root (1967). It is possible that the fundamental reason for this lack of agreement in the use of a common framework and terminology is that the guild concept is a theoretical construct rather than a natural unity in life. Therefore, instead of “discovering” a hidden entity, we are attempting to understand the way nature self-organizes in a complex, multidimensional and multiscalar setting.
Hence, given the multiplicity of approaches to assign guild membership and the lack of a unified terminology, we proposed a classification scheme for North American birds and mammals. Towards this end, we identified a variety of exclusive trophic guilds for mainland birds and mammals that show a clear, unequivocal separation among them regarding the use of available food resources. This classification is hierarchical and distinguishes 6 main levels of organization: 1) taxon (e. g., birds or mammal), 2) diet (e. g., granivore, insectivore), 3) foraging habitat (e. g., terrestrial, arboreal), 4) substrate used for foraging (e. g., ground, foliage), 5) foraging behavior (e. g., gleaner, hunter), and 6) activity period (e. g., nocturnal, diurnal). Certainly, this hierarchical classification would vary between birds and mammals, and not always the 6 levels are represented in guilds. However, our classification allows subdividing biotic communities in different levels and identifying ecological roles played by numerous species. Interestingly, in our classification bird guilds were subdivided mainly by the arboreal stratum, whereas mammal guilds were divided principally by activity period, indicating different mechanism of organization between these taxonomic groups.
Undoubtedly, our proposal does not escape to issues inherent in building a classification of guilds (a priori vs. a posteriori approach and the concept used). Our classification of guilds follows the concept proposed by Root (1967) and we used a posteriori classification (sensuJaksić, 1981), to make it applicable in other regions or other taxonomic groups. Variables used to assign species to guilds may be controversial, such as activity period; however, recent studies have highlighted the importance of temporal separation to promote species coexistence (e. g., Castro-Arellano and Lacher, 2009; Di Bitetti et al., 2009; Stuble et al., 2013). Some shortcomings that we have identified in our proposal include the fact that for birds we only considered the breeding season characteristics, and for mammals the gross feeding items to assign species into a guild, thus seasonal variations in feeding habits were not taken into account. As well, geographic variations in feeding habits were dismissed. Finally, we used only 3 qualitative variables for the classification. Certainly, the use of a larger set of variables, including quantitative ones (e. g., proportion of food items) would strengthen the statistical analyses; however, this information is lacking for a large proportion of species, thus including them in a classification would produce an incomplete scheme, reducing its applicability.
Another important aspect of Root’s definition is that guild classification should be independent of taxonomic relationship. In this regard, Jaksić (1981) suggested that a guild should include all kind of species that exploit the same resource (birds, mammals, reptiles or insects); he referred to these groups as community guild; otherwise, he defined assemblage guilds when the recognition of guilds were defined within taxonomic assemblages. He mentioned that restrict guild membership by arbitrary taxonomic boundaries may lead to a neglected of important influences among distantly related taxa. Even though, our classification may be considered an assemblage guild under these terms because guilds were built independently for birds and mammals, some of them may be considered as equivalent. For instance, carnivore birds and mammals that take their food on the ground can be considered one guild. Thus, quoting Jaksić (1981). It seems, then, that the study of guild structure within taxonomic assemblages is only a preliminary step for understanding the role of guilds in the organization of communities.
Community studies focused on guild composition bring greater clarity about assembly processes in contrast to studies focused on species composition. These may provide a more fruitful avenue for developing and testing general ecological hypotheses of community organization across biogeographic scales and processes of environmental change (e. g., Kissling et al., 2011). This standardization may provide means by which ecological studies can give us more robust information about relationships among species and their environment, and a better understand how species that form a guild might respond to environmental changes (Keddy, 1992; Mateos et al., 2011). For instance, analysis based on individual species may help to elucidate distribution patterns, whereas analyses using ecological groups (such as guilds) may identify assemblages according to habitat characteristics (Hoeinghaus et al., 2007). Sekercioglu et al., (2004) proposed a framework to characterize potential ecological consequences of avian declines using functional roles of birds and a stochastic model. This kind of studies provides a different perspective of the effects of environmental change on species and biotic communities.
Briefly, the misuse and abuse of the guild concept has driven to ad hoc interpretations and applications, where the usefulness of the concept depends more on the acuity of researchers (Jaksić, 1981; MacMahon et al. 1981; Hawkins and MacMahon, 1989). Certainly, it will be difficult to achieve a universally accepted classification for guilds; however, we believe that is possible to find a standardized nomenclature to identify ecological groups (Dale, 2001; Korňan and Adamík, 2007; Lešo and Kropil, 2007; Blaum et al., 2011). We hope that this study serve as a first step towards finding such common terminology for ecological guilds.
AcknowledgmentsWe deeply thank Adolfo Navarro Sigüenza, Pilar Rodríguez and Christopher Stephens for their review and suggestions to improve this manuscript. CG-S thanks the Programa de Posgrado en Ciencias Biológicas, UNAM for training and logistic support, and the Consejo Nacional de Ciencia y Tecnología for the doctoral scholarship (220650). We also thank Sophie Calmé and an anonymous referee for their valuable comments.