The family Profundulidae is one of the few families of freshwater fishes endemic to the Mesoamerican region. It includes the genus Profundulus that has been divided into two subgenera, Profundulus, with a wider distribution and Tlaloc, which presents a more limited range. The species currently recognized within the subgenus Profundulus are P. punctatus, P. oaxacae, P. guatemalensis and P. kreiseri. Results from a previous study using allozymes revealed a high degree of molecular differentiation of the populations inhabiting the Mixteca region (located at the north of Oaxaca in Mexico), compared to the rest of the species in the genus, especially regarding its geographically nearest neighbor (P. oaxacae). The main goal of this study was to evaluate the morphological distinctiveness and to assess the taxonomic identity of the populations from the Mixteca region in comparison with the morphological variation of P. oaxacae. Based on 22 measurements (17 morphometric and 5 meristic), we examined the morphological variation within and among species, including 50 individuals from 5 localities. Our results show a high degree of morphological differentiation between both populations in accordance to the molecular information reported before, supporting that populations from the Mixteca region are a new taxonomic entity, which is described in the present study.
La familia Profundulidae es una de las pocas familias de peces dulceacuícolas endémicas a la región de Mesoamérica. La familia incluye al género Profundulus, el cual a su vez incluye dos subgéneros: Profundulus, con una mayor distribución y Tlaloc, el cual presenta una distribución más restringida. Actualmente, dentro del género Profundulus se incluyen las siguientes especies: P. punctatus, P. oaxacae, P. guatemalensis y P. kreiseri. En un estudio previo basado en un análisis de aloenzimas para el subgénero Profundulus, se observó un alto grado de diferenciación entre las poblaciones de la región Mixteca, al norte del estado de Oaxaca en México, del resto de especies dentro del subgénero, particularmente de las poblaciones de la especie geográficamente más cercana (P. oaxacae). El objetivo principal del presente estudio es caracterizar la variación morfológica y evaluar la identidad taxonómica de las poblaciones de la región Mixteca comparándolas con la variación morfológica de P. oaxacae. Esta caracterización se llevó a cabo con base en 22 variables (17 morfométricas y 5 merísticas) en 50 individuos provenientes de 5 localidades. Nuestros resultados muestran que existe una clara diferenciación morfológica entre ambos grupos de poblaciones, en congruencia con evidencia molecular obtenida previamente, y que sustenta el reconocimiento de las poblaciones de la región de la Mixteca como una nueva especie, la cual es descrita en el presente estudio.
The family Profundulidae is one of few families of freshwater fishes endemic to the Mesoamerican region. It has been suggested that its history goes back to the Pliocene, or even the Miocene (Miller, 1955). Currently, most species in the family exhibit low abundances, and their distribution is restricted to the headwaters and spring-fed-tributaries of rivers in southern Mexico, El Salvador, Honduras and Guatemala, from both Pacific and Atlantic versants. Species diversity in the family is fairly low, consisting of 8 species in 2 genera: Profundulus and Tlaloc (Morcillo, Ornelas-García, Alcaraz, Matamoros, & Doadrio, 2015). In the genus Tlaloc, 4 species are currently recognized: T. candalarius, T. hildebrandi, T. labialis and T. portillorum, all of which exhibit limited distributions. The genus Profundulus also has 4 species, namely P. punctatus, P. oaxacae, P. guatemalensis and P. kreiseri, but has a decidedly wider distribution range.
In an early study dealing with systematics of Profundulidae, Miller (1955) pointed out a wide degree of morphological variation, being more evident within the P. punctatus “group”, in which more than one species might be recognized. Years later, within the P. punctatus “group” Miller (2005) corroborated the validity of P. oaxacae (Meek, 1902), whose distribution was restricted to altitudes above 1,550m in the Río Atoyac – Verde Basin, Oaxaca, in southern Mexico (Martínez-Ramírez, Doadrio-Villarejo, & de Sostoa-Fernández, 2004). Furthermore, in a genetic population analysis of the subgenus Profundulus based on allozymes (Doadrio, Carmona, Martínez, & De Sostoa, 1999), at least 5 different lineages were recovered. Of these, 2 distinct lineages were found in the Mixteca region and were well differentiated from P. punctatus. One of them corresponded to P. oaxacae and the other one included a clade named P. sp. nov. 1, which corresponded to populations from Mixteca headwaters (with an elevation over 700m) and showed a high degree of molecular divergence from the rest of species of the subgenus Profundulus (Nei's distances D=0.165−0.845). In fact, based on the aforementioned analysis, P. sp. nov. 1 was recovered as a sister clade to the rest of species in the P. punctatus “group”, where P. oaxacae is included.
As a consequence of the striking morphological differences between P. oaxacae and P. punctatus, Miller recognized the former as a valid species (Miller, 2005). These differences include the number of scales around the body (25–28 in P. punctatus vs. 30 in P. oaxacae), and the lateral color pattern (prominent round spots in P. punctatus vs. narrow weak and vertical marks in P. oaxacae).
Another aspect clearly separating this pair of species is their distribution, in which P. oaxacae is restricted to the upper part of the río Verde basin, Oaxaca while P. punctatus inhabits the lower part of the same basin. P. sp. nov. 1 also inhabits the upper part of the río Verde basin, but shows a high genetic distinctiveness regarding both, P. oaxacae and P. punctatus (Doadrio et al., 1999). Consequently, in this study we evaluated the morphological distinctiveness between P. oaxacae and P. sp. nov. 1, and determined whether morphological variation corresponds to the genetic differentiation previously documented by Doadrio et al. (1999).
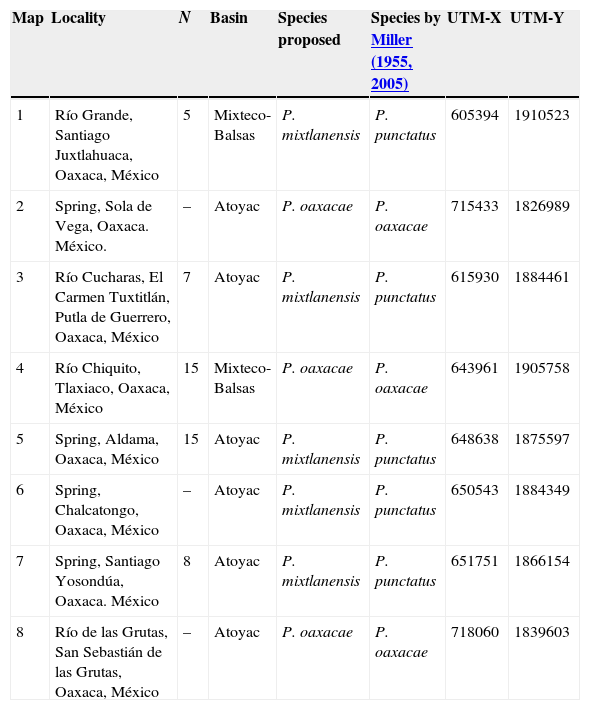
Materials and methodsSample collection and taxonomic determinationA total of 5 localities within the Mixteca region were surveyed using cast nets and electrofishing (Fig. 1). A total of 50 specimens were collected, with a range of individuals per locality from 5 to 15 (Table 1). Fin clips were obtained and individuals tagged. Fin clips and voucher specimens were preserved in 95% ethanol and deposited in Colección Nacional de Peces, Instituto de Biología, Universidad Nacional Autónoma de México (CNPE-IBUNAM), Colección de Peces, El Colegio de la Frontera Sur (ECOSC), Museo Nacional de Ciencias Naturales de Madrid, España (MNCN) and Colección de Peces del Centro de México Dr. Edmundo Díaz Pardo, Universidad Autónoma de Querétaro, Mexico (CPCMEDP-UAQ). Localities for P. sp. nov. 1 were determined based on a previous molecular study (Table 1; Doadrio et al., 1999). For P. oaxacae, we measured 15 specimens from Rio Chiquito, Tlaxiaco, in the Mixteco-Balsas basin. P. oaxacae were identified using Miller's keys (Miller, 2005).
Sampling localities, number of localities and number of individuals that were used in the morphologic analysis. Taxonomic designation is according to our study, and to Miller (1955, 2005).
| Map | Locality | N | Basin | Species proposed | Species by Miller (1955, 2005) | UTM-X | UTM-Y |
|---|---|---|---|---|---|---|---|
| 1 | Río Grande, Santiago Juxtlahuaca, Oaxaca, México | 5 | Mixteco-Balsas | P. mixtlanensis | P. punctatus | 605394 | 1910523 |
| 2 | Spring, Sola de Vega, Oaxaca. México. | – | Atoyac | P. oaxacae | P. oaxacae | 715433 | 1826989 |
| 3 | Río Cucharas, El Carmen Tuxtitlán, Putla de Guerrero, Oaxaca, México | 7 | Atoyac | P. mixtlanensis | P. punctatus | 615930 | 1884461 |
| 4 | Río Chiquito, Tlaxiaco, Oaxaca, México | 15 | Mixteco-Balsas | P. oaxacae | P. oaxacae | 643961 | 1905758 |
| 5 | Spring, Aldama, Oaxaca, México | 15 | Atoyac | P. mixtlanensis | P. punctatus | 648638 | 1875597 |
| 6 | Spring, Chalcatongo, Oaxaca, México | – | Atoyac | P. mixtlanensis | P. punctatus | 650543 | 1884349 |
| 7 | Spring, Santiago Yosondúa, Oaxaca. México | 8 | Atoyac | P. mixtlanensis | P. punctatus | 651751 | 1866154 |
| 8 | Río de las Grutas, San Sebastián de las Grutas, Oaxaca, México | – | Atoyac | P. oaxacae | P. oaxacae | 718060 | 1839603 |
For comparison between species groups, we performed 17 morphometric measurements and 5 meristic counts in 50 individuals from 5 localities (Fig. 1, Tables 1 and 2). In order to identify the variables that contributed the most to the variation among populations, we performed principal component analysis (PCA) on the morphometric dataset, based on a covariance matrix. Finally, we performed discriminant function analysis (DFA) and linear discriminant analysis (LDA) in order to evaluate each specimen, assign it to a species and assess the degree of separation between the groups.
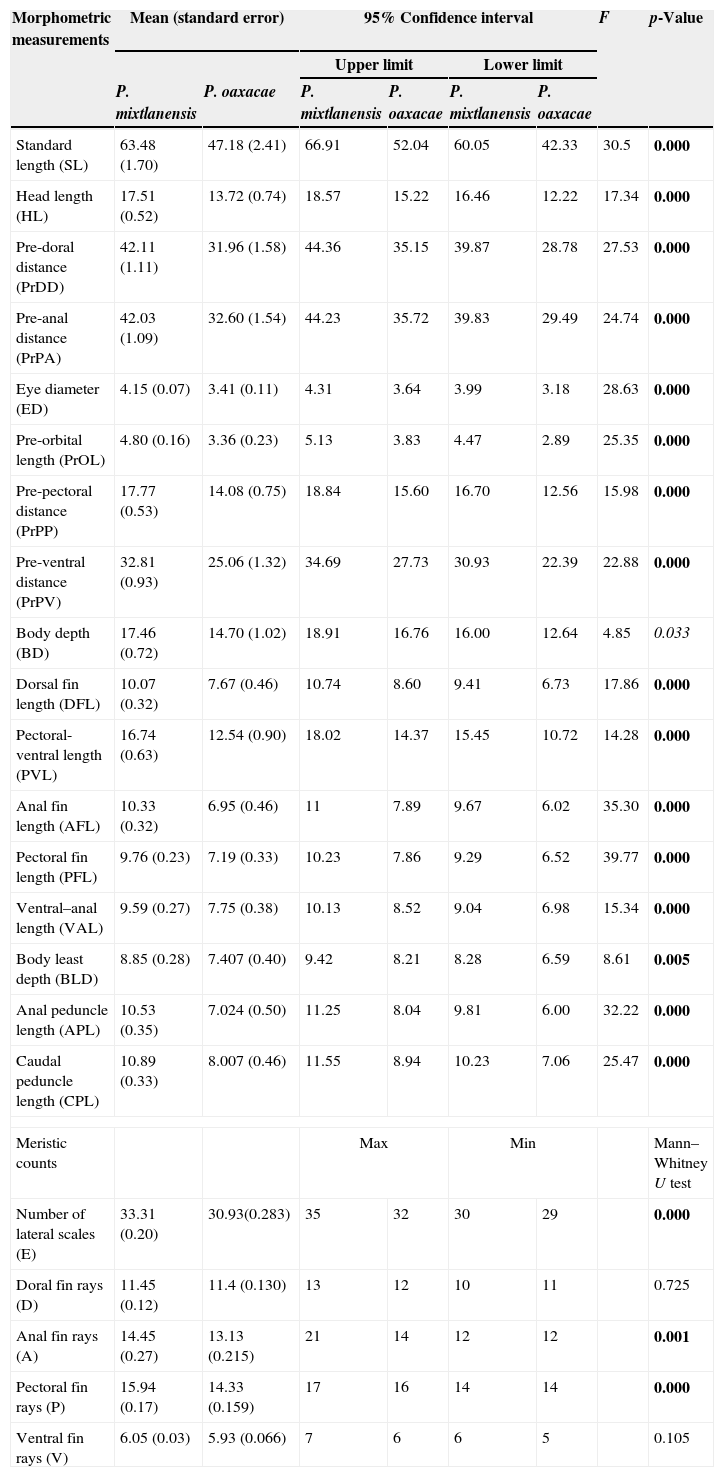
Mean, standard error and range values for morphometric measurements (mm) and meristic counts of P. mixtlanensis and P. oaxacae. p-Values from the ANOVA test for morphometric measurements and those from the Mann–Whitney U test for meristic counts are provided.
| Morphometric measurements | Mean (standard error) | 95% Confidence interval | F | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Upper limit | Lower limit | |||||||
| P. mixtlanensis | P. oaxacae | P. mixtlanensis | P. oaxacae | P. mixtlanensis | P. oaxacae | |||
| Standard length (SL) | 63.48 (1.70) | 47.18 (2.41) | 66.91 | 52.04 | 60.05 | 42.33 | 30.5 | 0.000 |
| Head length (HL) | 17.51 (0.52) | 13.72 (0.74) | 18.57 | 15.22 | 16.46 | 12.22 | 17.34 | 0.000 |
| Pre-doral distance (PrDD) | 42.11 (1.11) | 31.96 (1.58) | 44.36 | 35.15 | 39.87 | 28.78 | 27.53 | 0.000 |
| Pre-anal distance (PrPA) | 42.03 (1.09) | 32.60 (1.54) | 44.23 | 35.72 | 39.83 | 29.49 | 24.74 | 0.000 |
| Eye diameter (ED) | 4.15 (0.07) | 3.41 (0.11) | 4.31 | 3.64 | 3.99 | 3.18 | 28.63 | 0.000 |
| Pre-orbital length (PrOL) | 4.80 (0.16) | 3.36 (0.23) | 5.13 | 3.83 | 4.47 | 2.89 | 25.35 | 0.000 |
| Pre-pectoral distance (PrPP) | 17.77 (0.53) | 14.08 (0.75) | 18.84 | 15.60 | 16.70 | 12.56 | 15.98 | 0.000 |
| Pre-ventral distance (PrPV) | 32.81 (0.93) | 25.06 (1.32) | 34.69 | 27.73 | 30.93 | 22.39 | 22.88 | 0.000 |
| Body depth (BD) | 17.46 (0.72) | 14.70 (1.02) | 18.91 | 16.76 | 16.00 | 12.64 | 4.85 | 0.033 |
| Dorsal fin length (DFL) | 10.07 (0.32) | 7.67 (0.46) | 10.74 | 8.60 | 9.41 | 6.73 | 17.86 | 0.000 |
| Pectoral-ventral length (PVL) | 16.74 (0.63) | 12.54 (0.90) | 18.02 | 14.37 | 15.45 | 10.72 | 14.28 | 0.000 |
| Anal fin length (AFL) | 10.33 (0.32) | 6.95 (0.46) | 11 | 7.89 | 9.67 | 6.02 | 35.30 | 0.000 |
| Pectoral fin length (PFL) | 9.76 (0.23) | 7.19 (0.33) | 10.23 | 7.86 | 9.29 | 6.52 | 39.77 | 0.000 |
| Ventral–anal length (VAL) | 9.59 (0.27) | 7.75 (0.38) | 10.13 | 8.52 | 9.04 | 6.98 | 15.34 | 0.000 |
| Body least depth (BLD) | 8.85 (0.28) | 7.407 (0.40) | 9.42 | 8.21 | 8.28 | 6.59 | 8.61 | 0.005 |
| Anal peduncle length (APL) | 10.53 (0.35) | 7.024 (0.50) | 11.25 | 8.04 | 9.81 | 6.00 | 32.22 | 0.000 |
| Caudal peduncle length (CPL) | 10.89 (0.33) | 8.007 (0.46) | 11.55 | 8.94 | 10.23 | 7.06 | 25.47 | 0.000 |
| Meristic counts | Max | Min | Mann–Whitney U test | |||||
| Number of lateral scales (E) | 33.31 (0.20) | 30.93(0.283) | 35 | 32 | 30 | 29 | 0.000 | |
| Doral fin rays (D) | 11.45 (0.12) | 11.4 (0.130) | 13 | 12 | 10 | 11 | 0.725 | |
| Anal fin rays (A) | 14.45 (0.27) | 13.13 (0.215) | 21 | 14 | 12 | 12 | 0.001 | |
| Pectoral fin rays (P) | 15.94 (0.17) | 14.33 (0.159) | 17 | 16 | 14 | 14 | 0.000 | |
| Ventral fin rays (V) | 6.05 (0.03) | 5.93 (0.066) | 7 | 6 | 6 | 5 | 0.105 | |
In bold highly significant p-values (p<0.005) and in cursives significant p-values (p<0.05 and p>0.005).
For both analyses, measurements were standardized using the general allometric equation: Ms=Mo (Ls/Lo)b, where Ms=standardized measurement, Mo=measured character length (mm), Ls=overall (arithmetic) mean of the standard component (standard length – SL or head length – HL) for all individuals of each taxon, Lo=standard component of the specimen. For each character “b” was estimated using the non-linear equation, M=aLb as the slope of the regression of logMo on logLo (Elliot, Haskard, & Koslow, 1995; Ruiz-Campos, Camarena-Rosales, Varela-Romero, Sánchez-Gonzáles, & De La Rosa-Vélezv, 2003) (logistic regression in Supplementary material 1). Abbreviations for morphological measurements are given in Table 2.
Two-way analysis of variance (ANOVA) was carried out considering sex and species as factors to evaluate the influence of sexual dimorphism, and the sex×species interaction was included to test if dimorphism varied significantly between species groups for the 17 morphometric measurements. Proportions between measurements were calculated in relation to SL and HL (Table 3). Frequencies of the 5 meristic variables were compared between species groups. Mann–Whitney U tests were carried out on the 5 meristic variables as well as on the morphometric proportion measurements. Statistica v.6.0 (Statistica, 2001) was used for all statistical analyses.
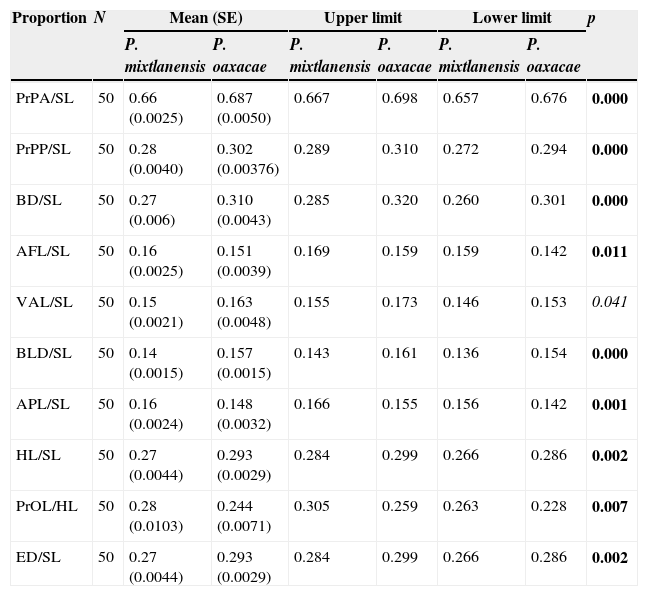
Mean, standard error and range values for proportions of morphometric variables between P. oaxacae and P. mixtlanensis. p-Values correspond to the Mann–Whitney U test.
| Proportion | N | Mean (SE) | Upper limit | Lower limit | p | |||
|---|---|---|---|---|---|---|---|---|
| P. mixtlanensis | P. oaxacae | P. mixtlanensis | P. oaxacae | P. mixtlanensis | P. oaxacae | |||
| PrPA/SL | 50 | 0.66 (0.0025) | 0.687 (0.0050) | 0.667 | 0.698 | 0.657 | 0.676 | 0.000 |
| PrPP/SL | 50 | 0.28 (0.0040) | 0.302 (0.00376) | 0.289 | 0.310 | 0.272 | 0.294 | 0.000 |
| BD/SL | 50 | 0.27 (0.006) | 0.310 (0.0043) | 0.285 | 0.320 | 0.260 | 0.301 | 0.000 |
| AFL/SL | 50 | 0.16 (0.0025) | 0.151 (0.0039) | 0.169 | 0.159 | 0.159 | 0.142 | 0.011 |
| VAL/SL | 50 | 0.15 (0.0021) | 0.163 (0.0048) | 0.155 | 0.173 | 0.146 | 0.153 | 0.041 |
| BLD/SL | 50 | 0.14 (0.0015) | 0.157 (0.0015) | 0.143 | 0.161 | 0.136 | 0.154 | 0.000 |
| APL/SL | 50 | 0.16 (0.0024) | 0.148 (0.0032) | 0.166 | 0.155 | 0.156 | 0.142 | 0.001 |
| HL/SL | 50 | 0.27 (0.0044) | 0.293 (0.0029) | 0.284 | 0.299 | 0.266 | 0.286 | 0.002 |
| PrOL/HL | 50 | 0.28 (0.0103) | 0.244 (0.0071) | 0.305 | 0.259 | 0.263 | 0.228 | 0.007 |
| ED/SL | 50 | 0.27 (0.0044) | 0.293 (0.0029) | 0.284 | 0.299 | 0.266 | 0.286 | 0.002 |
SL, standard length; HL, head length; PrPA, pre-anal distance; PrPP, pre-pectoral distance; BD, body depth; AFL, anal fin length; VAL, ventral–anal length; BLD, body least depth; APL, anal peduncle length; PrOL, pre-orbital length, ED, eye diameter. In bold highly significant p-values (p<0.005) and in cursives significant p-values (p<0.05 and p>0.005).
Diagnostic measurements were expressed as percent of the standard length (SL) (Álvarez del Villar, 1970). Additionally, the diagnosis was completed with other relevant features regarding morphology (i.e., body profile, fin shapes, among others), pigmentation and ecology.
DescriptionProfundulus mixtlanesis Ornelas-García, Martínez & Doadrio, new species (Fig. 2).
As a member of the Profundulus subgenus this species shows a preorbital region nearly covered with well-developed scales, a humeral spot, and a densely scaled basal half of the caudal fin. P. mixtlanensis sp. nov. differs from other species in the genus by having a pronounced lower jaw, which markedly differentiates it from P. oaxacae and the remaining species within the P. punctatus “group” sensuMiller (1955). Another striking difference between the new species and other species in the subgenus, especially P. oaxacae, is the absence of dark body spotting on the posterior half of the body caused by melanization of the base of the scales. In lateral view, the dorsal profile of the head shows a concavity just above the supra-occipital crest, which quickly reaches a flat profile posteriorly, an attribute that becomes more pronounced with age. In general terms, P. mixtlanesis depicts a longer body and has a lower height than the other species in the subgenus (see Table 3). Body height is <2/3 of SL, and pre-pectoral distance <1/3 of SL, in contrast the P. oaxacae proportions are significantly higher reflecting larger body height and pre-pectoral distance for the P. oaxacae. The distance between ventral and anal fins is 15% of SL, lower than that of P. oaxacae. Caudal peduncle depth is less than in P. oaxacae, being 13% of SL (15% of SL in P. oaxacae). The postanal length is 15% of SL, larger than the rest of species (data not shown). The head is shorter than in P. oaxacae, 26–28% of SL in contrast to 28–30% of SL in P. mixtlanensis. Preorbital length, 26–30% of HL, is longer than in P. oaxacae. Eye diameter is smaller than in P. oaxacae, 26–28% of SL. The anal fin is longer than in P. oaxacae, 16–17% of SL. Among meristic characteristics, the following are diagnostic: 30–35 lateral scales, 12–21 rays in the anal fin, and 14–17 rays in the pectoral fin.
Taxonomic summaryDisposition of types. Holotype CNPE-IBUNAM 19112 (Female, T1339) and allotype CNPE-IBUNAM 19113 (male, T1340), Spring system in Santiago Yosondúa, Oaxaca, México. The system is composed of five small springs, ∼1m in diameter and less than 1m deep each. Deposited at the CNPE-IBUNAM.
Paratypes. (45), Spring, Aldama, Oaxaca (15, CPCMEDP-UAQ:001/T1317-31); río Cucharas, El Carmen Tuxtitlán, Putla de Guerrero, Oaxaca (3, CPCMEDP-UAQ:002/1332-1335); Spring, Santiago Yosondúa, Oaxaca (6, 3 CNPE-IBUNAM 19114, 3; CPCMEDP-UAQ:003/T1340-1346-); río Grande, Santiago Juxtlahuaca, Oaxaca (3, ECOSC 7562-1/ECOSC 7562-3 and 2, CPCMEDP-UAQ:004/T1350-51, CPCMEDP-UAQ). Río Cucharas (10; MNCN:131746-53).
Distribution. Springs of the Pacific slope of the Mixteca region of Oaxaca, Mexico in the following river systems: Colorado River, Atoyaquillo River, the Atoyac river basin, and the Mixteco river basin (Fig. 1). Most of the populations for the species were found above 2,000m, however, they occur at altitudes between 710 and 2,300m.
Etymology. The epithet “mixtlanensis” derives from the word “Mixtlan” in the Nahuatl language. The Tenoch culture used to call Mixtlan the region where the new species is found. Mixtlan is a compound word, which is made up of the words Mixtli (cloud) and -Tlan (place). Hence it means “the place of the clouds” (Montemayor, 2008).
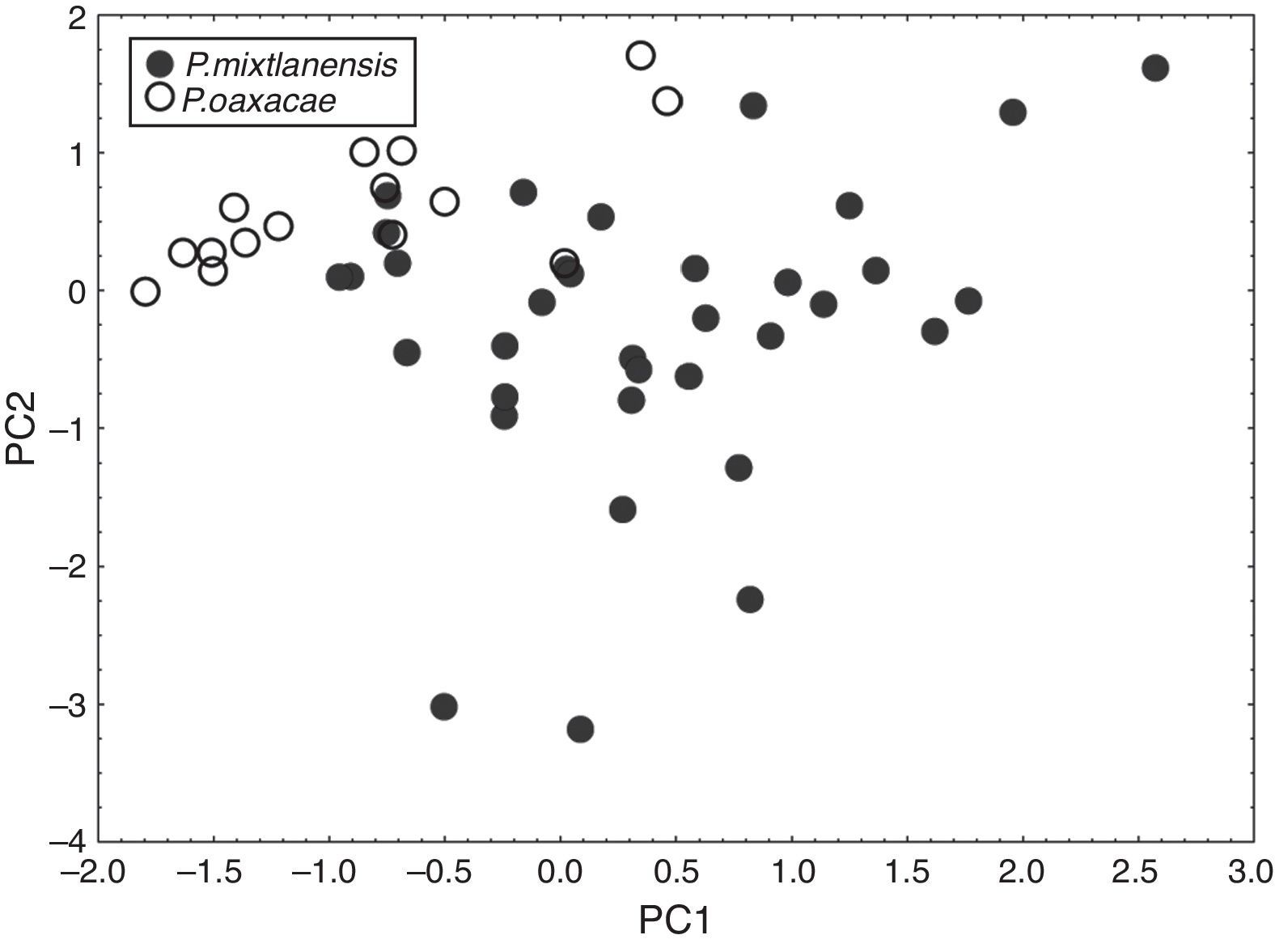
ResultsMorphometric analyses showed a separation between the two species groups tested. The two-way ANOVA using 17 morphometric measurements showed significant differences between species for most of the comparisons (p≤0.001, Table 2). Analyses however, did not disclose any significant differences in sex (p>0.05), or sex×species interaction (p>0.05, data not shown). Because results did not support the existence of sexual dimorphism within or between species, all posterior analyses were performed without sex separations. Ordination using PCA allowed for identification of two separate clusters of points. Each cluster corresponded to P. oaxacae and to P. sp. nov. 1 (Fig. 3). PCI explained 91.73% of the variance, where the most important variables in the formation of the groups were SL, PrOL and ED in the positive side of PCI, and PrPD, PrPA and PrPV in the negative side. PCII explained 2.79% of the variance, in which the most important variables were PrPD, PrPA, PrPP and BD, whereas PCIII explained 1.70% of the variance, with the most important variables PrPP, HL, PVL and VAL (Eigenvalues in Supplementary material 4).
DFA also showed significant discrimination between species. All individuals assigned to P. oaxacae were correctly classified whereas assignation to P. sp. nov. 1 was 97.14% correct (Fig. 4a, F(16,33)=7.0769 p<0.0001; posterior probabilities are shown in Supplementary materials 2 and 3). Linear discriminant factor from the DFA showed a contrasting distribution between the two groups of cases (Fig. 4). The root factor was ordered mostly according to the following variables: PrPD and AFL on the positive side and PrPA, DFL and BLD on the negative side.
Results from the discriminant function analysis (DFA): (a) proportions of correctly assigned individuals: 97% for P. mixtlanensis sp. nov. (individuals 1–35 in the graph) and 100% for P. oaxacae (individuals 36–50); (b) distribution frequencies of root factor from the DFA between species.
Results of the Mann–Whitney U test applied to meristic variables were also significant for three of five variables tested between species (Table 2), and when applied to proportions, comparisons were significant for 10 of 16 tested (Table 3). Highly significant differences were found in the following proportions: PrPA/SL, PrPP/SL, BD/SL, ADL/SL, BLD/SL, APL/SL, HL/SL, PrOL/HL and ED/SL.
Even when there is slight overlap, significant differences were found between species in the following meristic variables (Table 3; p<0.001): number of lateral scales, with a mean of 33 and range of 30–35 in P. mixtlanensis, and 31 mean and range of 29–32 in P. oaxacae; and with a lower number of anal-fin rays for P. oaxacae (12–14), while for P. mixtlanensis the anal-fin rays range from 12 to 21 (Fig. 5). Given the previous results, we conclude that P. mixtlanensis fulfills the criteria to be considered a different taxonomic unit, using both morphological and molecular (Doadrio et al., 1999) criterion.
DiscussionP. mixtlanensis and P. oaxacae occur at geographically distant locations in the Mixteco basin, and as we observed in the present description, the two species are easily distinguishable by their body shapes and coloration patterns. P. oaxacae shows a characteristic spotty pattern along the lateral part of the body while in P. mixtlanensis only shows a humeral spot. The molecular distinctiveness of P. mixtlanensis with respect to the other species within the Profundulus genus has been demonstrated by an allozyme study (Doadrio et al., 1999). In addition, phylogenetic analysis carried out by the authors showed that both, P. oaxacae and P. mixtlanensis depict a high degree of divergence from the rest of the species within the subgenus Profundulus (Dp=10.5±5.9 mtDNA and Dp=1.7±0.2 nDNA, respectively, data not shown).
We thank Carlos Pedraza for his helpful suggestions on an early version of the manuscript. We also thank Silvia Perea for her help in the present study. Funding for the present study was derived from the project CGL2010-15231 from the Consejo Superior de Investigaciones Científicas (CSIC). Two anonymous reviewers and the editor made useful suggestions to the manuscript.