The content of phytochemicals associated with the antioxidant activity of the fruits of species of hawthorn (Crataegus spp.; Rosacea) located in Mexico is unknown. The objective of the present study was to evaluate the content of phenolic compounds, flavonoids, vitamin C and the antioxidant activity in a selection of Mexican hawthorn species. A quantification was made of total phenols, flavonoids and vitamin C (expressed on mg of phenol, quercetin and ascorbic acid per 100g of fresh weight, respectively), in 10g of fruits selected from each genotype; a total of 20 genotypes were sampled, these located in the germplasm bank of the Universidad Autónoma Chapingo, Mexico. The antioxidant activity was evaluated by the DPPH method, expressed as mean inhibitory concentration (IC50). Content of total phenols, flavonoids and vitamin C cannot be associated with the origin and species of the samples. Some genotypes from the state of Chiapas could be considered to have a higher potential for commercial use and consumption due to their nutraceutical quality. Most of the fruits of the 20 genotypes of hawthorn presented a content of phenolic compounds higher than that described for other fruits (lychee fruits, peaches and strawberries); these nutraceutical characteristics provide an added value to the fruit.
El contenido de fitoquímicos asociado con la actividad antioxidante de los frutos de especies de tejocote (Crataegus spp.; Rosacea) localizadas en México es desconocido. El objetivo del presente estudio fue evaluar el contenido de compuestos fenólicos, flavonoides, vitamina C y la actividad antioxidante, en una selección de especies de tejocote mexicano. La cuantificación de fenoles totales, flavonoides y vitamina C (expresada en mg de ácido gálico, quercetina y ácido ascórbico por 100 gr de peso fresco, respectivamente) en 10 gr de frutos seleccionados de cada genotipo, se muestrearon 20 genotipos localizados en el banco de germoplasma de la Universidad Autónoma Chapingo, México. La actividad antioxidante se evaluó de acuerdo al método del DPPH y se expresó como concentración media inhibitoria (CI50). No se observó una relación del contenido de fenólicos, flavonoides y vitamina C con el origen y la especie de las muestras. Algunos genotipos del estado de Chiapas por su calidad nutracéutica podrían ser consideradas para uso comercial y consumo. La mayoría de los 20 genotipos de tejocote presentaron un contenido de compuestos fenólicos más alto que el descrito para otros frutos (lichi, durazno y fresa), estas características proporcionan un valor agregado a la fruta.
Recently in Mexico there has been a growing interest in knowledge and management of underutilized fruits, also known as minor, secondary or alternative fruits, such as the case of hawthorn fruits (Crataegus spp.) (Nieto-Ángel, 2007). There are descriptions of 150-200 species of this genus (Crataegus) in the world, approximately 13 have been identified in the north and center of Mexico, Phipps (1997) indicates 9 endemic species (Phipps, 1997; 1998). Nieto-Ángel (2007) mentions that there is great diversity and variability in its genotypic and phenotypic characteristics. Hawthorn belongs to the Rosacea family, is located principally in cold and temperate climates (Nieto-Ángel, 2007). Tejocote is the most widely used term and comes from the nahuatl language, in which tetl-xocotl means wild or hard sour fruit; the Nahoas (ancestors of the mexicas) referred to them as texococuahutl, which means the tree of Indian apple (Martínez, 1994) depending on the region where it is located, the tejocote has adopted different common names (Martínez, 1994).
In Mexico it is mainly used as animal feed, ornamental plant; in agro-industry it is used to make regional sweets, in the preparation of punches and conserves because of its high pectin content; it has a high demand mainly in the south-southeast-central region of Mexico in the traditional festivals of “All Saints” because it is put on the table as an offering and consumed as fruit (offerings and piñatas) (Nieto-Ángel, 2007). In traditional Mexican medicine the flowers, leaves, root and the fruits have numerous uses (Martínez, 1994; Ody, 1994).
There are a number of medicinally active phytochemicals that have been isolated from hawthorn plants with most of the data generated in studies of those species that are native to Europe and Asia. Little is known about the North American and Canadian (Edwards et al., 2012), and more specifically about the Mexican Crataegus species. One possible barrier to chemical studies of Crataegus, has been the perception that Mexican Crataegus are taxonomically problematic (Phipps, 1997). The high contents of phenolic compounds such as flavonoids, proanthocyanidins, catechins, phenolic acids, essential oils and terpenoids (Bahorum et al., 1994; Edwards et al., 2012; García-Mateos et al., 2012) explain their use as natural therapy for the treatment of neurodegenerative diseases, in some types of cancer, in the affectation of the immunological system and cardiovascular disorders (Craig, 1999; Chang et al., 2002; Cui et al., 2006). Hawthorn extracts exert a wide range of pharmaceutical properties, especially on the cardiovascular system, including cardiotonic, antiarrhythmic, hypotensive, hypolipidemic, and antioxidant activities (Craig, 1999; Barceloux, 2008; Arrieta et al., 2010).
The wide diversity and genotypic variability that exists in the Mexican hawthorn species demands the characterization of its fruits and the determination of its antioxidant properties to be recommended as a food of high nutraceutical value that permits a more efficient agro-industrial use and provides new economic alternatives for the producer. The nutraceutical content in products (jellies, preserves and candied fruit) made from the fruit is unknown. The objective of the present study was to evaluate the content of phenolic compounds, flavonoids, vitamin C and the antioxidant activity in a selection of Mexican hawthorn species.
Materials and methodsCollection of plant material. The present investigation was carried out with hawthorn fruits from the central plateau and the south of Mexico located in the hawthorn germplasm bank of the Universidad Autónoma Chapingo, located at 19°29' N, 98°53' W, at 2 240 m (García, 1981). The climate is C(Wo) (w)b (I')g, rainy moderate temperate and the driest of sub-humid climates, with rains in summer; the mean annual temperature is 17.8° C and rainfall is approximately 644.8mm annually. Ten grams of physiologically mature fruits were randomly selected from each genotype. A total of 20 genotypes were sampled; they were originally collected from 3 states of the center and south of Mexico (Puebla, State of Mexico and Chiapas). Preparation of the extract. One gram of fresh fruit pulp was weighed; each sample was dissolved in 25ml of ethanol at 95% v/v. After 24h the volume was adjusted to 25ml with ethanol at 80% v/v, and the mixture was centrifuged at 1 409g.
Quantification of total phenols. Quantification was made according to the method proposed by Waterman and Mole (1994). A mixture of phosphowolframic and phosphomolybdic acids in basic medium is used as reactive, and reduced by oxidizing the phenolic compounds, originating blue oxides of wolframic and of molybdenum. For the analysis, 0.5ml of ethanolic extract were taken, 10ml of a solution of Na2CO3 was added at 10% p/v, was homogenized and the mixture was incubated in darkness at 38° C for 15min. One ml of the mixture was taken, 3ml of water was added along with 1ml of the reactive of Folin Ciocalteu:water (1:1). The mixture was left to set for 15min in darkness. Finally, the absorbance reading was taken at 600nm in a Genesys 10s spectrophotometer. The concentration was obtained from a standard curve (y=0.0014x; R2=0.997) prepared with gallic acid. Total phenol values are expressed in mg equivalent of gallic acid per 100g of fresh weight. Each analysis was done in triplicate.
Quantification of flavonoids. One aliquot of 0.5ml of the supernatant of the ethanolic extract was previously prepared; 1.5ml of ethanol at 95% v/v was added, along with 0.1ml of a solution of AlCl3 at 10% p/v, 0.1ml of solution of 1M of potassium acetate and 2.8ml of distilled water. The mixture was incubated in darkness for 30min. Absorbance was read in a Genesy 10s spectrophotometer at a wave length of 415nm. For the quantification, a standard curve was made (y=0.0122x-0.0067; R2=0.965) based on the flavonol quercetin (Chang et al., 2002). Flavonoid values are expressed in mg equivalent of quercetin per 100g of fresh weight.
Quantification of vitamin C. Quantification of vitamin C was carried out through the determination of ascorbic acid (vitamin C). To a 1g of sample, 3ml of metaphosphoric acid at 3% v/v was added, and then the mixture was macerated for 3min and was filtered. One ml of the filtrate was taken and the volume brought to 10ml with the solution of metaphosphoric acid at 3% v/v. Two ml of the extract was taken and 2ml of the buffer solution, pH=4 (glacial acetic acid: sodium acetate 5%, 1:1) was added, along with 3ml of dichloroindophenol and 15ml of xylene, and the mixture was agitated vigorously. The organic phase was separated and dried with the addition of anhydrous Na2SO4, the mixture was filtered and absorbance was read in a Genesis 10s spectrophotometer at a wavelength of 520nm. From the standard curve (y=−2.666x+0.567; R2=0.995) the concentration of ascorbic acid present in each sample was obtained by means of the following equation: mg total ascorbic acid/mg= (C×V x100) / (A×P); where: C= ascorbic acid in the sample, V= final volume, A= ml of aliquot of the solution taken, P= weight or volume of the sample. The concentration of vitamin C was expressed in mg equivalent of ascorbic acid per 100g of fresh weight.
Evaluation of antioxidant activity. The analysis was performed using the free radical DPPH method (2,2-diphenyl-1-picrylhydrazyl, Sigma-Aldrich), described by Amico et al. (2008). The antioxidant capacity of the samples was observed at 516 nm from the gradual color change of the DPPH (purple) to reduced-DPPH (yellow) (Cotelle et al., 1996). For the analysis, 10g of sample were macerated (hawthorn fruit pulp) in methanol (GR) for 48h, the mixture was decanted and the plant residue was placed once more in methanol for the same period of time. The extracts were reunited and the dissolvent was evaporated in a Büchi evaporator. From the highest concentration of the methanolic extract (stock solution) the following dissolutions were prepared in methanol to obtain the concentrations of 0.2, 0.15, 0.1, 0.05mg ml−1. As references the concentrations 0.1, 0.001 and 0.0001mg ml−1 of quercetin and ascorbic acid were prepared separately. To each concentration of the extracts (1ml) and of the references 3ml were added separately of the solution of DPPH (0.1mM). The mixtures were left at room temperature during 30min and later the absorbance readings were made at 516nm. The percentage of DPPH was determined through the formula: % DPPH= (Acontrol Asample)*100/AbControl; where Acontrol is the absorbance of the control (DPPH 0.1mM) and Asample is the absorbance obtained after 30min of each sample with DPPH 0.1mM. The antioxidant activity of the samples was determined through the calculation of the mean inhibitory concentration (IC50), which is the concentration required by the sample to decrease the absorbance of DPPH to 50%. The low absorbance of the reaction mixture indicated high antioxidant activity.
To construct the standard curve (y=9.393×+0.006; R2=0.999) of DPPH, 3.93mg were weighed and dissolved in 100ml of methanol to obtain a concentration of 0.1mM. From this solution the different concentrations were prepared: 0.01, 0.02, 0.04, 0.06, 0.08 and 0.1mM of DPPH. Absorbance was measured at 516nm in a Genesys 10s spectrophotometer; the readings were taken by triplicate and methanol (GR) was used as reagent blank.
Statistical analysis. A Pearson's correlation and analysis of variance (Anova) were carried out, along with the comparison of means of Tukey (p≤0.05), using the program Statistical Analysis System (SAS, version 8.0) according to a completely randomized experimental design, where each selected genotype was considered as treatment of which 3 replicates were made.
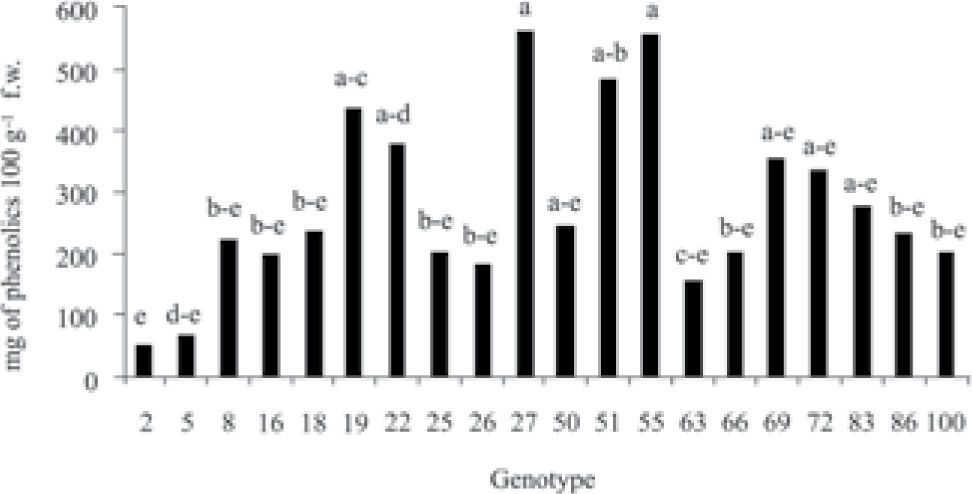
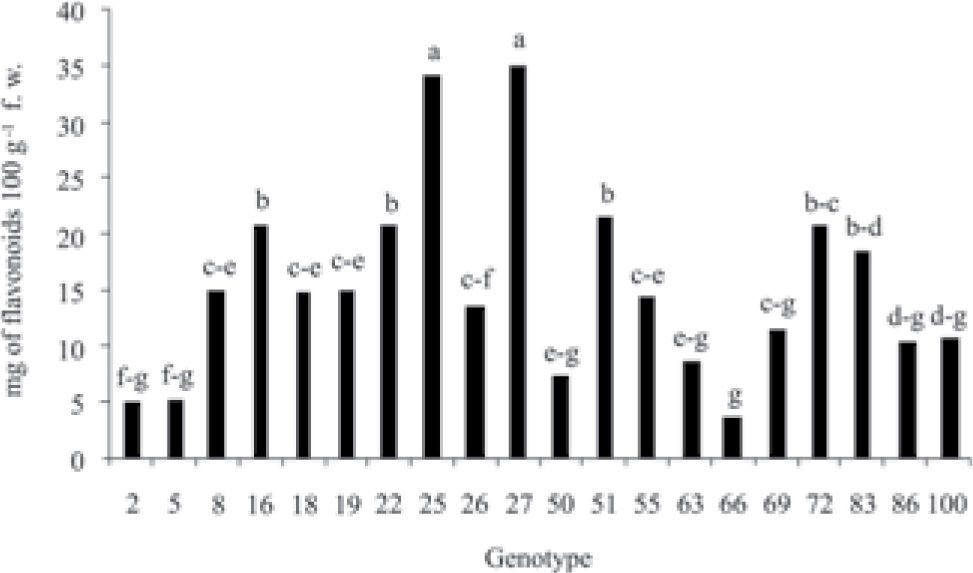
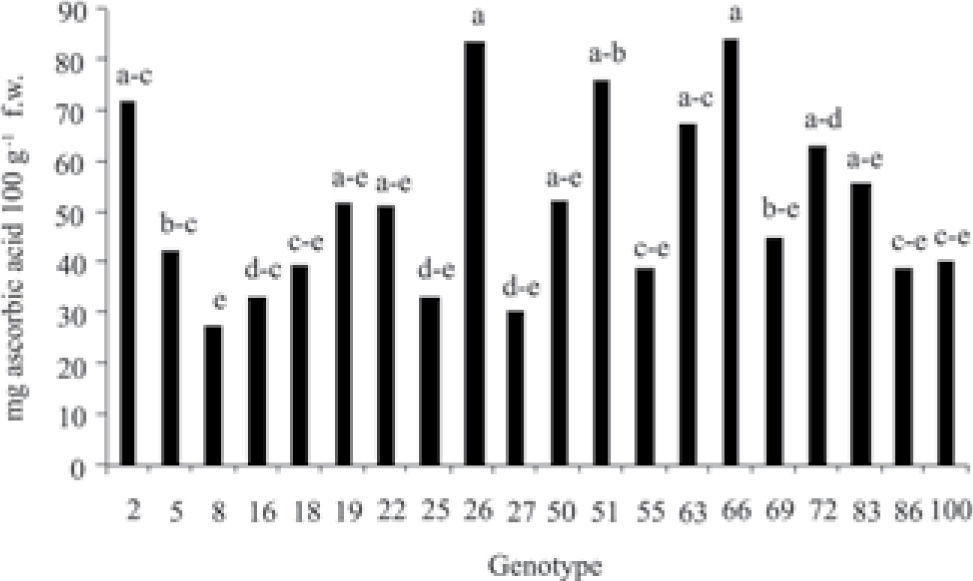
ResultsStatistical analysis showed significant differences (p≤0.05) in the concentration of phenolic compounds among the 20 genotypes of fruits of different species and origin (Fig. 1); the same tendency was observed in the content of flavonoids and vitamin C (ascorbic acid) (Figs. 2, 3). Genotypes 27, 51 and 55 were the ones that presented the highest concentration of phenols, genotypes 27 and 51 have a common geographic origin (Chiapas) in contrast to genotype 55 (Puebla). The lowest concentration was found in genotypes 2 (Crataegus rosei) and 5 (Crataegus aurescens), both have as origin the state of Puebla. Genotypes 25 and 27 presented the highest content of flavonoids, similarly, the content of these metabolites was also lower in genotypes 2 and 5. Results showed that the content of total phenols and flavonoids can not be associated with the origin and species of the samples (Fig. 1). Genotypes 2, 26 and 66 presented the highest concentration of vitamin C (Fig. 3).
The greatest antioxidant activity (media inhibitory concentrations 0.6−0.10μg ml−1 was significantly higher (p≤0.05) in the genotypes 18, 22, 51 and 100 than in the genotypes 26 and 27 (Table 1), due to the fact that a lower concentration of sample was required to inhibit in 50% of the solution (0.1mM) of the radical DPPH (Table 1). The references were quercetin (flavonoid) and ascorbic acid (vitamin C), which presented an antioxidant activity that was higher (IC50 of 0.00024 and of 0.00043 μg ml−1, respectively) than that found in the methanolic extracts of the fruits of the 20 genotypes selected for the study.
Antioxidant activity of 20 selected genotypes of hawthorn fruits of the germplasm bank of the Universidad Autónoma Chapingo, Mexico.
| Genotype | Species | State | Antioxidant activity (IC50= μg ml−1) |
|---|---|---|---|
| 2 | C. rosei Eggl rosei | Puebla | 0.16 e-i |
| 5 | C. aurescens Phipps | Puebla | 0.20 d-f |
| 8 | C. tracyi Ashe ex Eggl | Chiapas | 0.11 g-j |
| 16 | C. tracyi Ashe ex Eggl | Chiapas | 0.13 f-j |
| 18 | C. tracyi Ashe ex Eggl | Chiapas | 0.08 i-j |
| 19 | C. baroussana Eggl | Chiapas | 0.18 d-h |
| 22 | C. rosei Eggl parryana | Chiapas | 0.06 j |
| 25 | C. cuprina Phipps | Puebla | 0.13 f-j |
| 26 | C. gracillior Phipps | Chiapas | 0.22 c-e |
| 27 | C. gracillior Phipps | Chiapas | 0.27 b-c |
| 50 | C. greggiana Eggl | Edo. de México | 0.46 a |
| 51 | C. mexicana Moc y Sessé | Chiapas | 0.16 e-i |
| 55 | C. mexicana Moc y Sessé | Puebla | 0.10 h-j |
| 63 | C. mexicana Moc y Sessé | Edo. de México | 0.35 b |
| 66 | C. gracillior Phipps | Edo. de México | 0.14 e-j |
| 69 | C. cuprina Phipps | Chiapas | 0.20 d-g |
| 72 | C. baroussana Eggl | Chiapas | 0.35 b |
| 83 | C. tracyi Ashe ex Eggl | Chiapas | 0.20 d-g |
| 86 | C. mexicana Moc y Sessé | Puebla | 0.35 b |
| 100 | C. mexicana Moc y Sessé | Puebla | 0.10 h-j |
| CV. | 15.00 |
*Data with different letters in a column are statistically different (p≤0.05). IC50= mean inhibitory concentration; concentration required by the sample to inhibit 50% of DPPH after 30min.
Results showed that the content of phenolic compounds was higher than those of flavonoids in the 20 genotypes studied (Edwards et al., 2012; García-Mateos et al., 2012). Table 2 shows a positive correlation between the level of 0.01 total phenols and flavonoids, namely that with increasing phenol content also increases the level of flavonoids in fruits. Bahorum et al. (1994) observed the same tendency in the extracts of Crataegus monogyna. A small proportion of the content of phenolic compounds corresponds to flavonoids, the difference could be due to the presence of procyanidins and phenolic acids, as occurs in other plant species (Cui et al., 2006). Cui et al. (2006) point out a high content of polyphenols in the fruits of Crataegus pinnatifida due to the presence of procyanidins (19.7%) of phenols (1.27%; chlorogenic acid) and of flavonoids (0.48%). Svedstrom et al. (2002a; b) describe the presence of oligomeric procyanidins (condensed tannins) in leaves (1.58% dry weight), in flowers (1.15%) and the highest concentration detected was in fruits (0.15% dry weight) of Crataegus laevigata.
Correlation analysis between characters phenolic compounds, flavonoids, ascorbic acid and antioxidant activity in hawthorn fruits of different genotypes
| Character | Flavonoids | Vitamin C | Antioxidant activity | |||
|---|---|---|---|---|---|---|
| Phenolic compounds | 0.53123 | 0.01z | −0.17369 | 0.46 | −0.05720 | 0.81 |
| Flavonoids | −0.39661 | 0.08 | −1.13107 | 0.58 | ||
| Vitamin C | 0.18412 | 0.43 | ||||
Pearson's correlation coefficient and statistical significance between 4 variables. zSignificance level according to the Tukey test (p≤0.05).
For the 20 selected genotypes, the concentrations of phenols observed were found in the interval 52–558mg in 100g−1 f. w. Edwards et al. (2012) describe 1.84–248.18mg g−1 f. w. on fruits of several species of Crataegus.Georgé et al. (2005) point out that the concentration of phenols that they quantified in lychee fruits, peaches and strawberries (>180mg of gallic acid equivalent in 100g f. w.) is lower in comparison with some vegetables (celery, artichokes and Brussels sprouts, >250mg equivalent of gallic acid in 100g f. w.). In the present study most of the hawthorn samples surpassed the highest limit. Therefore, it is considered that they present a high concentration of phenols, but particularly some from Chiapas.
The content of phenolic compounds maintains a low positive correlation with the content of flavonoids (Table 2). In this respect, Bignami et al. (2003) mention that the ecological factors do not influence the concentration of phenolic compounds in the species that were analyzed; the authors point out that the variations of these metabolites may be of genetic origin. However, the environmental factors seem to have an influence on the presence of these metabolites, since Kirakosyan et al. (2003) mention that stress from both cold and drought increased the concentration of the polyphenols and of some flavonoids, as well as the antioxidant activity of the extracts of C. laevigata and C. monogyna. The phenolic composition of fruits is determined by genetic and environmental factors but may be modified by oxidative reactions during processing and storage (Robards et al., 1999).
In the present study no relationship was found in the genotypes of the antioxidant activity with respect to the content of flavonoids (negative correlation) (Table 2), probably because the flavonoids may be found as glycosides, not studied in the present work. Kirakosyan et al. (2003) indicate that the antioxidant activity is principally found associated to the content of flavonoids, but when these metabolites are found as aglycones, they present higher activity than their respective glycosides due to a structure-activity relationship (Robards et al., 1999). Those structures as quercetin (flavonoid), that present hydroxyl groups in positions 3' and 4' of the B ring and OH in C-3 permit stable structures that efficiently capture free radicals, a requirement for maximum antioxidant capacity (Bors et al., 1990; Cotelle et al., 1996; Robards et al., 1999). This information may explain the moderate antioxidant activity found in the extracts of the present study. In a previous study García-Mateos et al. (2012) identified 4 glycosides of quercetin in hawthorn flowers from 6 species located in the same germplasm bank as the fruits.
Due to the above, it is affirmed that the antioxidant activity of fruits and vegetables is not only associated with the presence of the phenolic compounds and flavonoids, but also is attributed to other metabolites such as carotenoids and vitamin C (Delgado-Vargas and Paredes-López, 2003; Georgé et al., 2005; Materska and Perucka, 2005; Brat et al., 2007). Nieto-Ángel (2007) mentions that the fruits from 16 genotypes from the germplasm bank have a high content of vitamin C (38.6–49.7mg 100g−1 f. w.). In the present study, for the 20 selected genotypes, the concentrations of vitamin C observed were found in the interval 27.51–84.15mg equivalent of ascorbic acid in 100g−1 f. w. These concentrations were higher than those described by Nieto-Ángel (2007) and genotypes 26 and 51 (origin Chiapas) and 66 (origin Puebla) presented the highest concentrations. However, no correlation was found for this variable with antioxidant activity (Table 2).
Scalbert and Williamson (2003) mention that phenols are the most abundant antioxidants in foods, and that the daily intake is equivalent to a gram of this type of metabolite, and represents 10 times more than the intake of vitamin C, 100 times more than that of vitamin E and 500 times more than that of carotene. Therefore, according to Scalbert and Williamson (2003) the phenolic compounds are the nutraceuticals that contribute the most to antioxidant activity of the fresh foods that are consumed, which makes it possible to infer that the antioxidant activity in some hawthorn fruits is due to the high content of phenols and in others possibly to a synergetic effect of vitamin C and of the flavonoids, as pointed out by Georgé et al. (2005).
The presence of components with medicinal properties gives importance to the exploitation of hawthorn fruits in the field of herbal preparations and food with medicinal and nutraceutical interest.
The fruits of most of the 20 selected genotypes of hawthorn species from the germplasm bank of the Universidad Autónoma Chapingo presented a higher content of phenolic compounds than that described for other fruits. Content of phenols, flavonoids and vitamin C cannot be associated with the origin and species of the genotypes. The fruits of genotypes 18 and 22 presented the highest antioxidant activity. The highest values of the content of phenolic compounds were identified in the fruits of genotypes 27, 51 and 55 and in genotypes 26, 51 and 66 the highest content of vitamin C was observed. Some of the genotypes from the state of Chiapas could be considered to have a higher potential for commercial use and consumption due to their nutraceutical quality. Phytochemicals were evaluated and provide evidence of the antioxidant activity of the fruits; their presence explains some of the medicinal properties attributed to species from the Mexican Crataegus. It is recommended to evaluate the nutraceuticals content in products made of hawthorn fruits (jellies, preserves and candied fruit) due to the cultural consumption activity in our country.