Through field work, bibliographic information and herbarium collections, a preliminary list of mosses for the state of Hidalgo was compiled. Records for 355 species were supported by 3 068 herbarium specimens at MEXU; when varieties are included along with taxa unsupported by local herbarium specimens, the number of taxa reaches 420. A collecting effort analysis indicates that 74.5% of the moss flora has been surveyed, that is, 56 taxa remain to be added. Species distribution modeling using 20 climatic variables from the WorldClim database for 150 species produced a map of potential distribution using a 5-minute cell network; it shows that the species potential richness is higher in central and southeastern Hidalgo, although most collections were obtained in southern and northwestern stations. Because large portions of the state land area are underexplored for mosses, no biodiversity hotspots are recognized. The Caribbean element is best represented in the Eastern Sierra Madre, but the confluence of the latter with the Neovolcanic Belt does not seem to show other major floristic differences between them, despite their geographical proximity.
Se presenta una lista preliminar de musgos del estado de Hidalgo basada en trabajo de campo, bibliografía y colecciones de herbario. Con base en 3 068 ejemplares en MEXU se registran 355 especies, pero esta cifra se incrementa a 420 si se incluyen las variedades y los taxa sin registros en los herbarios locales. El análisis del esfuerzo de recolecta señala que se ha registrado el 74.5% de la riqueza estatal, es decir, 56 taxa menos del valor esperado. Los modelos de distribución potencial de 150 especies, usando 20 variables climáticas de la base de datos de WorldClim y una división en celdas de 5minutos, indica que la riqueza potencial de musgos es más alta en el centro y sureste del estado, a pesar de que la mayoría de las colecciones provienen de sitios del sur y noroeste. Como partes importantes del centro del estado todavía están poco exploradas, no se reconocen zonas de alta diversidad. No se han detectado diferencias en patrones florísticos, excepto en el elemento del Caribe que está mejor representado en la sierra Madre Oriental que en el Eje Neovolcánico, a pesar de la cercanía geográfica de las 2 áreas.
Mosses of the state of Hidalgo have been collected and studied since the beginning of the XX century. Among the early collections are those obtained by Cyrus G. Pringle through several visits to the state (Davis, 1936). His moss collections were sent to J. Cardot (1909, 1910, 1911) for identification. In mid-XX century, Crum (1951) cited specimens by various collectors, including A. J. Sharp and his collaborators, referring to them by collector name and number; most of these specimens were deposited in MICH and other American herbaria. Sharp et al. (1994) included 281 moss taxa for Hidalgo, but the Moss Flora of Mexico did not cite specimens. In recent years, Alfaro and Castillo (1986) listed 169 species and varieties for Sierra de Pachuca; Cárdenas and Delgadillo (2009) cited specimens from localities bordering the Valley of Mexico that politically belong in Hidalgo; Delgadillo et al. (2011) listed 129 species and varieties from Los Mármoles National Park. The specimens derived from the last 3 contributions were deposited in the Bryophyte Collection at the National Herbarium (MEXU).
Despite the floristic information available, it seems that many areas in Hidalgo have not been explored for mosses, some sites are represented by many collections, and that a broader selection of sites should provide an adequate representation of the state’s moss flora. Because of its geological and geographical setting, especially at the point of contact between the Eastern Sierra Madre (ESM) and the Neovolcanic Belt (NVB), collections in the state of Hidalgo along with other nearby areas may be informative of the history of moss migration in this part of Mexico. In this contribution we offer a preliminary assessment of actual and potential species richness, and patterns of distribution; these may be in order to plan future field work in various parts of the state.
The state of Hidalgo in eastern Mexico has a surface area of nearly 21 000km2 (García and Falcón, 1984). Its rugged relief is dominated by the Eastern Sierra Madre that runs NW-SE; numerous sierras and isolated mountains are found in southern and western areas, several of them reaching more than 3 000m in elevation (cf. INFDM, 2005). Because of its geographical position, the state is also part of the Neovolcanic Belt area.
Materials and methodsRecently collected specimens and samples deposited in MEXU were examined along with records from the literature to produce a list of moss species from the state of Hidalgo. Major literature sources of floristic and geographical information were the updated electronic version of LATMOSS 2010 (Delgadillo, 2010) and Sharp et al. (1994) that complemented specimen data. The information for 3 068 moss specimens was compiled in a georreferenced database with records for 355 species that served to calculate cell width of the area of occupancy (AOO), according to IUCN criteria (IUCN, 2001). In this study, cell size is the longest axis between 2 collecting points divided by 10; the size of the grids (area of occupancy) was calculated by using the Conservation Assessment Tools designed for Arcview (Moat, 2007). The species cell width was averaged to obtain the width value applicable to all species; the value thus obtained, 8.4km, was transformed to arc minutes (about 5minutes). For further analysis, the state of Hidalgo was then divided into a network of 5-minute cells.
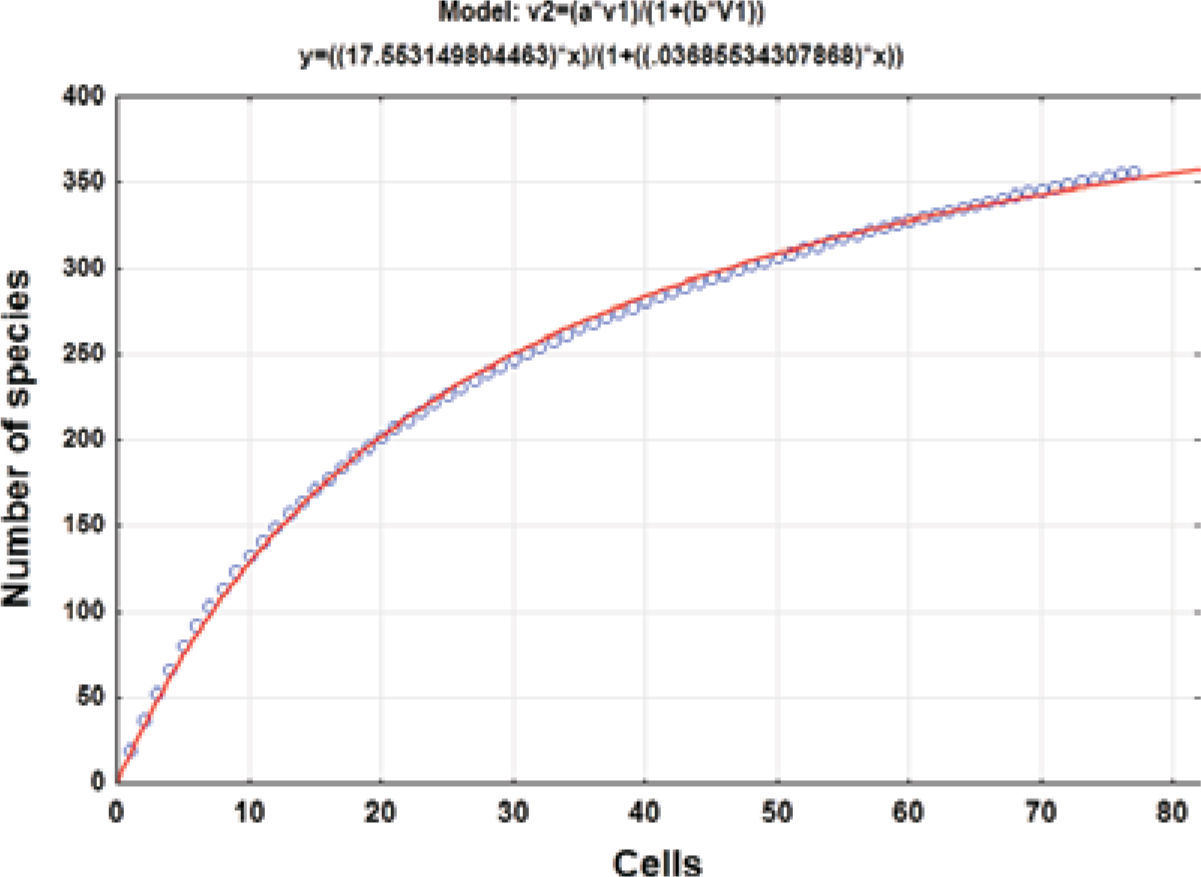
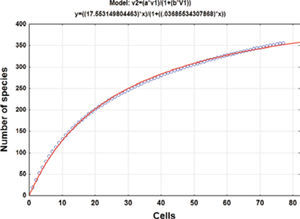
Collecting effortThe geographical data of the collecting records were used to produce a species accumulation curve (Gotelli and Colwell, 2001). Seventy-seven 5-minute cells with collecting records were used for the analysis. The asymptote of the accumulation curve (Fig. 1) is theoretically related to the number of species expected for the study area (Jiménez-Valverde and Hortal, 2003) and the number of cells is a measure of the collecting effort after randomly sorting these 50 times to produce a soft curve with EstimateS, version 8.2.0 (Colwell, 2009). The asymptote was estimated adjusting Clench’s equation to the accumulation curve (Soberón and Llorente, 1993; Colwell and Coddington, 1994) by the Simplex and Quasi-Newton method in the STATISTICA software (StatSoft, 2011); the predicted asymptotic value was used to estimate the precision of the inventory.
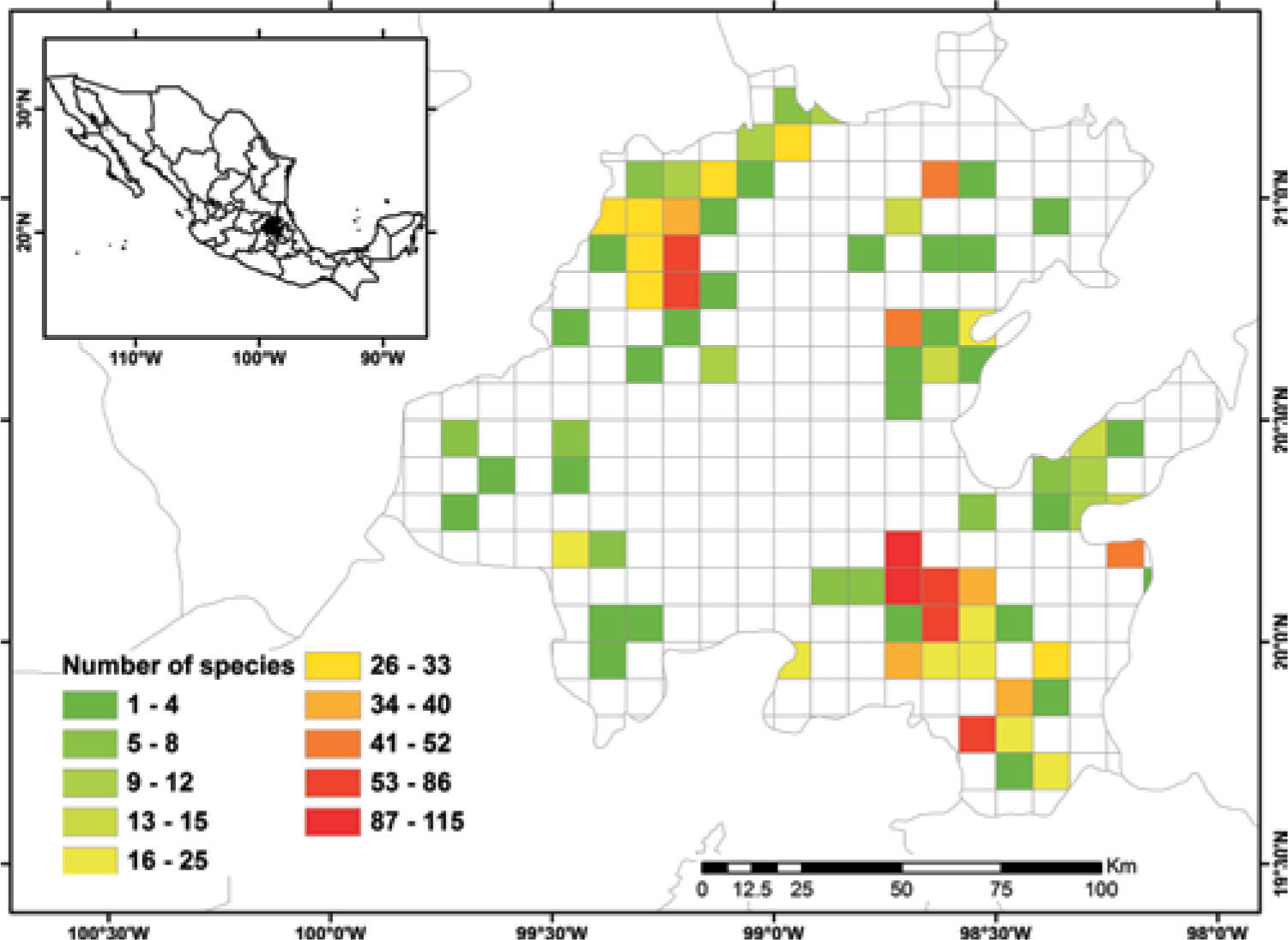
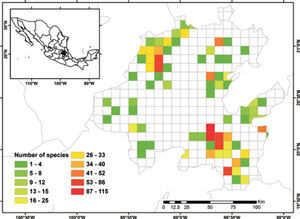
Known species richnessThe collecting data for 3 068 records were placed in the 5-minute cell network to identify the number of species per geographic unit and to produce a known species richness map (Fig. 2).
Species distribution modelingTwenty variables were thought to have potential predictive value for the distribution of plant species. Nineteen bioclimatic variables were obtained from the WorldClim website (Hijmans et al., 2005; http://www.worldclim.org/bioclim.htm) while the values of the twentieth, altitude, were obtained from an elevation digital model in the WorldClim website; spatial resolution was 1km2 for all variables.
Maxent was used to prepare models of potential distribution of 150 species (Phillips et al., 2006; Phillips and Dudik, 2008) each with at least five collecting records that were considered sufficient to obtain a reliable model. With Maxent’s predetermined configuration, 75% of the records were used in model’s training and the remaining 25% for the validation of the model. Models generated by Maxent show a logistic probability of 0.000 to 1.000 that may be transformed in presence-absence Boolean area maps by applying thresholds, i.e., all pixel values higher than the selected threshold are classified as “1” while a “0” value is given to the remaining pixels. The optimum threshold value has not been adequately established in Maxent (Phillips et al., 2006), however, in this contribution the threshold used was the logistic value equivalent to a 10% omission error to maintain a high proportion of correctly predicted presence records, as applied in Pearson et al. (2007), Suarez-Seoane et al. (2008), and Kumar and Stohlgren (2009).
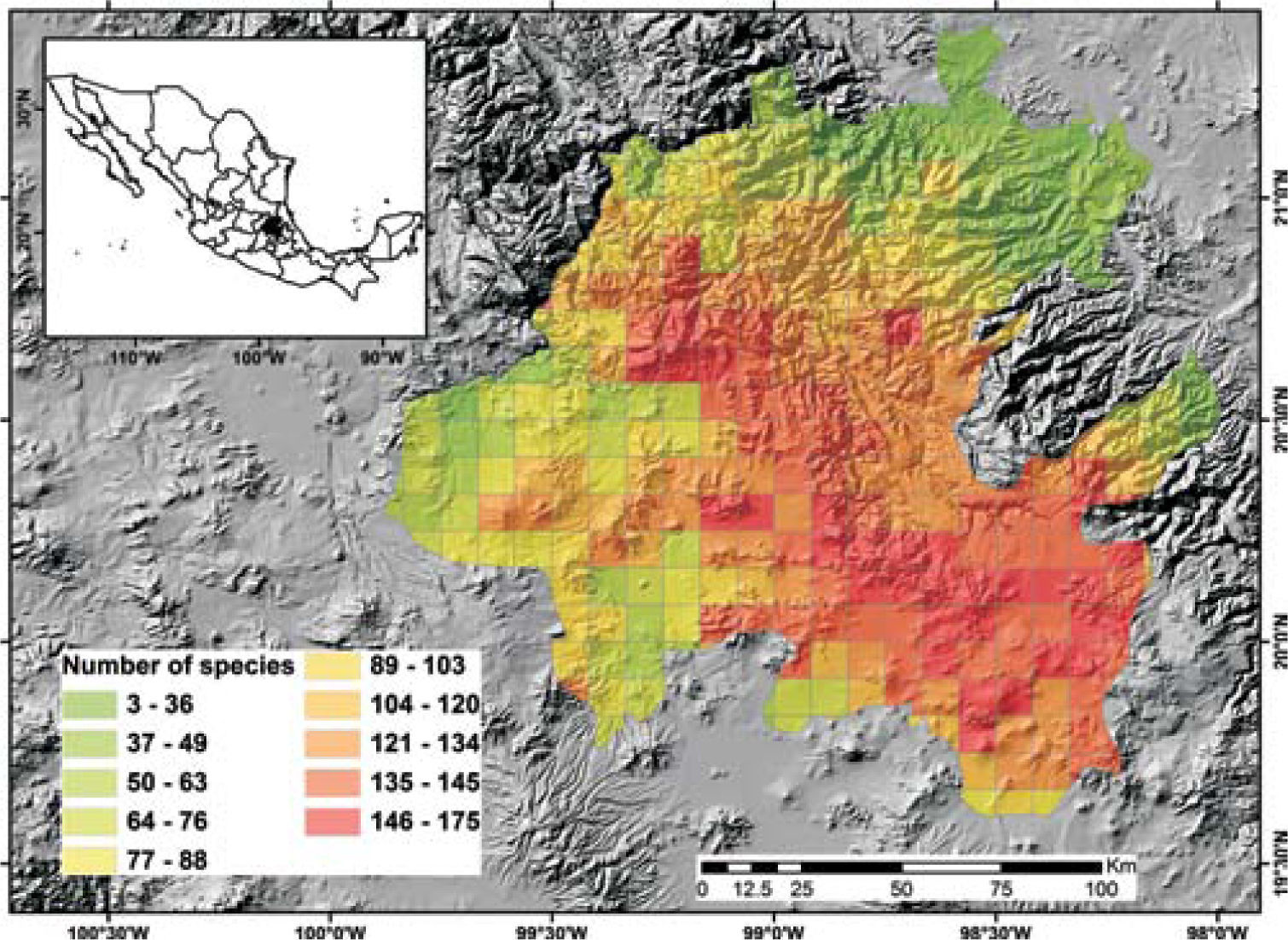
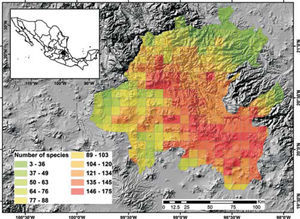
Potential richnessDistribution models for 150 species were placed in the 5-minute cell network to indicate the cells occupied by each species. A new richness map was produced with the models thus prepared and the records for 205 species without models supplemented the richness data for each cell. This information was used to prepare a map of potential richness (Fig. 3).
ResultsHerbarium and literature records produced a list of 420 species for the state of Hidalgo (Tables 1, 2). The species accumulation curve suggests that the moss flora of the state of Hidalgo should contain about 476 species, as indicated by the asymptote (Fig. 1). Since the presence of 355 species has been documented, the level of completeness of the flora under study is 74.5%. However, computation did not consider the varieties recognized for several species so that the actual number of taxa would be 371 (Table 1). Sharp et al. (1994) recorded 49 additional taxa (Table 2) that were represented in herbarium collections elsewhere in the world and should be expected to complement holdings in MEXU. This means that there are still 56 taxa lacking to fulfill the model prediction. The 420 moss taxa known from the state (Tables 1, 2) represent a high floristic number for the area. To be sure, published data indicate that such neighboring states as Guanajuato harbor 114 moss species and varieties (Delgadillo and Cárdenas, 1996), México 268 (Sharp et al., 1994), and Querétaro 212 (Herrera et al., 2008); it seems that a higher number may be found once the central dry lands and other forested areas are explored.
Moss taxa (371) in the state of Hidalgo, supported by herbarium specimens at MEXU. Neovolcanic Belt (NVB) and Eastern Sierra Madre (ESM) species are indicated by an X
| Taxon/Element | NVB | ESM |
| Northern | ||
| Anomobryum filiforme var. concinnatum (Spruce) Boul. | X | |
| Anomodon attenuatus (Hedw.) Huebener | X | |
| Anomodon rostratus (Hedw.) Schimp. | X | |
| Anomodon thraustus Müll. Hal. | X | |
| Atrichum angustatum (Brid.) Bruch and Schimp. | X | X |
| Barbula indica (Hook.) Spreng. | X | |
| Bryum erythroloma (Kindb.) Syed | X | |
| Campyliadelphus chrysophyllus (Brid.) Kanda | X | |
| Campylophyllum sommerfeltii (Myr.) Hedenäs | X | X |
| Campylopus fragilis (Brid.) Bruch and Schimp. | X | |
| Ceratodon purpureus subsp. stenocarpus (Bruch and Schimp.) Dixon | X | X |
| Claopodium pellucinerve (Mitt.) Best | X | |
| Dicranella varia (Hedw.) Schimp. | X | |
| Dicranoweisia cirrata (Hedw.) Lindb. | X | |
| Dicranum flagellare Hedw. | X | X |
| Diphyscium foliosum (Hedw.) Mohr | X | |
| Drepanocladus sordidus (Müll. Hal.) Hedenäs in W. R. Buck | X | |
| Encalypta ciliata Hedw. | X | |
| Entodon schleicheri (Schimp.) Demeter | X | |
| Fissidens dubius P. Beauv. | X | |
| Grimmia pilifera P. Beauv. | X | X |
| Haplocladium angustifolium (Hampe and Müll. Hal.) Broth. | X | X |
| Haplocladium microphyllum (Hedw.) Broth. | X | X |
| Heterophyllium affine (Hook. ex Kunth) M. Fleisch. | X | X |
| Hygroamblystegium fluviatile (Hedw.) Loeske | X | |
| Isopterygium tenerum (Sw.) Mitt. | X | |
| Kindbergia praelonga (Hedw.) Ochyra | X | |
| Mnium marginatum (With.) P. Beauv. | X | |
| Molendoa sendtneriana (Bruch and Schimp.) Limpr. | X | |
| Orthodontium gracile Schwägr. ex B.S.G. | X | |
| Oxyrrhynchium pringlei (Cardot) J. T. Wynns | X | |
| Plagiomnium cuspidatum (Hedw.) T. Kop. | X | |
| Platygyrium fuscoluteum Cardot | X | X |
| Pylaisia polyantha (Hedw.) Schimp. | X | X |
| Pylaisia selwynii Kindb. | X | |
| Rhodobryum roseum (Hedw.) Limpr. | X | X |
| Rhynchostegium pulchellum (Hedw.) H. Rob. | X | X |
| Rhynchostegium riparioides (Hedw.) Cardot | X | X |
| Rhynchostegium serrulatum (Hedw.) A. Jaeger | X | X |
| Rhytidium rugosum (Hedw.) Kindb. | X | X |
| Sematophyllum marylandicum (Müll. Hal.) E. Britton | X | |
| Syntrichia fragilis (Taylor) Ochyra | X | X |
| Syntrichia ruralis (Hedw.) Web. and Mohr | X | |
| Taxiphyllum deplanatum (Bruch and Schimp. ex Sull.) M. Fleisch. | X | |
| Thuidium delicatulum (Hedw.) Schimp. var. delicatulum | X | X |
| Thuidium delicatulum var. radicans (Kindb.) Crum, Steere and Anders. | X | X |
| Timmia megapolitana Hedw. var. bavarica (Hessl.) Brid. | X | |
| Tortella tortuosa (Hedw.) Limpr. | X | X |
| Tortula acaulon (With.) R. H. Zander | X | |
| Trichostomum crispulum Bruch | X | X |
| Trichostomum tenuirostre (Hook. and Taylor) Lindb. | X | X |
| Weissia condensa (Voit) Lindb. | X | |
| Zygodon viridissimus (Dicks.) Brid. | X | |
| Meso-American | ||
| Aloina hamulus (Müll. Hal.) Broth. | X | X |
| Aloinella catenula Cardot | X | |
| Anomobryum plicatum Cardot | X | |
| Atractylocarpus flagellaceus (Müll. Hal.) Williams | X | X |
| Atrichum oerstedianum (Müll. Hal.) Mitt. | X | X |
| Bartramia potosica Mont. | X | |
| Brachymenium spirifolium (Müll. Hal.) A. Jaeger | X | |
| Brachythecium cirriphylloides McFarland | X | X |
| Brachythecium conostomum (Taylor) A. Jaeger | X | |
| Brachythecium occidentale (Hampe) A. Jaeger | X | X |
| Braunia andrieuxii Lorentz | X | |
| Braunia squarrulosa (Hampe) Müll. Hal. | X | X |
| Bryoerythrophyllum recurvirostrum (Hedw.) Chen var. aeneum | X | X |
| Bryum chryseum Mitt. | X | |
| Bryum procerum Schimp. | X | X |
| Bryum richardsii Sharp | X | X |
| Campylopus anderssonii (Müll. Hal.) A. Jaeger | X | X |
| Campylopus reflexisetus (Müll. Hal.) Broth. | X | |
| Catagonium brevicaudatum Broth. | X | |
| Cryphaea apiculata Schimp. | X | |
| Cyclodictyon erubescens E. B. Bartram | X | |
| Cyclodictyon humectatum Cardot | X | |
| Cyrto-hypnum mexicanum (Mitt.) W. R. Buck and H. A. Crum | X | |
| Dicranum frigidum Müll. Hal. | X | X |
| Didymodon hampei R. H. Zander | X | |
| Didymodon rigidulus var. subulatus(Thér. and E. B. Bartram) R. H. Zander | X | X |
| Entodon jamesonii (Taylor) Mitt. | X | X |
| Epipterygium immarginatum Mitt. | X | |
| Fissidens excurrentinervis Williams | X | |
| Flowersia campylopus (Schimp. ex Müll. Hal.) Griffin and W. R. Buck | X | X |
| Globulinella globifera (Hampe) Steere ex Steere and Chapm. | X | |
| Herzogiella cylindricarpa (Cardot) Iwats. | X | |
| Horridohypnum mexicanum (Thér.) W. R. Buck | X | X |
| Leptodontium viticulosoides (P. Beauv.) Wijk and Margad. var. exasperatum(Cardot) R. H. Zander | X | X |
| Leskea angustata Taylor | X | X |
| Leucodon cryptotheca Hampe | X | X |
| Leucodon curvirostris Hampe | X | X |
| Lindbergia mexicana (Besch.) Cardot | X | X |
| Macromitrium fragilicuspis Cardot | X | |
| Meteorium teres Mitt. | X | |
| Mironia ehrenbergiana (Müll. Hal.) R. H. Zander | X | |
| Mironia stenotheca (Thér.) R. H. Zander | X | |
| Neckera chlorocaulis Müll. Hal. | X | X |
| Neckera ehrenbergii Müll. Hal. | X | X |
| Orthostichella pachygastrella (Müll. Hal. ex Aongstr.) B. H. Allen and Magill | X | |
| Orthotrichum bartramii Williams | X | |
| Orthotrichum pycnophyllum Schimp. ex Müll. Hal. | X | X |
| Orthotrichum pycnophyllum var. verrucosum (Müll. Hal.) Lewinsky | X | X |
| Physcomitrium subsphaericum Schimp. | X | |
| Platygyriella pringlei (Cardot) W. R. Buck | X | |
| Pohlia oerstediana (Müll. Hal.) Shaw | X | |
| Polytrichastrum tenellum (Müll. Hal.) G. Smith | X | |
| Pterobryopsis mexicana (Renauld and Cardot) M. Fleisch. | X | |
| Ptychomitrium serratum Bruch and Schimp. | X | X |
| Rauiella lagoensis (Hampe) W. R. Buck | X | |
| Rhexophyllum subnigrum (Mitt.) Hilp. | X | X |
| Rhynchostegium semiscabrum (E. B. Bartram) H. Rob. | X | |
| Rhynchostegium subrusciforme (Müll. Hal.) A. Jaeger | X | |
| Rozea andrieuxii (Müll. Hal.) Besch. Var. andrieuxii | X | X |
| Rozea andrieuxii var. bourgeana (Besch.) W. R. Buck | X | X |
| Sagenotortula quitoensis (Taylor in Hook.) R. H. Zander | X | |
| Schizymenium landii (Cardot) Shaw | X | |
| Schizymenium serratum (Cardot and Herz.) Shaw | X | |
| Sphaerotheciella pachycarpa (Schimp. Ex Besch.) Manuel | X | |
| Sphaerotheciella pinnata (Schimp.) Manuel | X | X |
| Syntrichia amphidiacea (Müll. Hal.) R. H. Zander | X | X |
| Syntrichia obtusissima (Müll. Hal.) R. H. Zander | X | X |
| Thuidium delicatulum var. peruvianum (Mitt.) H. A. Crum | X | X |
| Zygodon ehrenbergii Müll. Hal. | X | X |
| Zygodon liebmannii Schimp. Ex Müll. Hal. | X | |
| Caribbean | ||
| Archidium donnellii Austin | X | |
| Atrichum polycarpum (Müll. Hal.) Mitt. | X | X |
| Bartramia brevifolia Brid. | X | |
| Brachymenium mexicanum Mont. | X | X |
| Breutelia brittoniae Renauld and Cardot | X | |
| Breutelia inclinata (Hampe and Lorentz) A. Jaeger | X | |
| Breutelia subarcuata (Müll. Hal.) Schimp. | X | |
| Bryum limbatum Müll. Hal. | X | |
| Bryum pseudocapillare Besch. | X | |
| Campylopus albidovirens Herz. | X | |
| Campylopus tallulensis Sull. and Lesq. | X | X |
| Caribaeohypnum polypterum (Mitt.) Ando and Hig. | X | |
| Chryso-hypnum salleanum (Besch.) W. R. Buck | X | |
| Cryphaea patens Hornsch. | X | X |
| Ctenidium malacodes Mitt. | X | X |
| Chryso-hypnum diminutivum (Hampe) W. R. Buck | X | |
| Daltonia longifolia Taylor | X | |
| Dicranum sumichrastii Duby | X | X |
| Ditrichum rufescens (Hampe) Hampe | X | |
| Entodon beyrichii (Schwägr.) Müll. Hal. | X | X |
| Entodon hampeanus Müll. Hal. | X | |
| Entosthodon obtusifolius Hook. F. in Hook. | X | X |
| Erythrodontium longisetum (Hook.) Paris | X | |
| Fabronia ciliaris var. polycarpa (Hook.) W. R. Buck | X | X |
| Fabronia ciliaris var. wrightii (Sull.) W. R. Buck | X | X |
| Fabronia macroblepharis Schwägr. | X | |
| Fissidens crispus Mont. | X | X |
| Fissidens elegans Brid. | X | |
| Fissidens polypodioides Hedw. | X | |
| Holomitrium arboreum Mitt. | X | |
| Homalia glabella (Hedw.) Schimp. | X | |
| Hypnum amabile (Mitt.) Hampe | X | X |
| Isodrepanium lentulum (Wils.) Britt. | X | |
| Leptodontium viticulosoides var. sulphureum (Müll. Hal.) R. H. Zander | X | X |
| Leucobryum albidum (Brid. ex P. Beauv.) Lindb. | X | X |
| Leucobryum antillarum Schimp. | X | |
| Leucobryum polakowskii (Müll. Hal.) Cardot | X | |
| Leucodon julaceus (Hedw.) Sull. | X | |
| Leucoloma serrulatum Brid. | X | |
| Macromitrium cirrosum (Hedw.) Brid. | X | X |
| Macromitrium guatemaliense Müll. Hal. | X | |
| Macromitrium longifolium (Hook.) Brid. | X | X |
| Meteoridium remotifolium (Müll. Hal.) Man. | X | |
| Meteorium illecebrum Sull. | X | X |
| Microcampylopus curvisetus (Hampe) Giese and J.-P. Frahm | X | |
| Neckera urnigera Müll. Hal. | X | |
| Papillaria deppei (Hornsch. ex Müll. Hal.) A. Jaeger | X | |
| Papillaria imponderosa (Taylor) Broth. | X | |
| Philonotis longiseta (Mx.) E. Britton | X | |
| Philonotis sphaericarpa (Hedw.) Brid. | X | |
| Phyllogonium fulgens (Hedw.) Brid. | X | |
| Pilopogon guadalupensis (Brid.) J.-P. Frahm | X | |
| Pilotrichella mauiensis (Sull.) A. Jaeger | X | |
| Pireella pohlii (Schwägr.) Cardot | X | |
| Plagiothecium drepanophyllum Renauld and Cardot | X | |
| Pleuridium mexicanum Cardot | X | |
| Pogonatum campylocarpum (Müll. Hal.) Mitt. | X | X |
| Pogonatum tortile (Sw.) Brid. | X | |
| Pohlia richardsii Shaw | X | |
| Porotrichum korthalsianum (Dozy and Molk.) Mitt. | X | X |
| Porotrichum longirostre (Hook.) Mitt. | X | X |
| Porotrichum mutabile Hampe | X | |
| Pseudosymblepharis schimperiana (Paris) H. A. Crum | X | X |
| Pterobryon densum Hornsch. | X | X |
| Ptychomitrium lepidomitrium (Müll. Hal.) Schimp. in Besch. | X | X |
| Rhodobryum beyrichianum (Hornsch.) Müll. Hal. ex Hampe | X | X |
| Rhynchostegiopsis flexuosa (Sull.) Müll. Hal. | X | |
| Rhynchostegium scariosum (Taylor) A. Jaeger | X | |
| Rigodium toxarion (Schwägr.) A. Jaeger | X | |
| Schlotheimia jamesonii (Arnott) Brid. | X | |
| Schlotheimia rugifolia (Hook.) Schwägr. | X | |
| Sematophyllum cuspidiferum Mitt. | X | |
| Sematophyllum swartzii (Schwägr.) Welch and H. A. Crum | X | X |
| Sphagnum meridense (Hampe) Müll. Hal. | X | |
| Splachnobryum obtusum (Brid.) Müll. Hal. | X | |
| Squamidium nigricans (Hook.) Broth. | X | |
| Syrrhopodon prolifer Schwägr. | X | |
| Thuidium tomentosum Schimp. | X | X |
| Weissia jamaicensis (Mitt.) Grout | X | |
| Zelometeorium patulum (Hedw.) Manuel | X | |
| Zygodon campylophyllus Müll. Hal. | X | X |
| Southern | ||
| Braunia plicata (Mitt.) A. Jaeg. | X | X |
| Bryum microimbricatum Ochi | X | |
| Bryum radiculosum Brid. | X | |
| Campylopus heterostachys (Hampe) A. Jaeger | X | |
| Erpodium beccarii Müll. Hal. ex Vent. | X | |
| Leptodontium capituligerum Müll. Hal. | X | X |
| Orthotrichum aequatoreum Mitt. | X | X |
| Rhacocarpus purpurascens (Brid.) Paris | X | |
| Wide distribution | ||
| Aloina rigida (Hedw.) Limpr. | X | |
| Amphidium tortuosum (Hornsch.) Cufodontis | X | |
| Anacolia laevisphaera (Taylor) Flowers | X | X |
| Andreaea rupestris Hedw. | X | |
| Anoectangium aestivum (Hedw.) Mitt. | X | X |
| Anomobryum filiforme (Dicks.) Solms. in Rabenh. var. filiforme | X | |
| Anomodon tristis (Ces.) Sull. and Lesq. | X | |
| Aongstroemia orientalis Mitt. | X | X |
| Barbella pendula (Sull.) M. Fleisch. | X | |
| Barbellopsis trichophora (Mont.) W. R. Buck | X | |
| Barbula arcuata Griff. | X | |
| Barbula bolleana (Müll. Hal.) Broth. | X | X |
| Barbula convoluta Hedw. | X | |
| Brachymenium exile (Dozy and Molk.) Bosch and Sande Lac. | X | X |
| Brachymenium systylium (Müll. Hal.) A. Jaeger | X | X |
| Brachymitrion jamesonii Taylor | X | X |
| Brachythecium plumosum (Hedw.) Schimp. | X | X |
| Brachythecium ruderale (Brid.) W. R. Buck | X | X |
| Braunia secunda (Hook.) Bruch and Schimp. | X | X |
| Breutelia tomentosa (Brid.) A. Jaeg. and Sauerb. | X | |
| Bryoerythrophyllum campylocarpum (Müll. Hal.) H. A. Crum | X | X |
| Bryoerythrophyllum inaequalifolium (Taylor) R. H. Zander | X | |
| Bryoerythrophyllum recurvirostrum var. recurvirostrum | X | |
| Bryum argenteum Hedw. | X | X |
| Bryum billarderi Schwägr. | X | X |
| Bryum capillare Hedw. | X | X |
| Bryum muhlenbeckii Bruch and Schimp. | X | |
| Bryum pallescens Schleich. ex Schwägr. | X | |
| Bryum pseudotriquetrum (Hedw.) Gaertn., Meyer and Scherb. | X | |
| Bryum subapiculatum Hampe | X | |
| Campylopus flexuosus (Hedw.) Brid. | X | |
| Campylopus nivalis (Brid.) Brid. | X | X |
| Campylopus pilifer Brid. | X | X |
| Campylopus savannarum (Müll. Hal.) Mitt. | X | |
| Campylopus sinensis (Müll. Hal.) J.-P. Frahm | X | |
| Ceratodon purpureus (Hedw.) Brid. subsp. purpureus | X | X |
| Crossidium crassinervium (De Not.) Jur. | X | |
| Cryphaea jamesonii Taylor | X | |
| Cyrto-hypnum minutulum (Hedw.) W. R. Buck and H. A. Crum | X | |
| Desmatodon convolutus (Brid.) Grout | X | X |
| Didymodon australasiae (Hook. and Grev.) R. H. Zander | X | X |
| Didymodon ferrugineus (Schimp. ex Besch.) M.O. Hill | X | |
| Didymodon revolutus (Cardot) Williams | X | X |
| Didymodon rigidulus Hedw. var. gracilis (Schleich. ex Hook. and Grev.) R. H. Zander | X | X |
| Didymodon rigidulus var. icmadophilus(Schimp. ex Müll. Hal.) R. H. Zander | X | X |
| Didymodon rigidulus var. rigidulus | X | X |
| Didymodon vinealis (Brid.) R. H. Zander | X | |
| Distichium capillaceum (Hedw.) Bruch and Schimp. | X | |
| Drepanocladus aduncus (Hedw.) Warnst. | X | |
| Entodon macropodus (Hedw.) Müll. Hal. | X | |
| Entosthodon muhlenbergii (Turner) Fife | X | |
| Eustichia longirostris (Brid.) Brid. | X | |
| Fabronia ciliaris (Brid.) Brid. var. ciliaris | X | X |
| Fissidens asplenioides Hedw. | X | X |
| Fissidens curvatus Hornsch. | X | |
| Fissidens pellucidus Hornsch. | X | |
| Fissidens submarginatus Bruch in Krauss | X | |
| Fissidens taxifolius Hedw. | X | |
| Fissidens weirii Mitt. var. hemicraspedophyllus(Cardot) Pursell | X | |
| Forsstroemia producta (Hornsch.) Paris | X | X |
| Forsstroemia trichomitria (Hedw.) Lindb. | X | |
| Funaria hygrometrica Hedw. var. calvescens(Schwägr.) Mont. | X | X |
| Funaria hygrometrica var. hygrometrica | X | X |
| Grimmia longirostris Hook. | X | |
| Grimmia ovalis (Hedw.) Lindb. | X | |
| Grimmia trichophylla Grev. | X | |
| Groutiella tomentosa (Hornsch.) Wijk and Margad. | X | |
| Gymnostomum aeruginosum Sm. | X | X |
| Hedwigia ciliata (Hedw.) P. Beauv. | X | X |
| Hedwigidium integrifolium (P. Beauv.) Dixon | X | |
| Henicodium geniculatum (Mitt.) W. R. Buck | X | |
| Herpetineuron toccoae (Sull. and Lesq.) Cardot | X | |
| Homaliodendron flabellatum (Sm.) M. Fleisch. | X | |
| Hookeria acutifolia Hook. and Grev. | X | |
| Hymenostylium recurvirostrum (Hedw.) Dixon | X | |
| Hyophila involuta (Hook.) A. Jaeger | X | X |
| Hypnum cupressiforme Hedw. var. cupressiforme | X | X |
| Hypnum cupressiforme var. lacunosum Brid. | X | X |
| Hypnum revolutum (Mitt.) Lindb. | X | |
| Hypopterygium tamarisci (Sw.) Brid. ex Müll. Hal. | X | |
| Leptobryum pyriforme (Hedw.) Wilson | X | |
| Leptodictyum riparium (Hedw.) Warnst. | X | |
| Leptodontium brachyphyllum Broth. and Thér. | X | |
| Leptodontium flexifolium (Dicks. ex With.) Hampe | X | X |
| Leptodontium viticulosoides var. viticulosoides | X | X |
| Leptohymenium tenue (Hook.) Schwägr. | X | |
| Lescuraea arizonae (R.S. Williams) P.S. Wilson and D.H. Norris | X | X |
| Macrocoma orthotrichoides (Raddi) Wijk and Margad. | X | X |
| Macrocoma tenuis (Hook. and Grev.) Vitt subsp. sullivantii | X | X |
| Microbryum starkeanum (Hedw.) R. H. Zander | X | |
| Mittenothamnium reptans (Hedw.) Cardot | X | X |
| Orthostichella rigida (Müll. Hal.) B. H. Allen and Magill | X | X |
| Orthostichella versicolor (Müll. Hal.) B. H. Allen and Magill | X | |
| Orthotrichum anomalum Hedw. | X | |
| Orthotrichum diaphanum Schrad. ex Brid. | X | |
| Palamocladium leskeoides (Hook.) E. Britton | X | X |
| Papillaria nigrescens (Sw. ex Hedw.) A. Jaeger | X | X |
| Philonotis fontana (Hedw.) Brid. | X | |
| Philonotis glaucescens (Hornsch.) Broth. | X | |
| Philonotis marchica (Hedw.) Brid. | X | |
| Pilotrichella flexilis (Hedw.) Aongstr. | X | X |
| Plagiomnium rhynchophorum (Hook.) T. Kop. | X | X |
| Platygyriella densa (Hook.) W. R. Buck | X | |
| Pleuridium acuminatum Lindb. | X | |
| Pleurochaete squarrosa (Brid.) Lindb. | X | X |
| Pogonatum oligodus (Müll. Hal.) Mitt. | X | |
| Pohlia cruda (Hedw.) Lindb. | X | |
| Pohlia elongata Hedw. | X | X |
| Polytrichum commune L. ex Hedw. | X | |
| Polytrichum juniperinum Hedw. | X | X |
| Porotrichum usagarum Mitt. | X | |
| Prionodon densus (Hedw.) Müll. Hal. | X | X |
| Pseudocrossidium crinitum (Schultz) R. H. Zander | X | |
| Pseudocrossidium replicatum (Taylor) R. H. Zander | X | X |
| Pylaisia falcata Schimp. | X | X |
| Pylaisiadelpha tenuirostris (Bruch and Schimp.) W. R. Buck | X | X |
| Pyrrhobryum spiniforme (Hedw.) Mitt. | X | |
| Racomitrium subsecundum (Hook. and Grev. ex Harv.) Mitt. | X | |
| Racopilum tomentosum (Hedw.) Brid. | X | X |
| Rhabdoweisia fugax (Hedw.) Bruch and Schimp. | X | X |
| Rhodobryum huillense (Welw. and Duby) Touw | X | X |
| Schistidium apocarpum (Hedw.) Bruch and Schimp. | X | |
| Schistidium rivulare (Brid.) Podp. | X | |
| Sematophyllum adnatum (Mx.) E. Britton | X | X |
| Sematophyllum galipense (Müll. Hal.) Mitt. | X | X |
| Sematophyllum subpinnatum (Brid.) E. Britton | X | X |
| Sematophyllum subsimplex (Hedw.) Mitt. | X | |
| Sphagnum palustre L. | X | X |
| Sphagnum strictum Sull. | X | |
| Stereophyllum radiculosum (Hook.) Mitt. | X | |
| Symblepharis vaginata (Hook.) Wijk and Margad. | X | |
| Syntrichia chisosa (Magill, Delgad. and L. R. Stark) R. H. Zander | X | |
| Syntrichia pagorum (Milde) Amann | X | |
| Syntrichia papillosa (Wilson) Jur. | X | |
| Taxiphyllum taxirameum (Mitt.) M. Fleisch. | X | |
| Timmiella anomala (Bruch and Schimp.) Limpr. | X | X |
| Tortella humilis (Hedw.) Jenn. | X | |
| Trachypus viridulus (Mitt.) Broth. | X | |
| Trematodon sp. | X | |
| Trichostomum brachydontium Bruch | X | X |
| Weissia controversa Hedw. | X | X |
| Zygodon obtusifolius Hook. | X | X |
| Zygodon reinwardtii (Hornsch.) Braun | X | |
| Endemic | ||
| Brachymenium saint-pierrei Thér. | X | |
| Didymodon incrassatolimbatus Cardot | X | |
| Entodon abbreviatus (Schimp.) A. Jaeger | X | X |
| Grimmia involucrata Cardot | X | |
| Grimmia pulla Cardot | X | |
| Hennediella heteroloma (Cardot) R. H. Zander var. eckeliae R. H. Zander | X | |
| Homomallium sharpii Ando and Higuchi | X | |
| Jaffueliobryum arsenei (Thér.) Thér. | X | |
| Neckera angustifolia Müll. Hal. | X | |
| Oreoweisia delgadilloi H. Rob. and F. D. Bowers | X | X |
| Pylaisiadelpha duellii H. A. Crum | X | |
| Synthetodontium pringlei Cardot | X | |
| Weissia semidiaphana (Thér.) R. H. Zander | X | |
| Chihuahuan | ||
| Entosthodon apiculatopilosus (Cardot) Fife | X | |
| Homomallium mexicanum Cardot | X | X |
| Weissia ligulifolia (E. B. Bartram) R. H. Zander | X |
Moss taxa listed in Sharp et al. (1994), not supported by specimens at MEXU. Endemic taxa are indicated by “+”
| Taxon |
| Acroporium longirostre (Brid.) W. R. Buck |
| Aerobryopsis martinicensis (Broth.) Spessard-Schued. |
| Anomobryum prostratum (Müll.Hal.) Besch. |
| Aulacomnium palustre (Hedw.) Schwägr. |
| Barbula orizabensis Müll.Hal. |
| Brachymenium radiculosum (Schwägr.) Hampe |
| Breutelia austroarcuata (Müll. Hal.) Paris |
| Breutelia jamaicensis (Mitt.) A. Jaeger |
| Bryoerythrophyllum recurvirostrum(Hedw.) Chen var. aeneum (Müll. Hal.) R. H. Zander |
| Bryum apiculatum Schwägr. |
| Bryum caespiticium Hedw. |
| Bryum leptotorquescens Müll.Hal.ex Broth. |
| Bryum microchaeton Hampe |
| Calyptothecium duplicatum (Schwägr.) Broth. |
| Chryso-hypnum salleanum (Besch.) W. R. Buck |
| Cryphaea filiformis (Hedw.) Brid. |
| Cyclodictyon albicans (Hedw.) Kuntze |
| Daltonia tenuifolia Mitt. |
| +Dicranella barnesii Cardot |
| Dicranella hilariana (Mont.) Mitt. |
| Dicranella lindigiana (Hampe) Mitt. |
| +Dicranum lophoneuron Müll. Hal. |
| Didymodon tophaceus (Brid.) Lisa |
| Didymodon umbrosus (Müll. Hal.) R. H. Zander |
| Entodon serrulatus Mitt. |
| Entosthodon bonplandii (Hook.) Mitt. |
| Epipterygium mexicanum (Besch.) Broth. |
| Fissidens weirii Mitt.var. weirii |
| Grimmia elongata Kaulf. |
| Helicodontium capillare (Hedw.) A. Jaeger |
| Heterocladium macounii Best |
| Hyophiladelphus agrarius (Hedw.) R. H. Zander |
| Luisierella barbula (Schwägr.) Steere |
| Macromitrium punctatum (Hook. and Grev.) Brid. |
| Neckeropsis undulata (Hedw.) Reichardt |
| Philonotis cernua (Wilson) Griffin and W. R. Buck |
| Philonotis uncinata (Schwägr.) Brid. |
| Pilotrichella nudiramulosa Müll.Hal. |
| Plaubelia sprengelii (Schwägr.) R. H. Zander var. stomatodonta (Cardot) R. H. Zander |
| Pohlia papillosa (Müll. Hal. ex Jaeger) Broth. |
| Pohlia pseudobarbula (Thér.) H. A. Crum ex A. J. Shaw |
| Porotrichodendron lindigii (Hampe) W. R. Buck |
| Pylaisiadelpha deplanatula (Cardot) W. R. Buck |
| Rhachithecium perpusillum (Thwaites and Mitt.) Broth. |
| Schoenobryum concavifolium (Griff.) Gangulee |
| Streptopogon cavifolius Mitt. |
| Streptopogon matudianus H. A. Crum |
| Syntrichia bogotensis (Hampe) Mitt. |
| Trachypodopsis serrulata (P. Beauv.) M. Fleisch. var. crispatula (Hook.) Zant. |
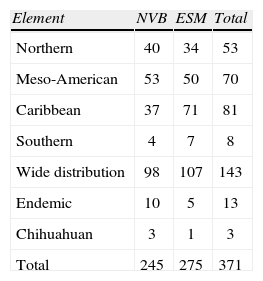
The list of species based on herbarium specimens (Table 1) also cites the taxa represented in the major mountain systems of the state, i.e., the Neovolcanic Belt with 245 taxa, and the Eastern Sierra Madre with 275. There are no strong differences in the number of taxa between mountain systems, but the Caribbean taxa are distinctly higher in the ESM (Table 3). Besides this, salient features of the moss flora include the large group of widely distributed and the Meso-American taxa. The endemic species, 12 in total, are not restricted to Hidalgo or to a topographic feature of the state and represent less than 3% of the entire moss flora. Because of their small number, it is also remarkable the presence of members of the Chihuahuan element.
Summary of number of species listed in Table 1. NVB=Neovolcanic Belt; ESM=Eastern Sierra Madre
| Element | NVB | ESM | Total |
| Northern | 40 | 34 | 53 |
| Meso-American | 53 | 50 | 70 |
| Caribbean | 37 | 71 | 81 |
| Southern | 4 | 7 | 8 |
| Wide distribution | 98 | 107 | 143 |
| Endemic | 10 | 5 | 13 |
| Chihuahuan | 3 | 1 | 3 |
| Total | 245 | 275 | 371 |
The species listed in table 2 may follow the same patterns of distribution as those listed in table 1, but should be added when their state distribution is confirmed.
Figure 2 shows the number of known species per cell in the state of Hidalgo. The distribution of cells with data also indicate the extent of field work thus far conducted; there are numerous collections from SE and NW areas, followed in order of importance, by the NE and SW areas. These were obtained along major highways and forested areas along the way. The empty cells in the map represent dry lands or scattered peaks, inaccessible areas, and major cities and industrial areas. The potential distribution map for 150 species (Fig. 3) suggests that many of the empty cells in figure 2 may contain rich moss floras, especially in central and southeastern parts of the state. However, potential species richness values become smaller toward the northeastern lowlands and, in the southwest, toward the lower areas of the Neovolcanic Belt.
DiscussionThe known moss flora of the state of Hidalgo is comparatively larger than that of adjacent states. Part of the size differences are undoubtedly due to insufficient bryological exploration in various states in central Mexico. The dry continental area north of the NVB and to the west of the ESM may indeed contain a reduced moss flora, but full diversity evaluations in Hidalgo require ample exploration in its central region. The size of the moss flora, with 420 species and varieties, is potentially more diverse as suggested in preceding paragraphs, but not as rich as that of Veracruz which includes more than 500 taxa (Delgadillo, 2011).
The presence of 2, NVB and ESM, groups of species of presumed different derivation might suggest a state flora with higher moss diversity or with peculiar geographical affiliation in the mountain areas. Neither hypothesis is confirmed by the results summarized in table 3; except for the values of the Caribbean element, the number of species in the NVB and in ESM is similar, perhaps due to the close proximity of the mountain ranges. The Caribbean species are an important constituent of the tropical floras of Mexico and these, along with the Meso-American and endemic taxa give a neotropical character to the flora of Hidalgo.
With respect to diversity, the number of species in Hidalgo is higher than in neighboring states, but no hotspots are readily identified with current data, although there are areas (e.g., between Zimapán and Jacala in the northwest, and in Sierra de Pachuca) where actual or potential species richness is higher (Figs. 2, 3). These areas, however, have been well collected and may not qualify for designation as hotspots.
The potential distribution map identifies areas of concentration of species; because of the habitat interrelationships between mosses and vascular plants, the map in figure 3 would be similar to the distribution of certain vascular plant communities. At present, it is unknown whether their distribution is comparable, but if field observations confirm this, mosses would be an additional criterion to justify conservation of diverse areas.