We explored the relationship between fragment area, topographic heterogeneity, and disturbance intensity with tree and shrub species diversity in seasonally dry oak forest remnants in the Mixteca Alta, Oaxaca, Mexico. The fragments are distributed in a matrix of eroded lands and crop fields, have a complex topography, and are disturbed by plant extraction and trail opening. Sampling was conducted in 12 fragments from 12-3 211 ha. Topographic heterogeneity was estimated by the fragment's standard deviation in slope-aspect, slope, and altitude. The density of stumps and roads were used as estimators of disturbance intensity. Fisher's α diversity ranked from 0.95 to 4.55 for the tree layer; and 2.99 to 8.51, for the shrub layer. A structural equation model showed that the diversity of woody plants increases with topographic heterogeneity and disturbance in the remnants. When these 2 variables were considered, diversity tended to decrease with fragment size probably because smaller fragments have a greater perimeter-to-area ratio and therefore proportionally offer more opportunities for pioneer species colonization. Indeed, the tree-to shrub-layer diversity ratio increased with fragment size. Conservation strategies in fragmented forests must consider the fragment's environmental heterogeneity, the disturbance type and intensity, and the species to be preserved.
Exploramos la relación entre el área, la heterogeneidad topográfica y el disturbio en remanentes de bosque de encino estacionales en la Mixteca Alta, Oaxaca, México. Una matriz de suelo erosionado y cultivos rodea los fragmentos, que están afectados por extracción vegetal y caminos y presentan topografía compleja. Muestreamos la vegetación en doce fragmentos de 12 a 3 211 ha. Estimamos la heterogeneidad ambiental con las desviaciones estándar en pendiente, orientación y altitud del fragmento, y la intensidad de disturbio, por la densidad de tocones y el área afectada por caminos. La diversidad α de Fisher varió entre 0.95 y 4.55 para el estrato arbóreo y 2.99 y 8.51 para el arbustivo. Un modelo de ecuaciones estructurales lineales mostró que la diversidad aumenta con la heterogeneidad topográfica y la perturbación humana. Al considerar estas dos variables, la diversidad disminuyó con el tamaño del fragmento probablemente porque proporcionalmente los fragmentos pequeños tienen mayor perímetro que los grandes y favorecen a las pioneras. La razón entre la diversidad del estrato arbóreo y el arbustivo aumentó con el tamaño del fragmento. Las estrategias de conservación en bosques fragmentados deben considerar la heterogeneidad ambiental, el disturbio y las especies que deben ser conservadas.
Fragmentation processes involve habitat losses and the splitting of the remaining habitat into pieces of various sizes and degrees of isolation (Laurance, 2008). Currently, a large part of the land surface is being affected by human activities, causing ecosystem fragmentation and jeopardizing biodiversity through habitat reduction, increased isolation, and alterations in biotic and abiotic factors in the remaining fragments (Saunders et al., 1991; Fahrig, 2003; Wade et al., 2003; Otálora et al., 2011). Several factors have been associated with biodiversity in fragmented landscapes. These include fragment size per se, based on the species-area theory (Arrhenius, 1921;Preston, 1962; MacArthur and Harris, 1984; Tjorve, 2003), environmental heterogeneity (Gaston, 2000; Tews et al., 2004; Clarke and Gaston, 2006), and disturbances, both of natural and anthropogenic origin (Bustamante and Grez, 1995; Williams-Linera et al., 2002; Wade et al., 2003; Davis, 2004).
A positive relationship between biodiversity and fragment or habitat area has been identified for nearly a century by the widely-known species-area relationship (Arrhenius, 1921; Preston, 1962; Bustamante and Grez, 1995). This relationship can be described in a probabilistic model following a geometric (Arrhenius, 1921) or logarithmic series (Preston, 1962), enabling the researcher to estimate the biodiversity of an ecosystem from a known area. Several theoretical and empirical studies in fragmented landscapes have found a close relationship between patch biodiversity and patch size (Hill and Curran, 2001, 2003; Echeverría et al., 2007; Pincheira-Ulbrich et al., 2008).
Although the species-area relationship is one of the main subjects in biogeography (Hill and Curran, 2001, 2003; Echeverría et al., 2007; Pincheira-Ulbricht et al., 2008; Blakely and Didham, 2010), it is not clear which mechanisms are at work. Numerous studies have suggested that environmental heterogeneity, which is usually positively correlated with area, is the main factor explaining biodiversity (Boecklen, 1986; Freemark and Merriam, 1986; Baz and García-Boyero, 1995; Brose 2001; Aström et al., 2007; Blakely and Didham, 2010). Indeed, heterogeneous environments offer greater diversity of niches for the establishment of different species (Boecklen, 1986; Baz and García-Boyero, 1995; Peterson et al., 1997; Tews et al., 2004; Hannus and von Numers, 2008). Complex topography is one of the most distinctive features of environmental heterogeneity in mountain ecosystems by altering soil depth, moisture content, stoniness, compaction, and permeability, among other environmental properties, thereby creating more niches per area than those occurring on a flat surface (Bunting, 1964; Balvanera and Aguirre, 2006; Aström et al., 2007).
Disturbance is another important factor affecting diversity. Disturbance has been defined as a more or less discrete event in time and space, altering the structure of populations, communities or ecosystems, causing drastic changes in resource availability or in the physical environment (Saunders et al., 1991; Bustamante and Grez, 1995; Laurance, 2004; di Bella et al., 2008), facilitating the spreading of short-lived early successional species (Saunders et al., 1991), and the invasion of exotic species that compete with native species for resources (Santos and Tellería, 2006; Stevenson and Rodriguez, 2008). Although disturbance is an important component of many ecosystems, there is no consensus on how it impacts biodiversity (Miller et al., 2011). Disturbance can be of natural origin, such as storms, telluric events, and tree falls, or anthropogenic, as is the case of human settlements, roads, deforestation, and fire. In this study we will focus on human disturbances, which often are difficult to measure directly, but can be estimated by their effects on the fragments of natural ecosystems. The occurrence of roads and stumps are signals of human disturbance (López, 2001; Williams-Linera et al., 2002; Herrera et al., 2004; Rudas et al., 2007), and they may modify the ecosystem structure and composition by: a) affecting microclimatic conditions (Gucinski et al., 2001); b) promoting the invasion of exotic species (Brown et al. 2004, 2006); c) allowing the uncontrolled extraction of natural products (Young, 1994; Verburg et al., 2004); d) setting up barriers between populations that may decrease gene flow and dispersal (Forman and Alexander, 1998), and e) reducing seed production (SEMARNAT, 2005; Alelign et al., 2007), all of which may jeopardize species persistence.
Disturbance is also related to fragment area and habitat heterogeneity. For instance, native species richness per unit area may decrease significantly in small-sized and highly disturbed fragments (Ross et al., 2002; Echeverría et al., 2007). Furthermore, road construction and deforestation induce habitat fragmentation, promote changes in the physical environment, and alter the biota balance (Saunders et al., 1991; Fahrig, 2003; Wade et al., 2003; Otálora et al., 2011). Environmental changes in fragmented communities are more dramatic at the edges than at the center of the fragments (Murcia, 1995; Laurance et al., 2000; Forero-Molina and Finegan, 2004). Some studies have shown that basal area significantly declines with decreasing patch size (Lezcano et al., 2004; Echeverría et al., 2007). The fragment species composition is also affected. The shrub layer diversity to tree layer diversity ratio could be an indicator of disturbance since these life forms usually have different environmental requirements. Shrubs, for instance, tend to have greater survivorship and biomass in open microsites (Asbjornsen et al., 2004b).
In summary, biodiversity may be directly or indirectly associated with fragment area, topographic heterogeneity, and disturbance intensity. For both conservation purposes and ecological studies, it is important to identify the major factors that influence biodiversity in remnant fragments. Few studies have explored simultaneously the role that each of the above factors plays on fragment diversity. Most of these studies have explored only fragment size and environmental heterogeneity (Freemark and Merriam, 1986; Baz and García-Boyero, 1995; Boecklen, 1986; Brose, 2001; Graham and Blake, 2001), but very few have included the effects of area and disturbance on biodiversity (Aström et al., 2007). We are only aware of one exploratory study that considers simultaneously the relationship between habitat size, environmental heterogeneity, and disturbance with diversity (Blakely and Didham, 2010). This study, carried out with insects, surprisingly found a negative relationship between biodiversity and habitat size under experimentally controlled conditions, due explicitly to experimental manipulation in which smaller habitats were modified to be more heterogeneous than larger habitats. This was accomplished by experimentally reversing resource concentration and enhancing drought disturbance, while holding constant colonization-extinction dynamics and habitat heterogeneity. The Mixteca Alta in southern Mexico provides a suitable landscape for exploring these relationships but in a natural habitat and with woody plants. This region is highly fragmented (Asbjornsen et al., 2004b; Martínez and Noriega, 2006), has a complex topography (González-Leyva, 2007), and has been affected by disturbances associated with the presence of nearby human settlements (Asbjornsen et al., 2004a). In this region, we aimed to explore the possible relationships between fragment area, topographic heterogeneity, and intensity of anthropogenic disturbance on fragment biodiversity of trees and shrubs. Based on the empirical evidence and theoretical studies described above, we expected (1) a positive relationship between fragment biodiversity and both area and topographic heterogeneity; and (2), a negative relationship between fragment biodiversity and disturbance intensity.
Materials and methodsStudy site and sampling design. The study site is located in the Nochixtlán District, Oaxaca, Mexico, at 17°0'-17°50' N, 97°0'-97°25' W, between 1 800 and 2 800m. The study area is mountainous, with a complex geology and topography. The climate is temperate and semi-humid. Annual rainfall varies between 500 and 800mm. A seasonally dry oak forest comprises most of the vegetation above 1 500m (Asbjornsen et al., 2004a). The main species are: Quercus liebmannii, Q. acutifolia, and Q. laurina. Endemic species are also relatively abundant (García-Mendoza et al., 1994). Paleontological evidence shows that the Mixteca Alta has been populated since the late Holocene by people who based their use of resources on a wise water management (Guerrero-Arenas et al., 2010). The Spanish conquest was accompanied by the introduction of sheep, goats, and diverse crops, causing an intense process of deforestation. After 500 years, deforestation has resulted in a highly fragmented landscape: 80% of its soils are affected by water erosion (González-Leyva, 2007; Guerrero-Arenas et al., 2010).
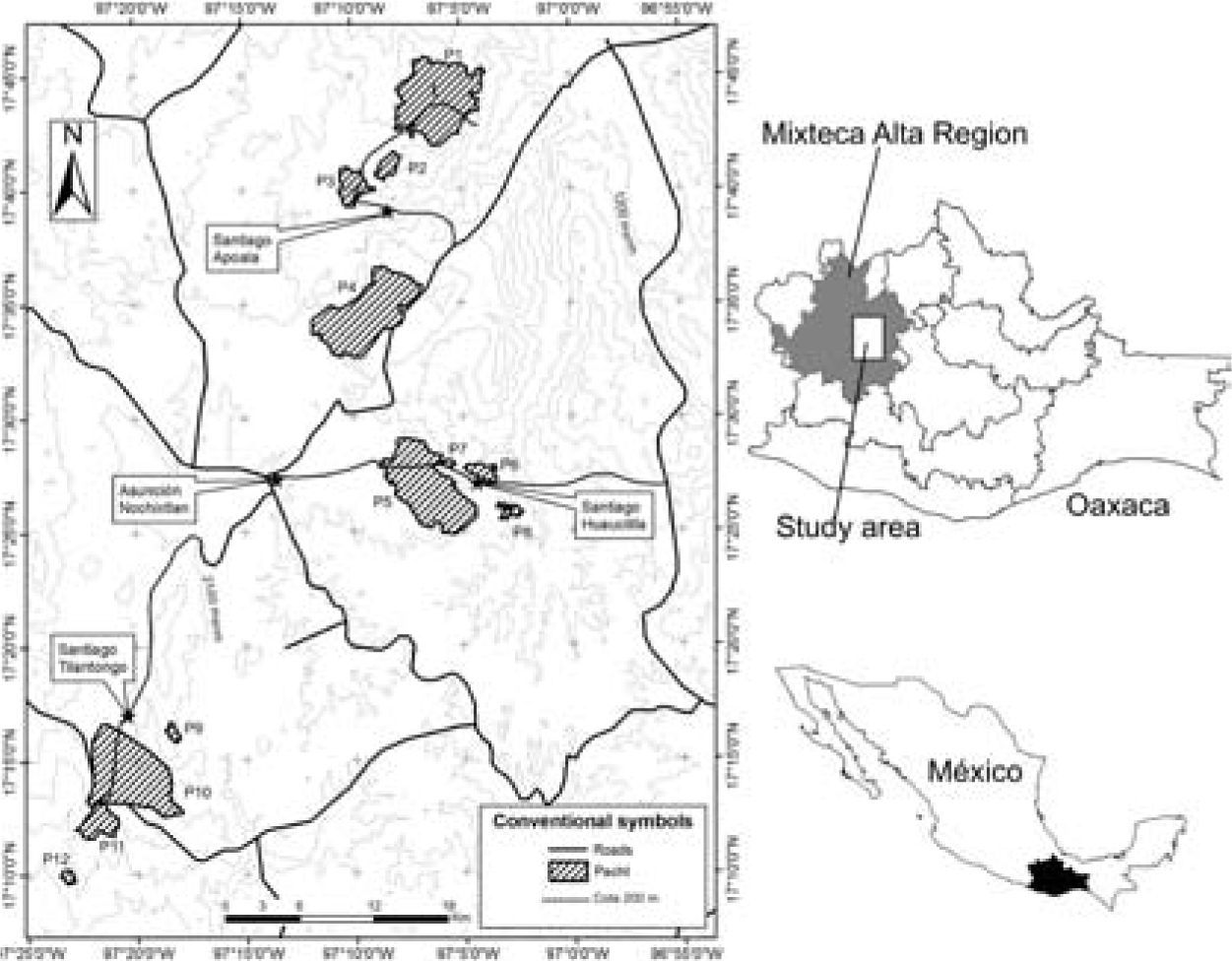
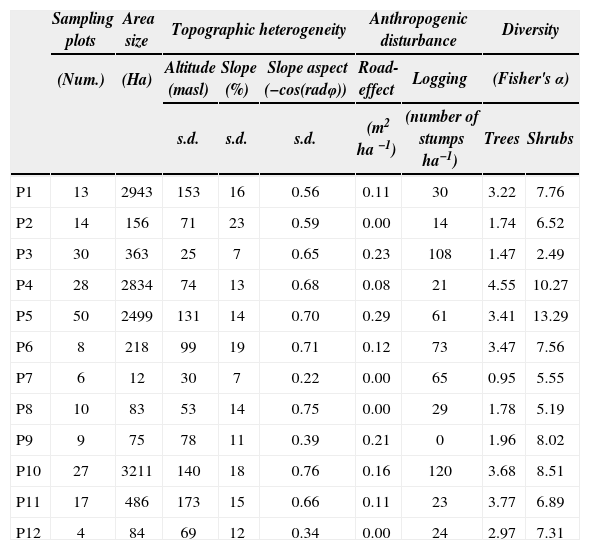
Data collection. We selected and characterized our study fragments using Landsat ETM+ satellite imagery (2005, path 24, Row 48, Band 4/7/1, pixel=30m). The geographic projection was UTM WGS84 14n zone. Geographic corrections were conducted with control points from digitalized 1: 50 000 road maps, and a second degree polynomial model (Cayuela et al., 2006b). Accuracy ranged from 0.25–0.45 pixels, corresponding to 7.5–13.5m. Atmospheric corrections were performed using the Chávez reflectivity model (Chuvieco, 2006; Cayuela et al., 2006b), which transforms the original digital numbers into reflectivity values in the corrected images. Elevation digital models were generated using ENVI 4.3 software. The topographic correction was performed using the Teillet et al. (1982) and Riaño et al. (2000) semi-empirical method and the PCI Geomatics software version 7.0. Classification was supervised with PCI Geomatics software version 7.0 using the maximum likelihood criterion. Six class signatures were obtained: 1) bare land, 2) water body, 3) grasslands-shrublands, 4) croplands, 5) urban areas, and 6) native forest. The obtained classification was checked with 300 independent control points located in the field with Google Earth software (image dates from 2004 to 2007). We haphazardly selected 12 seasonally dry oak forest fragments with contrasting areas ranging from 12 to 3 211 ha (Table 1). The area of the selected fragments was estimated using Fragstats (McGarigal and Marks, 1995). Sampling plots were randomly selected within each fragment using the extension Random Sites (Arc View 3.X, public domain), with the restriction that each sampling point should be located at least 70m from other sampling points or from the fragment edge to avoid overlapping and to decrease edge effects and the probability of autocorrelation between nearby sampling points (Fig. 1).
Environmental variables and diversity of trees and shrubs found in 12 fragments of seasonally dry oak forest at the Mixteca Alta of Oaxaca
| Sampling plots | Area size | Topographic heterogeneity | Anthropogenic disturbance | Diversity | |||||
|---|---|---|---|---|---|---|---|---|---|
| (Num.) | (Ha) | Altitude (masl) | Slope (%) | Slope aspect (−cos(radφ)) | Road-effect | Logging | (Fisher's α) | ||
| s.d. | s.d. | s.d. | (m2 ha −1) | (number of stumps ha−1) | Trees | Shrubs | |||
| P1 | 13 | 2943 | 153 | 16 | 0.56 | 0.11 | 30 | 3.22 | 7.76 |
| P2 | 14 | 156 | 71 | 23 | 0.59 | 0.00 | 14 | 1.74 | 6.52 |
| P3 | 30 | 363 | 25 | 7 | 0.65 | 0.23 | 108 | 1.47 | 2.49 |
| P4 | 28 | 2834 | 74 | 13 | 0.68 | 0.08 | 21 | 4.55 | 10.27 |
| P5 | 50 | 2499 | 131 | 14 | 0.70 | 0.29 | 61 | 3.41 | 13.29 |
| P6 | 8 | 218 | 99 | 19 | 0.71 | 0.12 | 73 | 3.47 | 7.56 |
| P7 | 6 | 12 | 30 | 7 | 0.22 | 0.00 | 65 | 0.95 | 5.55 |
| P8 | 10 | 83 | 53 | 14 | 0.75 | 0.00 | 29 | 1.78 | 5.19 |
| P9 | 9 | 75 | 78 | 11 | 0.39 | 0.21 | 0 | 1.96 | 8.02 |
| P10 | 27 | 3211 | 140 | 18 | 0.76 | 0.16 | 120 | 3.68 | 8.51 |
| P11 | 17 | 486 | 173 | 15 | 0.66 | 0.11 | 23 | 3.77 | 6.89 |
| P12 | 4 | 84 | 69 | 12 | 0.34 | 0.00 | 24 | 2.97 | 7.31 |
Study site in the Mixteca Alta, Oaxaca, Mexico, showing the seasonally dry oak forest fragments in which woody plants were sampled (see Materials and methods and Table 1).
Vegetation sampling was conducted on 216 plots distributed among the 12 selected fragments, using 4 to 50 sampling points per fragment, depending on fragment size. All plots were geo-referenced, using a GPS (GARMIN 60csx) with a 5m resolution. In order to analyze the diversity and structure of the vegetation, we sampled the individuals of both the tree and the shrub layer. All woody plants ≥ 2.5cm DBH and ≥ 2.5m height found in the sampling plots were included in the tree layer; whereas all woody plants < 2.5cm DBH or <2.5 m height were included in the shrub layer. Sampling plots of 102.06m2 and 12.56m2 were used for the tree and the shrub layer, respectively. Specimens of all species found in the sampling plots were deposited at the Herbarium OAX. The tree and shrub diversity per fragment was assessed using Fisher's α because it is relatively insensitive to sample size (Fisher et al., 1943; Magurran, 2004).
Topographic heterogeneity was evaluated by the standard deviation (sd) of altitude (m), slope (%), and slope-aspect (−cos φ, where φ is the slope angle in radians) of each fragment. Slope-aspect is defined as main compass direction that a slope faces (Physical Geography Dictionary, 2012). We used the Hawthtools extension of ArcGis to select randomly 50 points (pixel=30m), at least 70m apart. The selected points were overlapped on the slope, altitude, and slope-aspect layers from the digital elevation model to obtain the respective values for each point. Anthropogenic disturbance was estimated by assessing the intensity of logging and the proportion of the fragment area expected to be affected by roads or zone of influence of roads within each fragment. Logging intensity was evaluated by the density of stumps (ha−1) in each plot. Previous works have found that road effects on biodiversity depend on species, topography, and road type, but usually range between 100 and 200m on each side of the road (Forman et al., 1997). Based on these studies, we defined a buffer area of 150m width on both sides of the roads in the study area, to estimate the proportion of the fragment expected to be affected by roads. For this purpose, we used EPS data from Inegi (2011, scale 1:50 000). Road-effect zone was estimated as the ratio of the road buffer area to the total area of the fragment. In our study sites, all roads were of similar width, suggesting the same intensity of use.
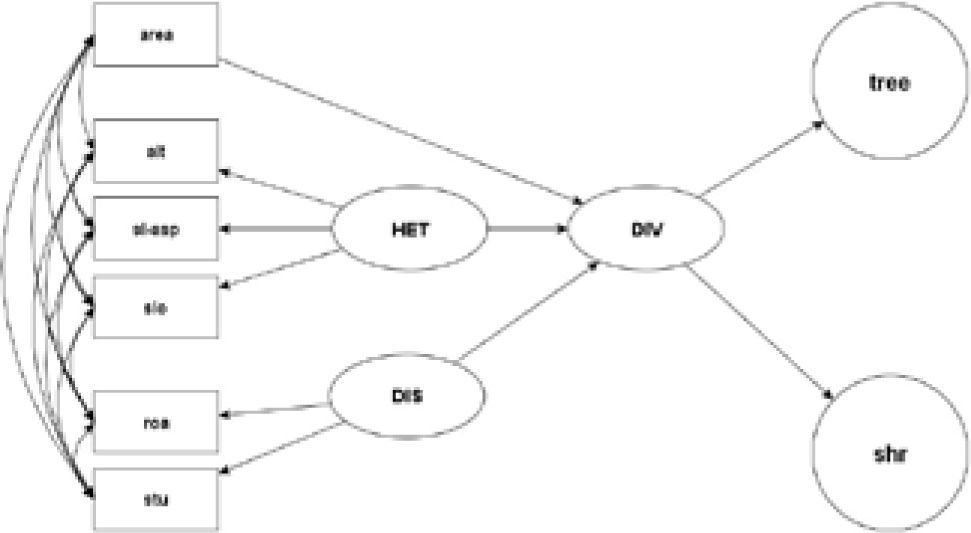
Data analysis. In order to disentangle the relationships between fragment area, topographic heterogeneity, anthropogenic disturbance, and woody plant species diversity, we developed a model based on structural equation modeling, using the CALIS procedure from the SAS 9.1 software package (SAS Institute, 1989). Since large fragments are probably both more heterogeneous and less disturbed than small fragments, it is important to explore to which extent biodiversity is directly affected by fragment area, topographic heterogeneity, and disturbance, or indirectly through the associations among these explanatory variables. Structural equation modeling (SEM) allows the testing of complex relationships among variables, partitioning direct and indirect effects, and making quantitative predictions about the relative contribution of each variable in the model (Grace and Pugesek, 1997). This method, based on covariance analysis, can be used to model multivariate relations and to test multivariate hypotheses (Bollen, 1989). An important attribute of structural equation modeling is that it allows the estimation of conceptual unmeasured (latent) variables based on a set of measurable (manifest) variables (Grace and Pugesek, 1997). The accepted models obtained from such analysis can indicate the role of different factors in a system and the strength of their relationships (Spitale et al., 2009). Further details of the SEM methodology can be found in Grace and Pugesek (1997), Spitale et al. (2009), Hayduk (1987) and Reed et al. (2009). In our model, biodiversity is the endogenous (response) latent variable, estimated by the tree and shrub diversity (manifest variables). Topographic heterogeneity, anthropogenic disturbance, and fragment area are our exogenous (explanatory) variables. Topographic heterogeneity is a latent variable estimated by the standard deviation of altitude, slope, and slope-aspect. Anthropogenic disturbance is a latent variable estimated by the exogenous manifest variables: road-effect zone, as defined above, and stump density (Fig. 2).
A priori structural equation model representing the possible effect of area, topographic heterogeneity, and disturbance on diversity of trees and shrubs in fragments of seasonally dry oak forest in the Mixteca Alta of Oaxaca. In rectangular shapes, we represent manifest variables (area= fragment area, alt= altitude standard deviation, sl-asp= slope-aspect standard deviation, slo= slope standard deviation, roa= road-effect zone, stu= stump density, tree= tree Fisher's α, shru= shrub Fisher's α), in oval shapes latent variables (HET= topographic heterogeneity, DIST= anthropogenic disturbance, DIV= fragment diversity). Single-headed arrows indicate one-way variance of the latent variable; double-headed arrows indicate covariance among manifest variables.
All the response and predictor variables were standardized (mean = 0, sd = 1) and did not show evidence of deviations from normality. Direct relations among variables (single-headed arrows in figures 2 and 4) were estimated as standardized coefficients from the covariance matrix. Non-directional standardized correlation coefficients were also calculated among explanatory manifest variables (double-headed arrows in figure 2). The initial structural model was reduced to the most parsimonious model by means of a stepwise specification search, eliminating in each step the path with the lowest coefficient (in absolute value) until all the remaining coefficient paths were significant (Hayduk, 1987; Grace and Pugesek, 1997; Reed et al., 2009; Blakely and Didham, 2010). The resulting model in each reduction step was checked by its goodness-of-fit index (GFI), its chi-square probability value (p), and its Akaike's information criterion (AIC) value (Hayduk, 1987). In each step, the fitted indices were compared against the previous model. The best model was the one with the GFI nearest to 0.9; the greatest p value, which should be >0.1, and the lowest AIC value (Mulaik et al., 1989; Stoelting, 2002).
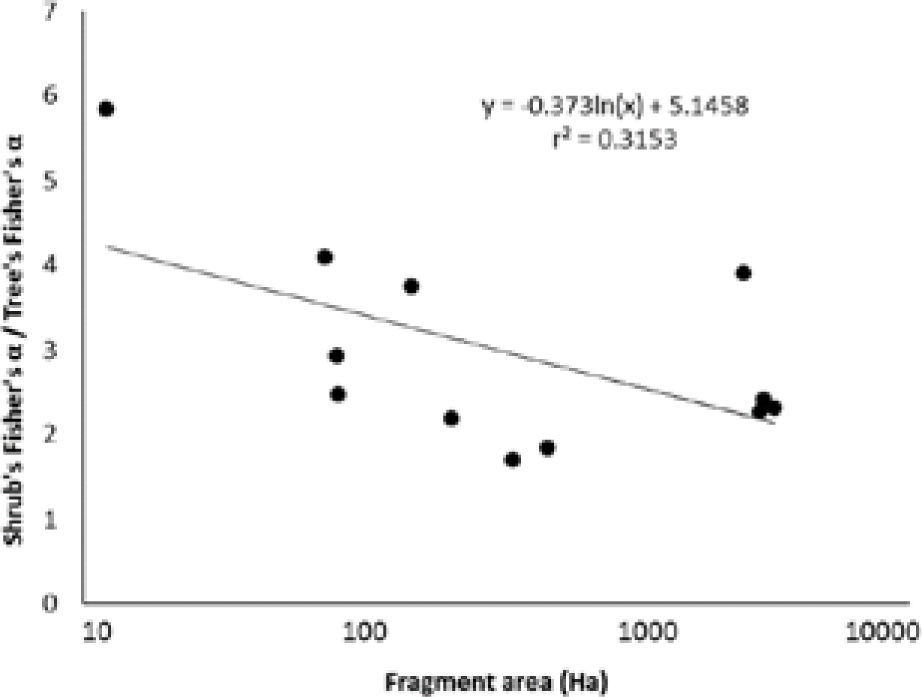
ResultsThe tree canopy sample was composed of 3 301 specimens from 46 species (Appendix 1), and the shrub layer was composed of 7 453 specimens from 116 species (Appendix 2). Fisher's α diversity ranged from 0.95 to 4.55 for the tree layer, and 2.99 to 8.51 for the shrub layer (Table 1). The shrub layer-to-tree layer diversity ratio decreased significantly with the size of the fragment (r2=0.315, p<0.01, Fig. 3). The final and most parsimonious model for species diversity in the seasonally dry oak forest remnants of the Mixteca Alta region had acceptable goodness-of-fit indices (GFI=0.899, p=0.171, AIC=−1.595), following Hayduck (1987 [Fig. 4]).
Floristic list of tree layer species (DBH≥2.5cm and height≥2.5m), recorded in 12 seasonally dry oak forest remnants in the Mixteca Alta of Oaxaca, Mexico. P1 to P12=fragments in consecutive order.
| Scientific name | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cupressaceae | ||||||||||||
| Juniperus flaccida Schltdl. | X | X | — | X | X | X | X | X | X | — | X | — |
| Pinaceae | ||||||||||||
| Pinus sp. | — | — | — | X | — | — | — | — | — | — | — | — |
| Anacardiaceae | ||||||||||||
| Actinocheita filicina (DC.) F.A. Barkley | — | — | — | — | — | — | — | — | — | — | — | X |
| Pistacia mexicana Kunth | — | — | — | — | — | X | — | X | — | — | — | X |
| Rhus schiedeana Schltdl. | — | — | — | — | — | — | — | — | X | — | — | X |
| R. standleyi F.A. Barkley | X | — | — | X | X | X | — | X | — | — | — | — |
| Asteraceae | ||||||||||||
| Ageratina mairetiana (DC.) R.M. King et H. Rob. | — | — | — | — | — | — | — | — | — | — | X | — |
| Critonia hebebotrya DC. | — | — | — | — | — | — | — | — | — | X | — | — |
| Montanoa frutescens (Mairet ex DC.) Hemsl. | — | — | — | — | — | X | — | — | — | — | — | — |
| Betulaceae | ||||||||||||
| Alnus jorullensis Kunth | — | — | — | — | — | — | — | — | — | X | — | — |
| Buddlejaceae | ||||||||||||
| Buddleja parviflora Kunth | — | X | — | X | X | X | — | — | — | — | X | — |
| Burseraceae | ||||||||||||
| Bursera bipinnata (DC.) Engl. | — | — | — | — | — | — | — | — | — | — | — | X |
| Ericaceae | ||||||||||||
| Arbutus xalapensis Kunth | X | X | X | X | X | — | — | — | — | X | X | — |
| Comarostaphylis discolor (Hook.) Diggs | — | — | — | X | — | — | — | — | — | X | X | — |
| C. polifolia (Kunth) Zucc. ex Klotzsch | — | — | — | — | X | X | — | — | X | — | — | — |
| Fabaceae | ||||||||||||
| Acacia pennatula (Schltdl. et Cham.) | — | — | — | — | — | — | — | — | — | — | — | X |
| Benth. subsp. pennatula | ||||||||||||
| Brongniartia mollis Kunth | — | — | — | — | X | — | — | — | — | — | — | — |
| Calliandra grandiflora (L'Hér.) Benth. | — | — | — | — | X | — | — | — | — | — | — | — |
| Eysenhardtia polystachya (Ortega) Sarg. | — | — | — | X | X | X | — | — | — | — | — | X |
| Leucaena diversifolia (Schltdl.) Benth. | — | — | — | — | x | — | — | — | — | — | — | — |
| Mimosa lactiflua Delile ex Benth. | — | — | — | — | — | — | — | — | — | — | — | x |
| Rhynchosia discolor M. Martens et Galeotti | — | — | — | — | x | — | — | — | — | — | — | — |
| Fagaceae | ||||||||||||
| Quercus acutifolia Née | x | x | — | x | x | x | — | x | — | x | x | — |
| Q. candicans Née | x | — | — | — | — | — | — | — | — | — | — | — |
| Q. castanea Née | x | x | x | x | — | — | — | — | — | x | x | — |
| Q. crassifolia Humb. et Bonpl. | x | — | x | — | — | — | — | — | — | x | — | — |
| Q. deserticola Trel. | — | — | — | — | — | — | — | — | — | x | x | — |
| Q. dysophylla Benth. | — | — | — | x | — | — | — | — | — | — | — | — |
| Q. laeta Liebm. | — | x | x | x | x | — | — | — | — | — | — | — |
| Q. laurina Bonpl. | — | — | x | x | — | — | — | — | — | x | x | — |
| Q. liebmannii Oerst. ex Trel. | X | X | X | X | X | X | X | X | X | X | X | X |
| Q. obtusata Bonpl. | X | X | X | X | — | — | — | — | — | X | — | — |
| Q. rugosa Née | X | — | X | X | X | — | — | — | — | X | X | — |
| Garryaceae | ||||||||||||
| Garrya laurifolia Hartw. ex Benth. | — | — | — | X | X | X | — | X | — | X | — | — |
| Lauraceae | ||||||||||||
| Litsea glaucescens Kunth | — | — | — | — | X | — | — | — | — | — | — | — |
| Rhamnaceae | ||||||||||||
| Ceanothus caeruleus Lag. | — | — | — | — | X | — | — | — | X | — | — | — |
| Rhamnus serrata Humb. et Bonpl. ex Willd. | — | — | — | — | — | — | — | — | X | — | — | X |
| Rosaceae | ||||||||||||
| Cercocarpus macrophyllus C.K. Schneid. | X | — | — | X | — | — | — | — | — | X | X | — |
| Malacomeles denticulata (Kunth) G.N. Jones | — | — | — | — | — | X | — | — | — | X | — | — |
| Prunus serotina subsp. capuli (Cav.) McVaugh | — | — | — | X | — | — | — | — | — | X | X | — |
| Vauquelinia australis Standl. | — | — | — | — | — | — | — | — | — | X | — | — |
| Sapindaceae | ||||||||||||
| Dodonaea viscosa Jacq. | — | — | — | — | X | X | X | X | X | — | — | |
| Solanaceae | ||||||||||||
| Cestrum anagyris Dunal | — | — | — | — | — | — | — | — | — | X | — | — |
| No determinated | ||||||||||||
| n.d. 2 | — | — | — | — | — | — | — | — | — | — | X | — |
| Arecaceae | ||||||||||||
| Brahea dulcis (Kunth) Mart. | X | — | — | — | — | — | X | X | — | — | X | X |
| Nolinaceae | ||||||||||||
| Nolina longifolia (Karw. ex Schult. f.) Hemsl. | X | — | — | X | X | — | — | — | — | — | — | — |
| Total species in fragment | 13 | 8 | 8 | 19 | 19 | 12 | 4 | 8 | 7 | 18 | 14 | 10 |
Floristic list of shrub layer species (DBH<2.5cm or height<2.5m) recorded in 12 seasonally dry oak forest remnants in the Mixteca Alta of Oaxaca, Mexico. P1 to P12= fragments in consecutive order.
| Scientific name | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cupressaceae | ||||||||||||
| Juniperus flaccida Schltdl. | X | X | X | X | X | — | X | X | X | — | — | — |
| Pinaceae | ||||||||||||
| Pinus sp. | — | — | — | — | x | — | — | — | — | — | x | — |
| Anacardiaceae | ||||||||||||
| Actinocheita filicina (DC.) F.A. Barkley | — | — | — | — | — | — | — | — | — | — | — | x |
| Asclepias linaria Cav. | — | x | — | — | — | — | — | — | — | — | — | — |
| Pistacia mexicana Kunth | — | — | — | — | — | x | — | x | — | — | — | x |
| Rhus schiedeana Schltdl. | — | — | — | — | — | — | — | — | X | X | — | X |
| R. standleyi F.A. Barkley | X | X | — | X | X | X | X | X | X | X | X | — |
| Asteraceae | ||||||||||||
| Ageratina calophyla (Greene) R.M. King et H. Rob. | — | X | — | X | X | X | X | X | X | X | X | X |
| A. espinosarum (A. Gray) R.M. King et H. Rob. | — | X | — | — | — | — | X | — | X | — | — | X |
| A. mairetiana (DC.) R.M. King et H. Rob. | — | — | — | — | — | — | — | — | — | — | X | — |
| A. petiolaris (Moc. ex DC.) R.M. King et H. Rob. | X | X | — | X | X | — | — | — | — | X | X | — |
| A. scorodonioides (A. Gray) R.M. King et H. Rob. | — | — | — | — | X | — | — | — | — | — | — | — |
| Archibaccharis serratifolia (Kunth) S.F. Blake | — | — | — | — | X | — | — | — | — | — | — | — |
| Baccharis conferta Kunth | — | — | X | — | X | — | — | — | — | — | — | — |
| B. serrifolia DC. | X | X | — | X | X | — | — | — | — | — | X | — |
| Bidens pilosa L. | X | X | X | — | X | X | — | X | — | X | X | — |
| Brickellia secundiflora (Lag.) A. Gray | — | — | — | X | X | — | — | — | — | — | X | — |
| B. veronicifolia (Kunth) A. Gray | — | X | — | X | X | X | X | X | X | — | X | X |
| Coreopsis mutica DC. | — | — | — | X | — | — | — | — | — | — | — | — |
| Critonia hebebotrya DC. | — | — | — | — | — | — | — | — | — | X | — | — |
| Eupatorium sp. | — | — | — | X | — | — | — | — | — | — | — | — |
| Lagascea helianthifolia Kunth | — | — | — | — | — | X | — | — | — | — | — | — |
| Perymenium discolor Schrad. | — | — | — | X | X | X | X | — | — | — | — | — |
| Pittocaulon praecox (Cav.) H. Rob. et Brettell | — | — | — | — | — | — | — | — | — | X | — | — |
| Roldana barba—johannis (DC.) H. Rob. et Brettell | — | — | — | — | — | — | — | — | — | X | — | — |
| R. oaxacana (Hemsl.) H. Rob. et Brettell | — | — | — | X | X | — | — | — | — | — | — | — |
| Rumfordia floribunda DC. | — | — | — | — | — | — | — | — | — | X | — | — |
| Senecio callosus Sch. Bip. | — | — | — | — | X | — | — | — | — | — | — | — |
| Stevia lucida var. oaxacana (DC.) Grashoff | — | X | — | X | X | — | — | — | — | X | — | — |
| S. ovata Willd. | — | — | — | — | — | — | — | — | — | — | X | — |
| Tagetes lucida Cav. | — | — | — | — | X | — | — | — | — | — | — | — |
| Verbesina oncophora B.L. Rob. et Seaton | X | X | — | X | X | — | X | — | — | — | X | X |
| V. virgata Cav. | — | — | — | — | X | — | — | — | — | — | — | — |
| Viguiera benziorum B.L. Turner | — | — | — | — | X | — | — | — | — | X | — | — |
| Asteraceae sp. | X | — | — | — | — | — | — | — | — | — | — | — |
| Berberidaceae | ||||||||||||
| Berberis moranensis Schult. et Schult. f. | — | — | — | — | — | X | X | — | — | X | — | — |
| Bignoniaceae | ||||||||||||
| Tecoma stans (L.) Juss. ex Kunth | — | X | — | — | — | — | — | — | X | — | — | — Boraginaceae |
| Lithospermum calycosum (J.F. Macbr.) I.M. Johnst. | — | — | — | x | x | — | — | — | — | — | — | — |
| Buddlejaceae | ||||||||||||
| Buddleja parviflora Kunth | — | — | — | — | — | x | — | — | — | — | x | — |
| Burseraceae | ||||||||||||
| Bursera bipinnata (DC.) Engl. | — | — | — | — | — | — | — | — | — | — | — | — |
| Cactaceae | ||||||||||||
| Ferocactus macrodiscus (Mart.) Britton et Rose | — | x | — | — | x | — | — | — | — | — | — | — |
| Mammillaria haageana Pfeiff. | — | x | — | x | — | — | — | — | x | — | x | x |
| M. kraehenbuehlii (Krainz) Krainz | — | — | — | — | x | — | x | — | — | — | — | — |
| Mammillaria sp. | — | x | — | — | x | — | — | — | — | — | — | — |
| Opuntia lasiacantha Pfeiff. | x | x | — | x | — | — | — | — | — | — | — | — |
| O. streptacantha Lem. | — | — | — | — | X | — | — | X | — | — | — | — |
| Ericaceae | ||||||||||||
| Arbutus xalapensis Kunth | X | — | X | — | X | X | — | — | — | X | X | — |
| Chimaphila maculata (L.) Pursh | — | — | X | — | — | — | — | — | — | — | — | — |
| Comarostaphylis discolor (Hook.) Diggs | — | — | — | X | — | — | — | — | — | X | X | — |
| C. polifolia (Kunth) Zucc. ex Klotzsch | — | — | — | — | X | X | X | X | X | X | — | — |
| Fabaceae | ||||||||||||
| Acacia pennatula (Schltdl. et Cham.) Benth. subsp. pennatula | — | — | — | — | — | — | — | — | — | — | — | X |
| A. tequilana S. Watson | — | — | — | X | X | — | — | — | — | — | — | — |
| Brongniartia mollis Kunth | — | — | — | — | X | — | — | — | — | — | — | — |
| Calliandra grandiflora (L'Hér.) Benth. | X | — | — | X | X | — | — | X | — | — | — | — |
| Dalea aff. lutea (Cav.) Willd. | X | X | — | X | X | — | — | — | — | — | — | — |
| Desmodium sp. | — | — | — | X | X | — | — | — | — | — | — | — |
| Eysenhardtia polystachya (Ortega) Sarg. | — | — | — | — | X | — | — | — | — | — | — | T |
| Harpalyce formosa DC. | — | — | — | — | — | X | X | X | — | — | — | — |
| Leucaena diversifolia (Schltdl.) Benth. | — | — | — | — | X | — | — | — | — | — | — | — |
| Lysiloma acapulcense (Kunth) Benth. | — | — | — | — | X | — | — | — | — | — | — | — |
| L. divaricatum (Jacq.) J.F. Macbr. | — | — | — | X | — | — | — | — | — | — | — | — |
| Mimosa lactiflua Delile ex Benth. | — | — | — | — | — | — | — | — | — | — | — | X |
| Rhynchosia discolor M. Martens et Galeotti | X | — | — | — | X | — | — | X | — | — | — | — |
| Tephrosia sp. | — | — | — | X | — | — | — | — | — | — | — | — |
| Fabaceae sp. | — | — | — | — | X | — | — | — | — | — | — | — |
| Fagaceae | ||||||||||||
| Quercus acutifolia Née | X | X | — | X | X | X | — | — | — | X | X | X |
| Q. castanea Née | X | — | — | — | — | — | — | — | — | X | X | — |
| Q. crassifolia Humb. et Bonpl. | — | — | X | — | — | — | — | — | — | — | — | — |
| Q. deserticola Trel. | — | — | — | — | — | — | — | — | — | X | — | — |
| Q. laeta Liebm. | — | X | X | — | — | — | — | — | — | — | — | — |
| Q. laurina Bonpl. | — | — | X | — | X | — | — | — | — | X | X | — |
| Q. liebmannii Oerst. ex Trel. | X | X | — | X | X | X | — | X | — | X | — | X |
| Q. obtusata Bonpl. | — | — | — | X | — | — | — | — | — | X | — | — |
| Q. rugosa Née | — | — | X | X | X | — | — | — | — | X | X | — |
| Garryaceae | ||||||||||||
| Garrya laurifolia Hartw. ex Benth. | — | — | — | X | X | X | X | X | — | X | — | — |
| Lamiaceae | ||||||||||||
| Clinopodium macrostemum (Moc. et Sessé ex Benth.) Kuntze | — | — | — | — | — | — | — | — | — | X | — | — |
| Salvia aff. fruticosa Mill. | — | — | — | — | — | — | — | — | X | — | — | — |
| S. cinnabarina M. Martens et Galeotti | — | X | — | X | X | — | — | — | — | X | X | X |
| S. macrophylla Benth. | — | — | — | — | — | — | — | — | — | X | — | — |
| S. melissodora Lag. | X | X | — | — | X | X | X | X | — | — | X | — |
| S. mexicana L. | — | — | — | — | — | X | — | — | — | — | — | — |
| S. stolonifera Benth. | — | — | X | — | — | — | — | — | — | — | — | — |
| Lauraceae | ||||||||||||
| Litsea glaucescens Kunth | X | — | — | X | X | X | — | — | — | X | — | — |
| Lythraceae | ||||||||||||
| Cuphea cyanea DC. | — | X | — | X | X | — | — | — | — | X | — | — |
| Oleaceae | ||||||||||||
| Fraxinus purpusii Brandegee | — | — | — | — | — | — | X | X | — | — | — | — |
| Onagraceae | ||||||||||||
| Fuchsia encliandra Steud. | — | X | X | X | X | — | — | — | — | X | — | — |
| Polygalaceae | ||||||||||||
| Monnina xalapensis Kunth | X | — | — | X | X | X | — | — | — | X | — | — |
| Rhamnaceae | ||||||||||||
| Ceanothus caeruleus Lag. | X | — | — | — | X | — | — | — | — | — | — | — |
| Rhamnus serrata Humb. et Bonpl. ex Willd. | X | X | — | X | X | — | — | — | X | — | — | X |
| Rosaceae | ||||||||||||
| Cercocarpus macrophyllus C.K. Schneid. | — | — | — | X | X | X | — | — | X | — | — | — |
| Malacomeles denticulata (Kunth) G.N. Jones | X | — | — | — | X | X | — | — | X | X | X | — |
| Prunus serotina subsp. capuli (Cav.) McVaugh | — | — | — | X | — | — | — | — | — | X | — | — |
| Rubus trilobus Moc. et Sessé ex Ser. | — | — | — | — | — | — | — | — | — | X | X | — |
| Vauquelinia australis Standl. | — | — | — | — | — | — | — | — | — | — | X | — |
| Rubiaceae | ||||||||||||
| Bouvardia longiflora (Cav.) Kunth | — | — | X | — | X | — | X | X | X | X | X | — |
| B. ternifolia (Cav.) Schltdl. | X | — | — | X | X | — | — | — | X | X | — | — |
| Chiococca pachyphylla Wernham | — | — | — | — | X | — | — | — | — | — | — | — |
| Sapindaceae | ||||||||||||
| Dodonaea viscosa Jacq. | — | X | — | — | X | X | X | X | X | — | — | — |
| Scrophulariaceae | ||||||||||||
| Castilleja tenuiflora Benth. | — | — | — | — | — | — | — | — | — | — | X | — |
| Lamourouxia rhinanthifolia Kunth | — | — | — | X | — | — | — | — | — | — | — | — |
| Penstemon roseus (Cerv. ex Sweet) G. Don | X | — | — | — | — | — | — | — | — | — | X | — |
| Solanaceae | ||||||||||||
| Cestrum anagyris Dunal | — | — | — | — | X | — | — | — | — | X | — | — |
| Solanum cervantesii Lag. | — | — | — | — | — | — | — | — | — | X | — | — |
| S. lanceolatum Cav. | X | X | — | — | — | — | — | — | — | — | X | X |
| Verbenaceae | ||||||||||||
| Lantana camara L. | X | X | — | X | X | X | — | — | X | X | — | — |
| No determinated | ||||||||||||
| n.d. 1 | — | — | — | — | — | — | — | — | X | — | — | — |
| n.d. 3 | — | — | — | — | — | — | — | — | — | X | — | — |
| n.d. 4 | — | X | — | — | — | — | — | — | — | — | — | — |
| n.d. 5 | — | — | — | — | — | — | — | — | — | — | X | — |
| Agavaceae | ||||||||||||
| Agave potatorum Zucc. | — | — | — | — | X | — | — | X | — | — | — | X |
| Arecaceae | ||||||||||||
| Brahea dulcis (Kunth) Mart. | — | — | — | — | X | — | X | X | — | — | X | X |
| Asparagaceae | ||||||||||||
| Beaucarnea gracilis Lem. | — | — | — | — | X | — | — | — | — | — | — | — |
| Dasylirion serratifolium (Karw. ex Schult. f.) Zucc. | — | — | — | — | — | — | — | — | X | — | — | — |
| Bromeliaceae | ||||||||||||
| Hechtia aff. sphaeroblasta B.L. Rob. | X | — | — | — | — | — | — | — | X | — | — | X |
| Nolinaceae | ||||||||||||
| Nolina longifolia (Karw. ex Schult. f.) Hemsl. | X | — | — | — | X | — | — | — | — | — | — | — |
| Smilacaceae | ||||||||||||
| Smilax moranensis M. Martens et Galeotti | — | — | — | — | X | — | — | — | — | — | — | — |
| Total species in fragment | 27 | 29 | 12 | 39 | 63 | 23 | 17 | 19 | 20 | 39 | 30 | 18 |
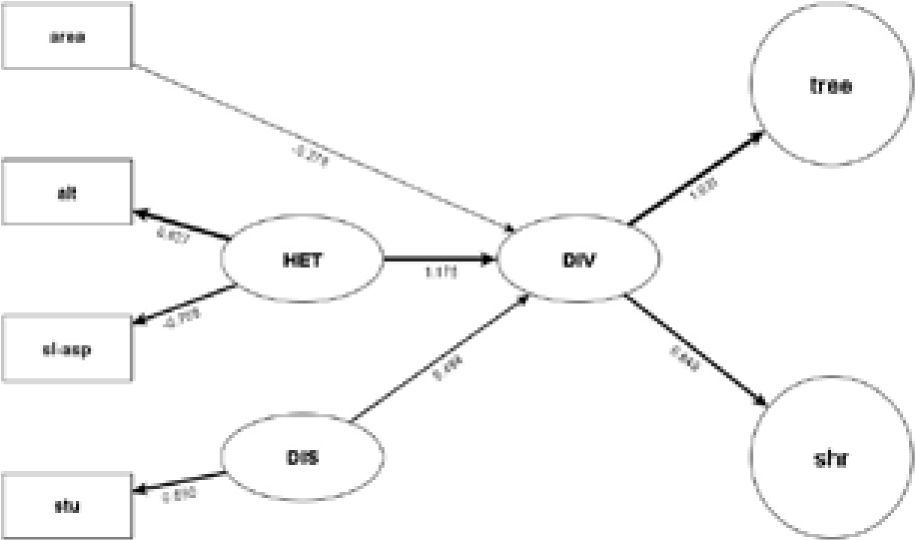
Reduced structural equation model used to disentangle the effect of area, topographic heterogeneity, and disturbance on diversity of trees and shrubs in seasonally dry oak forest fragments in the Mixteca Alta region of Oaxaca, Mexico. Numbers on the arrows are the standardized coefficients for each of the paths. Only significant relationships are noted in the diagram (p<0.05). The size of the arrows is proportional to the strength of the path. In rectangular shapes, we represent manifest variables (area= fragment area, alt= altitude standard deviation, sl-asp= slope-aspect standard deviation, slo= slope standard deviation, roa= road-effect zone, stu= stump density, tree= tree Fisher's α, shru= shrub Fisher's α), in oval shapes latent variables (HET= topographic heterogeneity, DIST= anthropogenic disturbance, DIV= fragment diversity). Single-headed arrows indicate oneway variance of the latent variable; double-headed arrows indicate covariance among manifest variables.
Our SEM analysis revealed that disturbance, habitat heterogeneity, and fragment area have significant relationships with species diversity. Of these drivers of species diversity, habitat heterogeneity, here estimated in terms of topographic variables, is the most important and has a positive effect on species diversity. When habitat heterogeneity is considered, the size of the fragment has a significant but negative effect on diversity. Two out of the 3 explanatory variables used to estimate topographic heterogeneity were significant: the slope-aspect heterogeneity and the altitude heterogeneity. According to our SEM analysis, the Fisher's α diversity in the fragment tends to increase in fragments with high variation in elevation and low variation in slope-aspect. Human disturbance also affected significantly and positively the diversity of the fragments. Of the manifest variables used to estimate disturbance, only stump density, an indicator of the intensity of plant extraction, was significant.
DiscussionStructural equation modeling revealed the effect of anthropogenic disturbance, fragment area, and topographic heterogeneity in woody plant species diversity in remnants of seasonally dry oak forests in the Mixteca Alta, southern Mexico. In accordance with our hypotheses, woody plant species diversity per fragment can be explained directly by the topographic heterogeneity, the intensity of anthropogenic disturbance, and the fragment area, as has been shown in other studies (Ross et al., 2002; Cayuela et al., 2006a; Echeverría et al., 2007). Our results indicate that, when topographic heterogeneity and human disturbance are taken into account, the effect of fragment size on diversity is negative in the seasonally dry oak forest of the Mixteca region. More specifically, smaller fragments with similar altitude and slope-aspect and human disturbance level tend to be more diverse than large fragments.
Edge effects provide a possible explanation for this result since the perimeter to area ratio is greater in small fragments. Small fragments provide more opportunities for light tolerant species to become established, favoring a greater diversity of species. Some species may find the habitat of the edges of the fragment more suitable for survival and reproduction than the center of the fragment (e.g., Bernabe et al., 1999; Asbjornsen et al., 2004b). Indeed, in our study shrubs were proportionally more diverse than trees in smaller than in larger fragments. Shrubs are usually more light-demanding than trees, and their abundance has been found to decrease significantly with the abundance of trees in oak forest in the adjacent Sierra Norte (Zacarías-Eslava and del Castillo, 2010). More attention should be paid to edge effects in future studies to explore the possible role of edge effects on this result.
The negative effect of area on diversity obtained in our analysis suggests that we have not omitted any important diversity driver that is positively associated with fragment area, in which case, a positive, not a negative effect of area on diversity would be obtained. Our results, therefore, suggest that environmental factors associated with topography are among the most important diversity drivers for woody plants in the seasonally dry oak forest remnants of the Mixteca Region.
Both heterogeneity in altitude and heterogeneity in slope-aspect within the fragments showed a significant relationship with habitat heterogeneity. However, their combined effect on diversity effects are opposite, according to our SEM analysis. This result suggests that a greater effect of topographic heterogeneity on species diversity can be achieved with a combination of high heterogeneity in altitude with low heterogeneity in slope-aspect. Thus, these 2 variables should be considered together when analyzing the impact of habitat heterogeneity on diversity. The involvement of climatic effects affecting species diversity and associated with topography may help to interpret this result. The mean annual temperature, for instance, is well known to decrease linearly with altitude (e.g., Zacarías-Eslava and del Castillo, 2010). Throughout the same slope-aspect, a given mean temperature is expected to be found only at a unique elevation point, ignoring microclimatic differences caused by variations in shading by vegetation or micro topography. However, the same mean temperature can be found at different elevations on a mountain if the orientation of the slope changes. North-facing slopes, for instance, are usually colder than south-facing slopes, at the same elevation in the Northern Hemisphere. The same temperature that is found on the north face of a mountain at a given elevation is expected to be found at a higher elevation at other slope orientations. In this way, the combination of high heterogeneity in both slope-aspect and elevation may reduce the total environmental variation of the fragment because different combinations of altitude and orientation can render the same climate.
Disturbance is a factor that undoubtedly alters ecosystem biodiversity, even if there is no consensus on how it works (Mackey and Currie, 2000, 2001). Most studies have developed indices to assess the total effect of disturbance without distinguishing the partial effect of each source of disturbance on biodiversity (Ross et al., 2002). Stump density reveals logging activities in the fragments, whereas road-effect zone is an indicator of the accessibility of the fragment to anthropogenic activities (Forman et al., 1997), as well as potential invasion by exotic species. Only logging, as a disturbance indicator, was significant in our study. In the study site, logging is manual and selective. Logged trees are scattered over the landscape, creating small gaps in the fragments. In seasonally dry oak forests, each small opening may decrease soil moisture, creating inappropriate conditions for native sapling development (Asbjornsen et al., 2004a, 2004b; Brown et al., 2004). In turn, the new conditions in these open spaces may allow the establishment of resilient and short-lived species, such as pioneer species. The above leads to species turnover and an increased biodiversity, by allowing, to a certain extent the coexistence of pioneer and shade-tolerant species in the same fragment. The effect of roads on diversity in the study area was not significant, probably because the roads in the area are used primarily for communication between indigenous villages, which are characterized by very low population densities. As a result, the effect of human disturbance in this area is probably due mainly to logging, either for fuel or for the small-scale production of wood products for local construction or tool manufacturing.
Conservation implications. Our results provide evidence of the importance of selecting fragments with high variation in topographic heterogeneity, which may favor a great diversity of species. The kinds and intensities of disturbance are also crucial since they may not have a common effect on species. The consideration of the species to be preserved is also crucial in developing conservation strategies, since different species may have different requirements, and some strategies may benefit only a limited number of species and harm others.
Using data of 12 fragments and structural equation modeling techniques, we were able to test and confirm a 3 factor model that characterized the diversity of woody plant species of a seasonally dry oak forest in the Mixteca Alta, Oaxaca, Mexico. Topographic heterogeneity, human disturbance, and fragment size, in that order of importance, play a significant role on woody plant diversity of these fragments. Topographic heterogeneity had a positive relationship with diversity. Fisher's α diversity increased significantly with fragment heterogeneity in slope-aspect or altitude. Disturbance, here estimated as a fragment's stump density, also showed a positive relationship with diversity. Small fragments with similar levels oftopographic heterogeneity and disturbance tend to be more diverse than large fragments, probably because small fragments have a greater perimeter-to-area ratio and therefore convey more opportunities for the successful establishment of species that are benefited by open, less dense habitats such as edge, resilient, and pioneer species. Structural equation modeling was shown to be an appropriate technique for disentangling the contribution of several factors related with biodiversity. Conservation strategies of fragmented landscapes must consider not only fragment size, but the type and intensity of disturbance affecting the fragments and the species that need to be preserved.
We thank Raúl Rivera for field and cartographic work, and Adriadna Ferrer, Soledad Iglesias, Remedios Martínez, Hugo Nieto, Adriana Pacheco, and Victoria Pérez for field work. Suzanna Elkin helped us with the English version. The project was funded by grants from the Instituto Politécnico Nacional (SIP and COFAA), and the European Commission INCO V Program (REFORLAN FP6-2004-INCO-DEV-3 032132). We acknowledge the valuable comments of María Luisa Martínez and two anonymous reviewers. We are especially grateful to the people of Santiago Huauclilla, Santiago Tilantongo, and San Antonio Nduayaco (Santiago Apoala municipal agency), Nochixtlán District villages, who allowed us to carry out this work in their municipalities.