Guadalupe Island represents a unique ecosystem. Its volcanic origin and remoteness from the Baja California peninsula have allowed for the successful establishment of its distinctive flora and fauna. However, the difficulty in accessing the island has precluded the study of its biotic communities, mainly the marine ones. Consequently, no studies on benthic or planktonic diatoms have been hitherto published. Thus, the first records of marine benthic diatom species (epiphytic, epilithic, epizoic) from Guadalupe Island in the NW Mexican Pacific are here provided. One hundred and nineteen diatom taxa belonging to the Bacillariophyceae and Fragilariophyceae were identified, including species and varieties. The former with 87 taxa was the most diverse. Thirteen taxa are new records for Mexico; photographic images of these are provided. Because this is the first study for the benthic diatoms of Isla Guadalupe, a particular bio-geographical affinity is not proposed. However, the best represented genus was Mastogloia which has tropical affinity, and Cocconeis thalassiana was also identified, a new species recently recorded for the Mexican Caribbean.
Isla Guadalupe es única como ecosistema; su origen volcánico y lejanía de la península de Baja California han permitido que el desarrollo evolutivo de sus distintivas flora y fauna haya sido exitoso. La dificultad para acceder a la isla es causa de que algunas comunidades, principalmente marinas, no hayan sido estudiadas aún. Consecuentemente, a la fecha no existía algún estudio publicado sobre diatomeas. Así, se presenta el primer registro de diatomeas bentónicas (epilíticas, epifitas y epizoicas) de isla Guadalupe en el noroeste de México. Se identificaron 119 taxa (incluyendo especies y variedades) de diatomeas pertenecientes a las clases Bacillariophyceae y Fragilariophyceae. Las primeras fueron más diversas con 87 taxa. Del total, 13 taxa son nuevos registros para México y se proveen imágenes fotográficas de ellos. Dado que se trata apenas del primer estudio para la flora de diatomeas bentónicas de isla Guadalupe, no se propone una afinidad biogeográfica. Sin embargo, uno de los géneros con mayor número de especies es de afinidad tropical, i.e., Mastogloia y se identificó Cocconeis thalassiana, una especie nueva recientemente registrada para el Caribe mexicano.
Isla Guadalupe is a natural reserve that represents a unique ecosystem as other Mexican islands. Its volcanic origin and distance from the Baja California peninsula have allowed for a successful evolutionary development of both its particular land and marine flora and fauna, as can be inferred by the high number of cases of endemism recorded (Aguirre-Muñoz et al., 2003; García-Gutiérrez et al., 2005). However, studies on biodiversity, particularly of marine life, are lacking for Isla Guadalupe, mainly because of the difficulty to access the island. The available studies on marine flora of Isla Guadalupe date back to the late 1800s and were published by Setchell and Gardner (1930) in a list that included 90 species of macroalgae. The last review of the macroalgae of Isla Guadalupe was carried out by Stewart and Stewart (1984) and included 212 species, with 24 new records. Both studies recorded a large number of genera of subtropical affinity, indicating that the marine flora in the island is more characteristic of a subtropical than a temperate environment.
The difficulty in accessing an area such as Isla Guadalupe to carry out scientific research is the main cause that many marine communities are yet to be studied, even in their most basic aspects such as species composition. Thus, no published work existed hitherto on diatoms from that area, nor planktonic or benthic forms, in spite of being the most diverse, abundant and productive algal group in marine ecosystems.
In general, studies on benthic diatoms from the Mexican NW are scarce, and these are related either with their role in the feeding habits of abalone (Haliotis spp.) and grazing intertidal molluscs (Siqueiros-Beltrones & Valenzuela-Romero, 2004), or the structure of epiphytic forms assemblages found on macroalgae and marine plants (Argumedo-Hernández & Siqueiros-Beltrones, 2008; Siqueiros-Beltrones, Serviere-Zaragoza, & Argumedo-Hernández, 2002). Another study describes assemblages of epipelic diatoms characteristic of mangrove environments (López-Fuerte, Siqueiros-Beltrones, & Navarro, 2010).
In this study we begin the construction of a taxonomic basis that serves for monitoring and assessing the environmental health of any ecosystem, and provide the first floristic account of benthic diatoms from Isla Guadalupe.
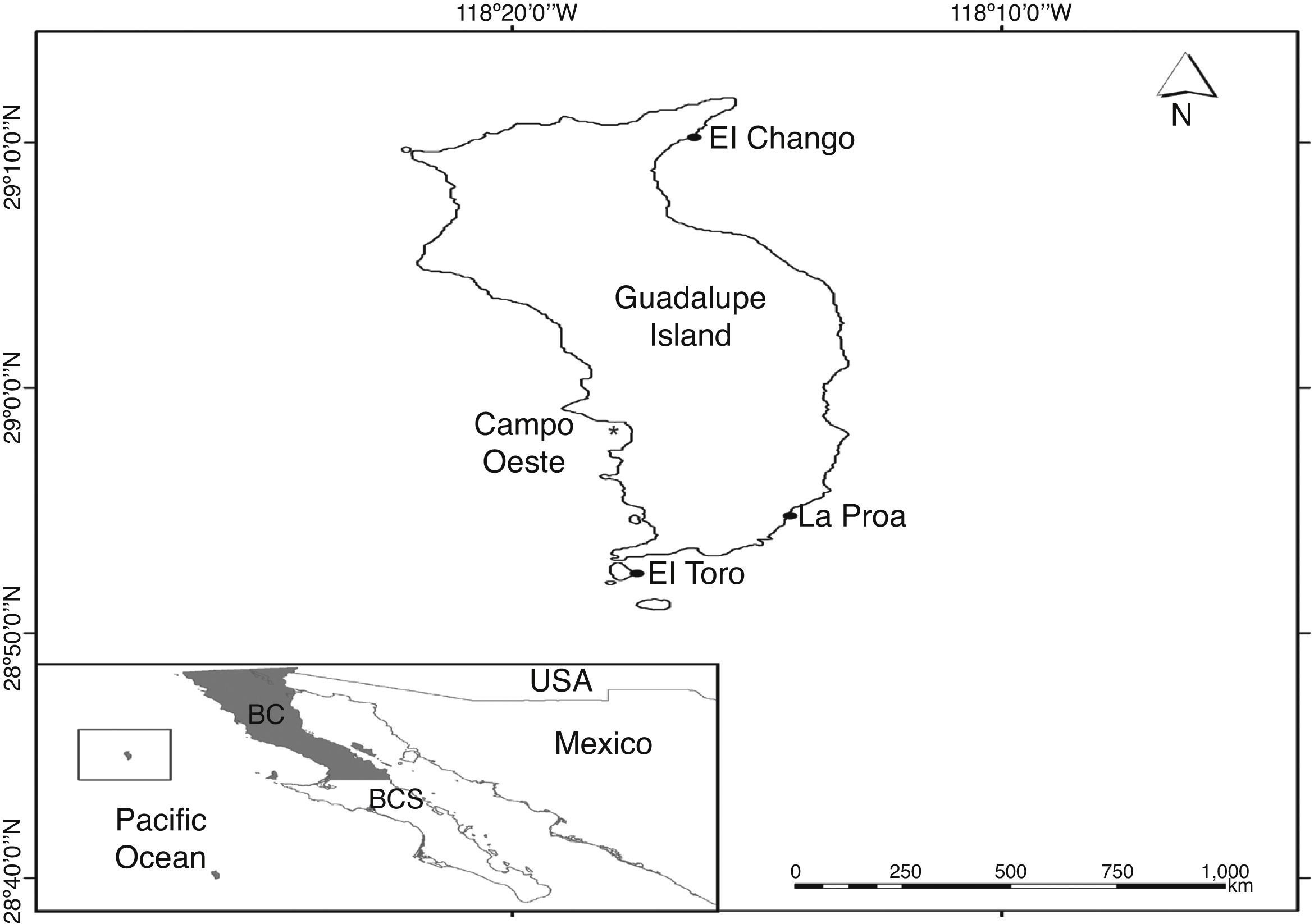
Materials and methodsIsla Guadalupe is located in the Mexican Pacific Ocean approximately 256km off the coast of the Baja California peninsula at 29°N, 118°W, within the Guadalupe Island Biosphere Reserve (Fig. 1). It is influenced by the California Current which is characterized by low temperature and salinity (Lynn & Simpson, 1987). Surficial water temperature ranges between 15 and 20°C during winter and between 20 and 22°C in summer. Its ocean volcanic nature and its remote distance from the coast confer it an abrupt topography and a unique biodiversity. The coastal zone physiography consists of loose basalts, blocks, dikes, cliffs, and few sandy beaches (Pierson, 1987).
Sample collectionSurficial temperature, salinity and pH were measured in situ using a field multi-sensor (Horiba U10). Benthic diatoms were collected at Guadalupe Island in one sampling site on January 18, 2013. Epiphytic diatoms were scraped off from specimens of Eisenia desmarestioides Setchell and N.L. Gardner, 1930 (Ochrophyta; Laminariales), and Codium latum subsp. palmeri (E.Y. Dawson) P.C. Silva, 1962 (Chlorophyta; Bryopsidales), using a glass slide for each sampling. Epilithic and epizoic diatoms from the shell of the sea-snail Megastraea undosa W. Wood, 1828 were brushed off from an area of 5cm2 in each substrate using a toothbrush. Afterwards, a compound sample was formed for the site and preserved in commercial ethanol (70%); concomitant observations of fresh diatom samples were made. In order to clean the diatom frustules for identification the organic matter was oxidized with a mixture of sample, commercial ethanol and nitric acid at a 1:3:5 proportion (Siqueiros-Beltrones, 2002). The samples were then rinsed with drinking water until reaching a pH >6. From each compound sample three permanent slides were mounted for each substrate using Zrax® (RI: 1.7) as mounting medium. All species names and authorities were revised, and in certain cases nomenclatural updates were made, confirming all of the currently accepted taxonomic names. In order to revise the taxonomic names and their synonymies, we consulted Round, Crawford, and Mann (1990) along with the data bases on www.algaebase.org (Guiry & Guiry, 2014) and www.marinespecies.org (WoRMS Editorial Board, 2014). Taxonomic keys used for species identification included: Schmidt et al. (1874–1959), Peragallo and Peragallo (1897–1908), Foged (1984), Witkowski, Lange-Bertalot, and Metzeltin (2000), Siqueiros-Beltrones (2002), and López-Fuerte et al. (2010). Photographic images of newly recorded taxa and others were acquired using an electronic ocular lens.
ResultsSurficial water temperature in the sampling site was measured at 17.7°C; salinity was recorded at 38psu, which renders the environment as polyhalobous. The measured pH was close to neutral at 7.4.
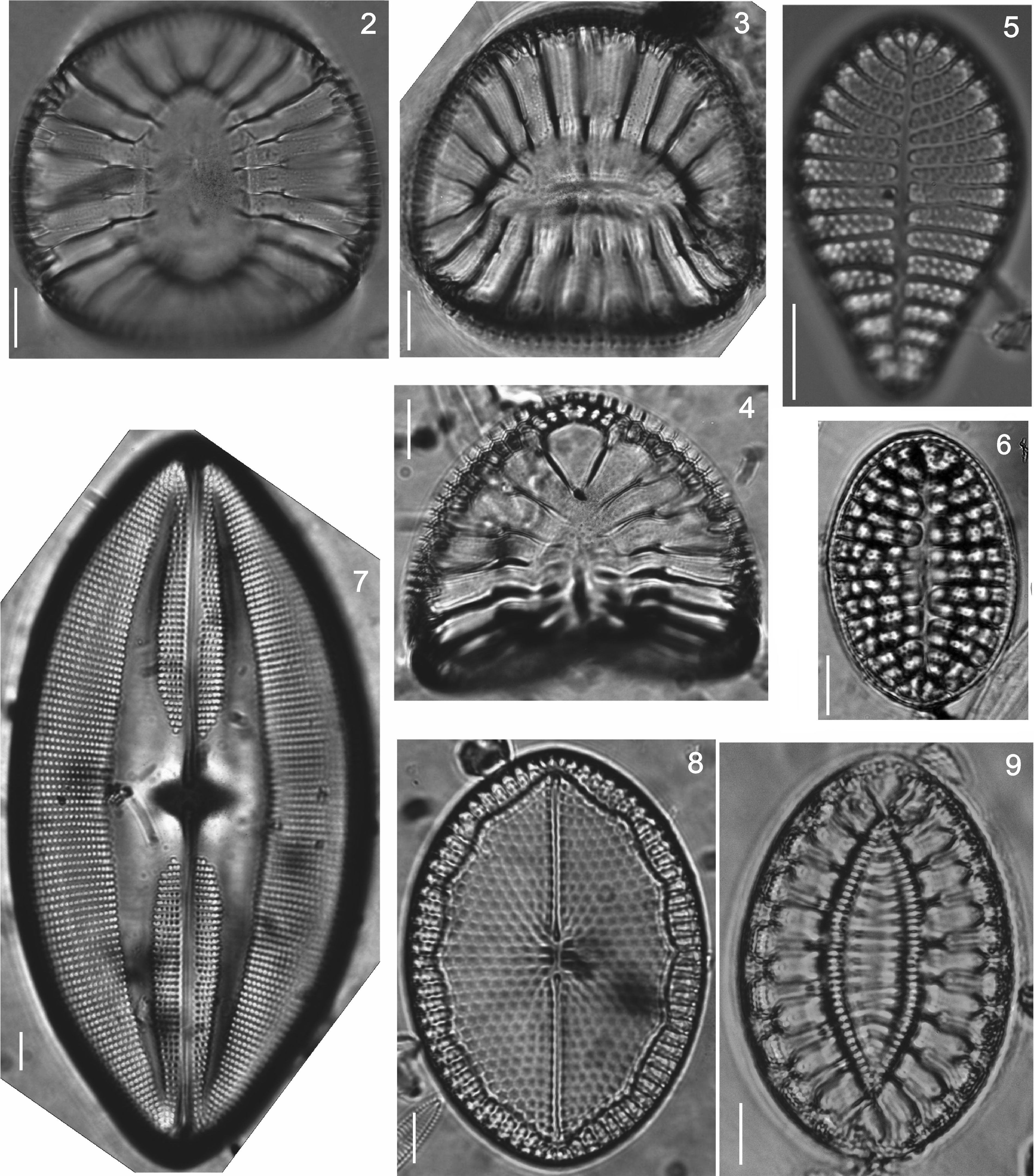
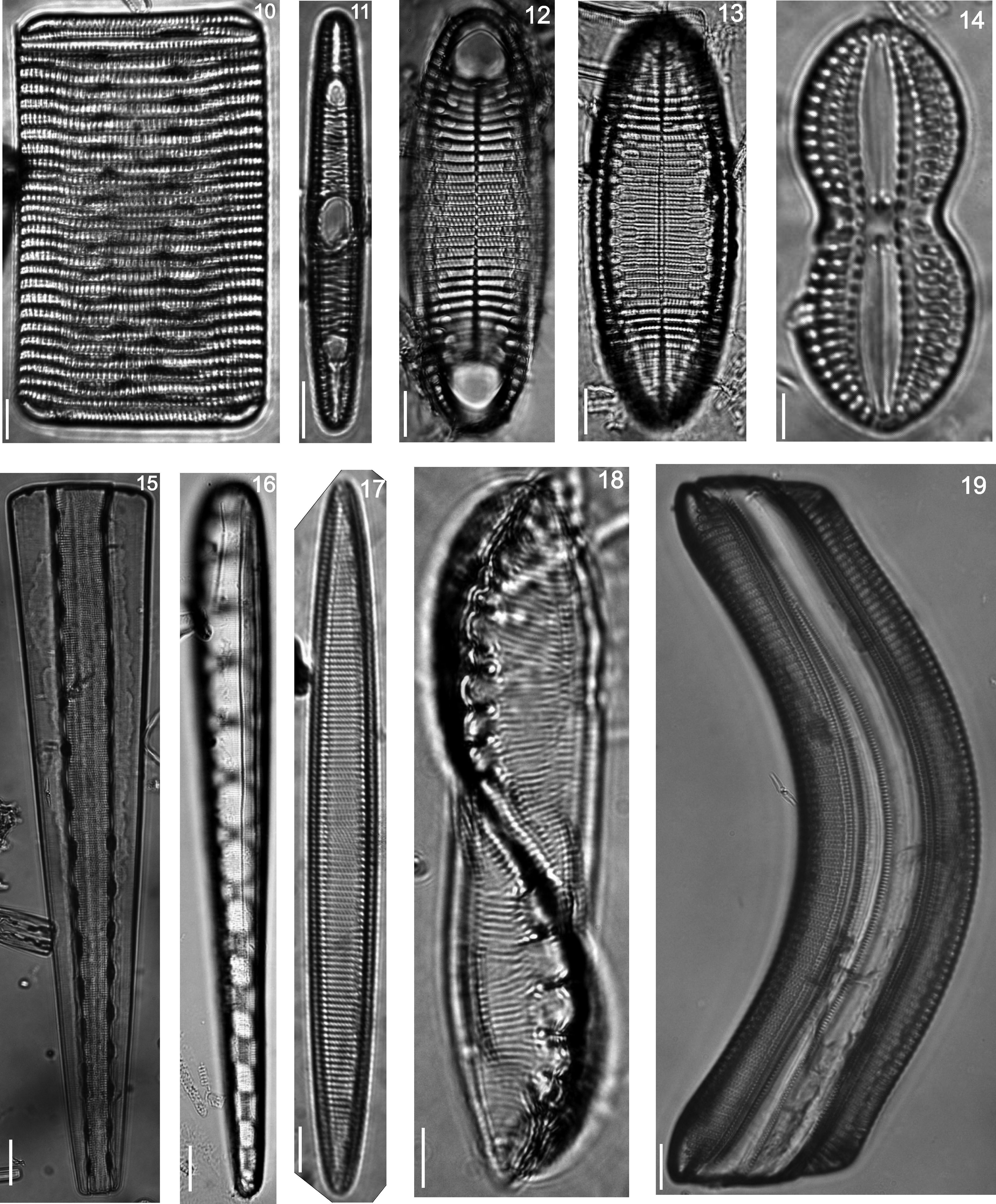
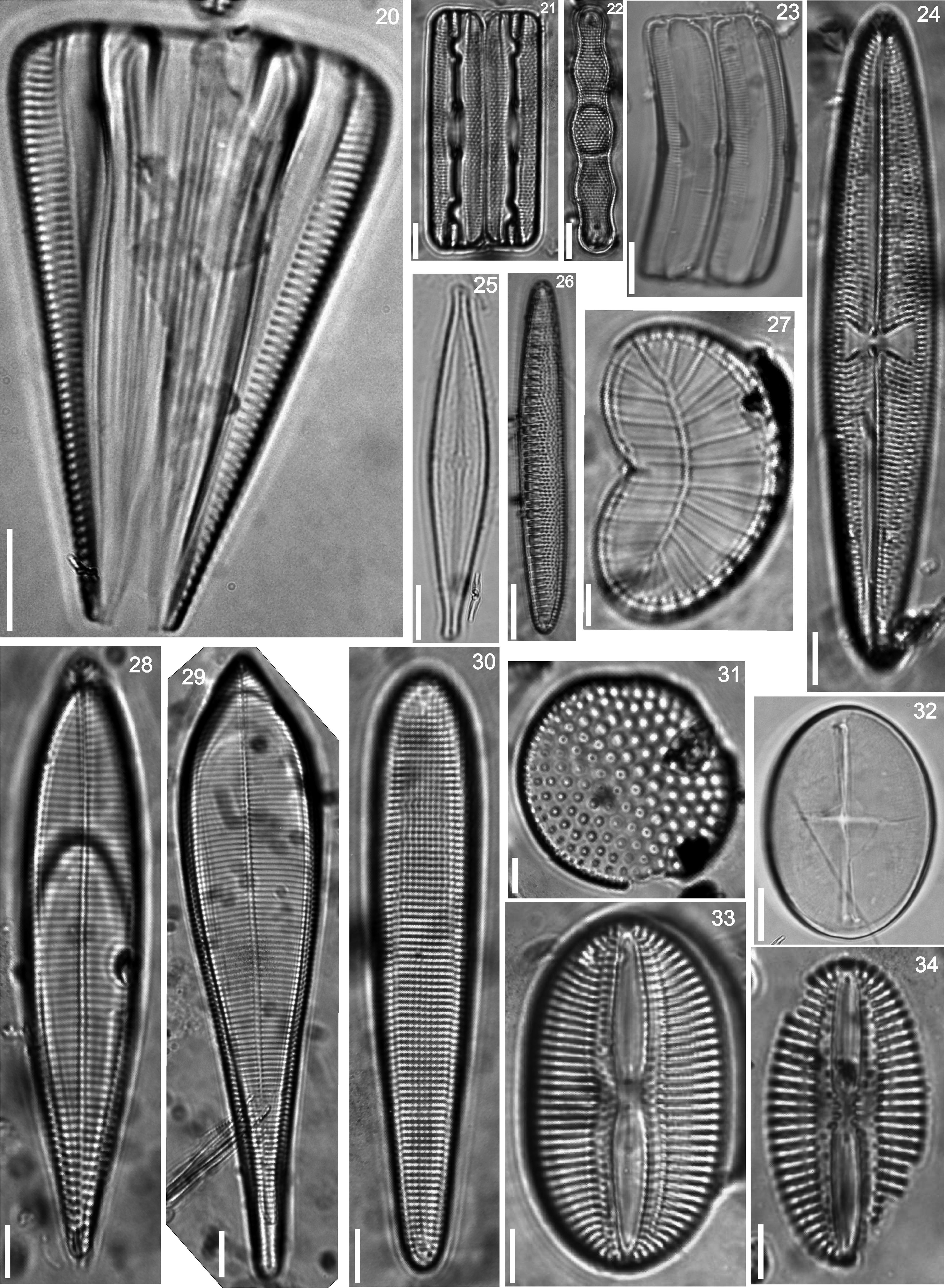
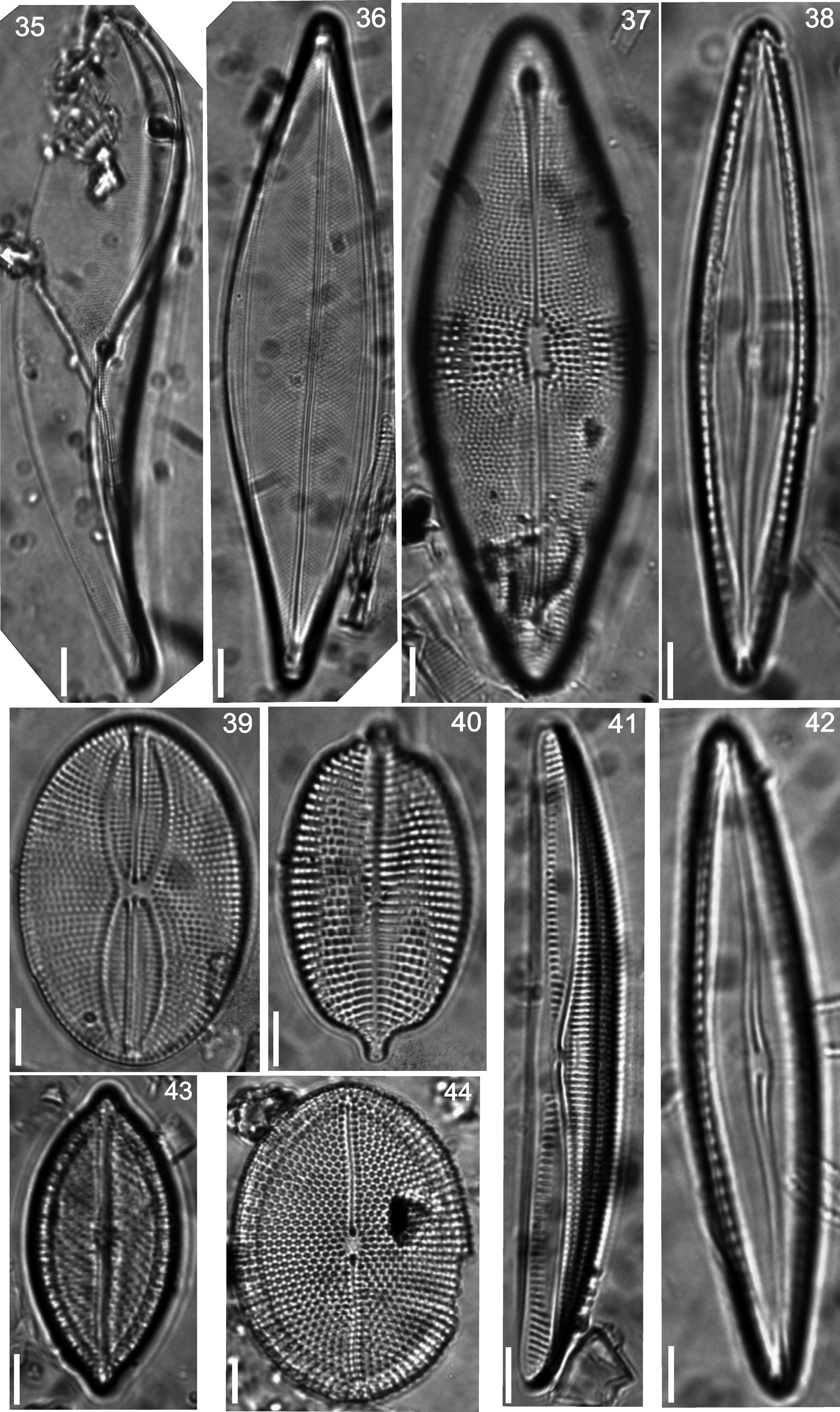
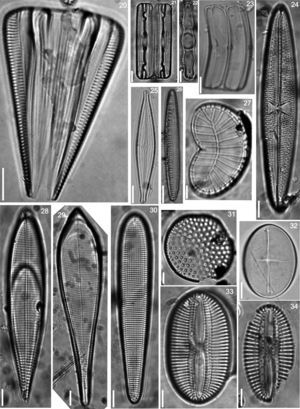
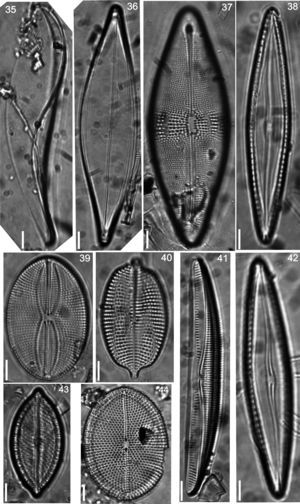
Floristic analysis yielded 119 taxa including species and varieties of benthic diatoms, including epiphytic, epilithic and epizoic forms (Appendix). All diatoms appear alive in the fresh mountings (Fig. 2). The class Bacillariophyceae with 87 taxa was much more diverse than the Fragilariophyceae which yielded 32 taxa. Out of the 45 identified genera, those with higher number of species were Mastogloia (13), Diploneis (11 species), Nitzschia (10), Cocconeis (9), Grammatophora (6) and Licmophora (5). These represent 46% of the species recorded in this study. In contrast, 25 genera were represented by a single species. Thirteen taxa are new records for Mexico: Achnanthes citronella (A. Mann) Hustedt (Fig. 40), Amphiprora conspicua Greville (Fig. 18), Amphora proteus var. oculata H. Peragallo (Fig. 41), Campylodiscus ambiguus Greville (Fig. 3), Diploneis coffaeiformis (Schmidt) Cleve (Fig. 34), D. suborbicularis var. constricta Hustedt (Fig. 33), Donkinia reticulata Norm. (Fig. 35), Lyrella perplexoides (Hustedt) D.G. Mann (Fig. 39), Mastogloia asperuloides Hustedt (Fig. 43), M. ciskeiensis Giffen (Figs. 38 and 42), M. punctatissima (Greville) Ricard (Fig. 44), Parlibellus delognei (Van Heurck) E. J. Cox (Fig. 37) and Psammodiscus calceatus T. Watanabe, T. Nagumo and J. Tanaka (Fig. 31). Only 11 taxa occurred in all three surveyed substrates. The higher number of taxa was observed on the rocky substrate (57), and the lowest on the shell of Megastraea undosa. On the other hand, the higher number of exclusive taxa occurred on macroalgal substrate, particularly on Eisenia desmarestioides with 29 taxa, which renders it as a proper substrate for diatoms, while M. undosa had the lowest (6) number of taxa.
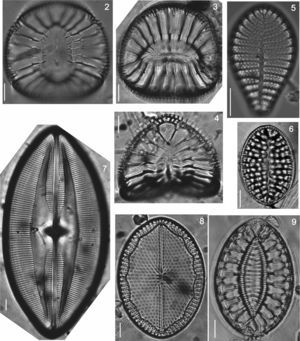
Iconographic sample of the diatoms observed on different coastal substrata from Isla Guadalupe. All images by Folf. Bar=10μm. (2) Campylodiscus crebrecostatus var. speciosa, (3) Campylodiscus ambiguus, (4) Campylodiscus fastuosus, (5) Podocystis adriatica, (6) Campyloneis grevillei, (7) Lyrella approximata, (8) Mastogloia fimbriata, (9) Surirella fastuosa.
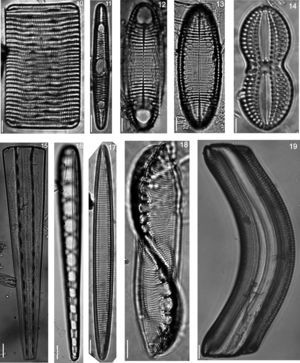
(20) Licmophora abbreviata, (21) Grammatophora marina, (22) Grammatophora undulata, (23) Campylopyxis garkeana, (24) Trachyneis aspera, (25) Brachysira aff. neoexilis, (26) Denticula kuetzingii, (27) Plagiodiscus nervatus, (28) Licmophora communis, (29) Licmophora ehrenbergii, (30) Licmosoma squamosum, (31) Psammodiscus calceatus, (32) Cocconeis molesta var. crucifera, (33) Diploneis suborbicularis var. constricta, (34) Diploneis coffaeiformis.
Research on benthic marine diatoms in Mexico dates barely to the 1980s, and most studies have been carried out for the NW region. Recently, however, some have been carried out in the Mexican Caribbean area (Hernández-Almeida, Herrera-Silveira, & Merino-Virgilio, 2013; López-Fuerte, Siqueiros-Beltrones, & Hernández-Almeida, 2013; Siqueiros-Beltrones, Argumedo-Hernández, & Hernández-Almeida, 2013). Nonetheless, there are still extensive areas in the country where the most basic floristic studies on benthic diatoms are lacking and are badly needed.
Our present work falls within this category, inasmuch no research on diatoms, whether scientific or of any sort, existed for Mexican islands, in spite the fact that 1365 islands are distributed within the Mexican territory (Comité Asesor Nacional sobre el Territorio Insular Mexicano, 2012). Thus, this constitutes the first floristic list of benthic diatoms for any oceanic island located in the exclusive economic zone of Mexico, and it targets the Guadalupe Island Biosphere Reserve.
Although only three substrates were examined, the number of identified taxa is high, and over 11% are new records for the whole country. Some of the genera with a higher number of species are of tropical affinity, e.g., Mastogloia. Others such as Cocconeis thalassiana, primarily described for the Mexican Caribbean (Romero & López-Fuerte, 2013), leads us to suggest that, as with the terrestrial flora, Isla Guadalupe does not have a diatom flora with a particular bio-geographical affinity, i.e., temperate, subtropical, or tropical. Moreover, due to the distance of the island with the continent it is likely that further observations based on a more exhaustive sampling may render new records of diatom species or varieties. This, and on the basis that Isla Guadalupe is not influenced by coastal upwelling, shows a biogeographically mixed macroalgal flora that includes species from California, the Mexican tropics, and the insular Indo-Pacific, plus a conspicuous group of endemic taxa derived from the California flora. All of these are evidence of their effective isolation and the ecological divergence in a process of speciation (Dawson, 1960).
In comparison with E. desmarestioides that stands out as a proper substrate for diatoms with 29 exclusive taxa and the second most number of taxa overall, Siqueiros-Beltrones et al. (2002) did not find diatom epiphytes on Eisenia arborea Areschoug, although recent research has shown that older blades may harbor monospecific proliferations of diatoms. Along the West coast of the Baja California Peninsula, Macrocystis pyrifera (Linnaeus) C. Agardh is considered the main food source of abalone (Haliotis spp.), together with its numerous epiphytic diatom species (Argumedo-Hernández & Siqueiros-Beltrones, 2008). However, in Isla Guadalupe M. pyrifera is absent, thus its ecological role may be replaced by E. desmarestioides, which is the largest macroalgae in the area and, according to the local fishermen, its abundance relate to the that of abalone.
Aside from this being the first publication on the diatom flora of Isla Guadalupe or any other Mexican island, the high number of new records either for the region or the whole country gives an insight to a highly diverse flora hitherto unknown as an assemblage. Thus, the generated information is relevant both in bio-geographical as well as in environmental health terms. We are confident that further exhaustive research will provide much valuable information that is hard to come by, given the remoteness of the studied area. The differential distribution of exclusive diatom taxa on distinct substrates strongly supports the expectation of increasing the species richness for the island as more substrates are examined.
This study was financed by the Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, through project Conabio-JF170. Conanp permit, Folio No: F00.DRPBCPN.000025. Semarnat notice, Folio No. SGPA/DGVS/05604/12. Sagarpa permit, Folio No. PPF/DGOPA-215/2013. We thank the Semar and Cooperativa de abuloneros y langosteros de isla Guadalupe for maritime transportation. The second author is EDI and COFAA fellow of the IPN.
List of benthic diatoms recorded for the Biosphere Reserve Isla Guadalupe, Mexico. Symbols indicate the type of substrate from which it was sampled. Epiphytic (Eisenia desmarestioides*; Codium latum subsp. palmeri ■); epilithic ●; epizoic ▴ (Megastraea undosa); ♦ new records for Mexico.
| Class Bacillariophyceae Haeckel, 1878 | ||||||
| Subclass: Bacillariophycidae D.G. Mann, 1990 | ||||||
| Order: Achnanthales Silva, 1962 | ||||||
| Family: Achnanthaceae Kützing, 1844 | ||||||
| Achnanthes Bory de Saint Vincent, 1822 | ||||||
| 1. Achnanthes brevipes var. angustata (Greville) Cleve, 1894 | ● | ▴ | ||||
| 2. Achnanthes brevipes var. intermedia (Kützing) Cleve, 1895 | ▴ | |||||
| 3. Achnanthes citronella (A. Mann) Hustedt, 1937 | ♦ | ● | ||||
| 4. Achnanthes yaquinensis McIntire et Reimer, 1974 | ● | |||||
| Order: Bacillariales Hendey, 1937 | ||||||
| Family: Bacillariaceae Ehrenberg, 1831 | ||||||
| Alveus Kaczmarska et Fryxell, 1995 | ||||||
| 5. Alveus marinus (Grunow) Kaczmarska et Fryxell, 1996 (Fig. 17) | ■ | ● | ||||
| Denticula Kützing, 1844 | ||||||
| 6. Denticula kuetzingii Grunow, 1862 (Fig. 26) | * | |||||
| Nitzschia Hassall, 1845 | ||||||
| 7. Nitzschia angularis W. Smith, 1853 | ■ | |||||
| 8. Nitzschia dissipata (Kützing) Grunow, 1862 | * | ■ | ● | ▴ | ||
| 9. Nitzschia macilenta W. Gregory, 1859 | * | ■ | ● | ▴ | ||
| 10. Nitzschia marginulata var. didyma Grunow, 1880 | * | |||||
| 11. Nitzschia punctata var. coarctata (Grunow) Hustedt, 1921 | ▴ | |||||
| 12. Nitzschia sigma (Kützing) W. Smith, 1853 | ■ | |||||
| 13. Nitzschia spathulata W. Smith, 1853 | ● | |||||
| 14. Nitzschia tryblionella Hantzsch, 1860 | * | |||||
| 15. Nitzschia ventricosa Kitton, 1873 | ● | |||||
| Tryblionella W. Smith, 1853 | ||||||
| 16. Tryblionella hungarica (Grunow) Frenguelli, 1942 | * | |||||
| Order: Cymbellales D.G. Mann, 1990 | ||||||
| Family: Rhoicospheniaceae Chen et Zhu, 1983 | ||||||
| Campylopyxis L. K. Medlin, 1985 | ||||||
| 17. Campylopyxis garkeana (Grunow) L. K. Medlin, 1985 (Fig. 23) | * | |||||
| Gomphonemopsis L.K. Medlin, 1986 | ||||||
| 18. Gomphonemopsis pseudexigua cf. (R. Simonsen) L.K. Medlin, 1986 | * | |||||
| Order: Lyrellales D.G. Mann, 1990 | ||||||
| Family: Lyrellaceae D.G. Mann, 1990 | ||||||
| Lyrella Karajeva, 1978 | ||||||
| 19. Lyrella approximata (Greville) D.G. Mann, 1990 (Fig. 7) | * | |||||
| 20. Lyrella perplexoides (Hustedt) D.G. Mann, 1990 | ♦ | ● | ||||
| Order: Mastogloiales D.G. Mann, 1990 | ||||||
| Family: Mastogloiaceae Mereschkowsky, 1903 | ||||||
| Mastogloia Thwaites ex W. Smith, 1856 | ||||||
| 21. Mastogloia asperuloides Hustedt, 1933 | ♦ | ● | ||||
| 22. Mastogloia binotata (Grunow) Cleve, 1895 | ■ | ● | ||||
| 23. Mastogloia borneensis Hustedt, 1927 | * | |||||
| 24. Mastogloia ciskeiensis Giffen, 1967 | ♦ | ● | ||||
| 25. Mastogloia crucicula (Grunow) Cleve, 1895 | ● | |||||
| 26. Mastogloia crucicula var. alternans Zanon, 1948 | ■ | ● | ||||
| 27. Mastogloia erythraea Grunow, 1860 | ● | |||||
| 28. Mastogloia fimbriata (T. Brightwell) Grunow, 1863 (Fig. 8) | * | ■ | ● | ▴ | ||
| 29. Mastogloia gieskesii Cholnoky, 1963 | ■ | ● | ||||
| 30. Mastogloia mediterranea Hustedt, 1933 | * | |||||
| 31. Mastogloia obliqua Hagelstein, 1939 | ■ | |||||
| 32. Mastogloia punctatissima (Greville) Ricard, 1975 | ♦ | ● | ||||
| 33. Mastogloia rostrata (Wallich) Hustedt, 1933 | ● | |||||
| Order: Naviculales Bessey, 1907 | ||||||
| Family: Amphipleuraceae Grunow, 1862 | ||||||
| Amphiprora Ehrenberg, 1844 | ||||||
| 34. Amphiprora conspicua Greville, 1861 (Fig. 18) | ♦ | * | ||||
| Family: Brachysiraceae D. G. Mann, 1990 | ||||||
| Brachysira Kützing, 1836 | ||||||
| 35. Brachysira aff. neoexilis Lange-Bertalot, 1994 (Fig. 25) | * | |||||
| Family: Pinnulariaceae D.G. Mann, 1990 | ||||||
| Oestrupia Heiden ex Hustedt, 1935 | ||||||
| 36. Oestrupia musca (Gregory) Hustedt, 1935 | + | |||||
| Suborder: Diploneidineae D.G. Mann, 1990 | ||||||
| Family: Diploneidaceae D.G. Mann, 1990 | ||||||
| Diploneis Ehrenb. ex Cleve, 1844 | ||||||
| 37. Diploneis aestuarii Hustedt, 1939 | * | |||||
| 38. Diploneis bombus (Ehrenb.) Ehrenberg, 1853 (Fig. 14) | ■ | ● | ||||
| 39. Diploneis chersonensis (Grunow) Cleve, 1894 | ■ | ● | ||||
| 40. Diploneis coffaeiformis (Schmidt) Cleve, 1894 | ♦ | * | ||||
| 41. Diploneis crabro (Ehrenb.) Ehrenberg, 1854 | * | ■ | ● | ▴ | ||
| 42. Diploneis nitescens (Gregory) Cleve, 1894 | * | |||||
| 43. Diploneis papula (A.W.F. Schmidt) Cleve, 1894 | ■ | ● | ||||
| 44. Diploneis papula var. constricta Hustedt, 1937 | ■ | ● | ||||
| 45. Diploneis suborbicularis var. constricta Hustedt, 1937 | ♦ | * | ||||
| 46. Diploneis vacillans var. renitens (A. Schmidt) Cleve, 1894 | * | ■ | ||||
| 47. Diploneis vacillans var. vacillans (A. Schmidt) Cleve, 1894 | * | ■ | ||||
| Suborder: Naviculineae (Bessey) Hendey, 1937 | ||||||
| Family: Naviculaceae Kützing, 1844 | ||||||
| Caloneis Cleve, 1894 | ||||||
| 48. Caloneis excentrica (Grunow) Boyer, 1927 | ■ | |||||
| 49. Caloneis linearis (Grunow) Boyer, 1927 | ■ | |||||
| Navicula Bory de Saint-Vincent, 1822 | ||||||
| 50. Navicula agnita Hustedt,1955 | ■ | |||||
| 51. Navicula cancellata Donkin, 1872 | * | |||||
| 52. Navicula longa var. irregularis Hustedt, 1955 | * | |||||
| 53. Navicula zostereti Grunow, 1860 | * | |||||
| Trachyneis Cleve, 1894 | ||||||
| 54. Trachyneis aspera (Ehrenb.) P.T. Cleve, 1894 (Fig. 24) | * | ■ | ● | ▴ | ||
| 55. Trachyneis velata A. Schmidt, 1876 | * | ■ | ● | ▴ | ||
| Family: Pleurosigmataceae Mereschkowsky, 1903 | ||||||
| Pleurosigma W. Smith, 1852 | ||||||
| 56. Pleurosigma salinarum (Grunow) Grunow, 1880 | ■ | |||||
| Family: Sellaphoraceae Mereschkowsky, 1902 | ||||||
| Fallacia Stickle et D.G. Mann, 1990 | ||||||
| 57. Fallacia forcipata (Greville) Stickle et Mann, 1990 | * | |||||
| Family: Berkeleyaceae D.G. Mann, 1990 | ||||||
| Parlibellus E. J. Cox, 1998 | ||||||
| 58. Parlibellus delognei (Van Heurck) E. J. Cox, 1988 | ♦ | * | ■ | ▴ | ||
| Family: Pleurosigmataceae Mereschkowsky, 1903 | ||||||
| Donkinia Ralfs, 1861 | ||||||
| 59. Donkinia reticulata Norman, 1861 | ♦ | * | ||||
| Order: Rhopalodiales D.G. Mann, 1990 | ||||||
| Family: Rhopalodiaceae (G. Karst.) Topachevs’kyj et Oksiyuk, 1960 | ||||||
| Rhopalodia Otto Müll., 1895 | ||||||
| 60. Rhopalodia pacifica Krammer, 1987 | ■ | |||||
| Epithemia Kützing, 1844 | ||||||
| 61. Epithemia turgida (Ehrenb.) Kützing, 1844 | * | ■ | ||||
| Order: Surirellales D. G. Mann, 1990 | ||||||
| Family: Surirellaceae Kützing, 1844 | ||||||
| Campylodiscus Ehrenb. ex Kützing, 1840 | ||||||
| 62. Campylodiscus ambiguus Greville, 1860 | ♦ | * | ||||
| 63. Campylodiscus crebrecostatus var. speciosa T. Eulenstein, 1875 (Fig. 2) | ● | |||||
| 64. Campylodiscus fastuosus Ehrenberg, 1845 (Fig. 4) | ▴ | |||||
| 65. Campylodiscus simulans Gregory, 1857 | ■ | ● | ||||
| Family: Cocconeidaceae Kützing, 1844 | ||||||
| Campyloneis Grunow, 1862 | ||||||
| 66. Campyloneis grevillei Petit, 1877 (Fig. 7) | ■ | ● | ||||
| Cocconeis Ehrenberg, 1836 | ||||||
| 67. Cocconeis costata var. hexagona Grunow, 1880 | ■ | ● | ||||
| 68. Cocconeis dirupta var. flexella (Janisch et Rabenhorst) Grunow, 1880 | ● | |||||
| 69. Cocconeis discrepans A.W.F. Schmidt, 1894 | ■ | ● | ||||
| 70. Cocconeis molesta var. crucifera Grunow, 1880 (Fig. 32) | ■ | ● | ||||
| 71. Cocconeis molesta var. molesta Kützing, 1844 | ■ | ● | ||||
| 72. Cocconeis pediculus Ehrenberg, 1838 | * | |||||
| 73. Cocconeis cf. pseudomarginata Gregory, 1857 | ● | |||||
| 74. Cocconeis scutellum Ehrenberg, 1838 | * | ■ | ● | ▴ | ||
| 75. Cocconeis thalassiana O.E. Romero et F.O. López-Fuerte, 2013 | * | ■ | ● | ▴ | ||
| Psammodictyon D.G. Mann, 1990 | ||||||
| 76. Psammodictyon panduriforme (W. Gregory) D.G. Mann, 1990 | ■ | ● | ▴ | |||
| Order: Thalassiophysales D.G. Mann, 1990 | ||||||
| Family: Catenulaceae Mereschkowsky, 1902 | ||||||
| Amphora Ehrenb. ex Kützing, 1844 | ||||||
| 77. Amphora angusta Gregory, 1857 | * | ▴ | ||||
| 78. Amphora clevei Grunow, 1875 | ▴ | |||||
| 79. Amphora immarginata Nagumo, 2003 | ▴ | |||||
| 80. Amphora laevissima W. Gregory, 1857 | ● | |||||
| 81. Amphora libyca Ehrenberg, 1840 | * | |||||
| 82. Amphora proteus Gregory, 1857 | ▴ | |||||
| 83. Amphora proteus var. oculata H. Peragallo, 1898 | ♦ | ▴ | ||||
| 84. Amphora rhombica var. intermedia Cleve, 1895 | ● | |||||
| Halamphora (Cleve) Levkov, 2009 | ||||||
| 85. Halamphora coffeaeformis (C. Agardh) Levkov, 2009 | * | |||||
| 86. Halamphora costata (W. Smith) Levkov, 2009 | ● | |||||
| 87. Halamphora cymbifera (Gregory) Levkov, 2009 | ● | |||||
| Class Fragilariophyceae Round et R.M. Crawford, 1990 | ||||||
| Order: Climacospheniales Round, 1990 | ||||||
| Family: Climacospheniaceae Round, 1990 | ||||||
| Climacosphenia Ehrenberg, 1843 | ||||||
| 88. Climacosphenia moniligera Ehrenberg, 1843 (Figs. 15 and 16) | * | ■ | ● | ▴ | ||
| Order: Cyclophorales Round, 1990 | ▴ | |||||
| Family: Entopylaceae Round, 1990 | ||||||
| Gephyria Arnott, 1858 | ||||||
| 89. Gephyria media Arnott, 1860 (Fig. 19) | * | ■ | ● | ▴ | ||
| Subclass Fragilariophycidae Round, 1990 | ||||||
| Order: Fragilariales Silva, 1962 | ||||||
| Family: Fragilariaceae Greville, 1833 | ||||||
| Hyalosynedra D.M. Williams et F.E. Round, 1986 | ||||||
| 90. Hyalosynedra laevigata (Grunow) D.M. Williams et Round, 1986 | * | |||||
| Opephora Petit, 1889 | ||||||
| 91. Opephora schwartzii (Grunow) Petit ex Pelletan, 1889 | ■ | |||||
| Podocystis J. W. Bailey, 1854 | ||||||
| 92. Podocystis adriatica (Kützing) Ralfs, 1861 (Fig. 5) | * | ▴ | ||||
| Synedra Ehrenberg, 1830 | ▴ | |||||
| 93. Synedra fulgens (Greville) W. Smith, 1853 | * | ■ | ● | ▴ | ||
| 94. Synedra parva Kützing, 1844 | ● | |||||
| Tabularia (Kützing) D.M. Williams et Round, 1986 | ||||||
| 95. Tabularia fasciculata (C. Agardh) D.M. Williams et Round, 1986 | ■ | ● | ||||
| Order: Licmophorales Round et R.M. Crawford, 1990 | ||||||
| Family: Licmophoraceae Kützing, 1844 | ||||||
| Licmophora C. Agardh, 1827 | ||||||
| 96. Licmophora abbreviata C. Agardh, 1831 (Fig. 20) | * | ■ | ||||
| 97. Licmophora communis (Heiberg) Grunow, 1881 (Fig. 28) | * | |||||
| 98. Licmophora ehrenbergii (Kützing) Grunow, 1867 (Fig. 29) | * | |||||
| 99. Licmophora gracilis (Ehrenb.) Grunow, 1867 | * | |||||
| 100. Licmophora paradoxa (Lyngbye) C. Agardh, 1828 | ● | |||||
| Licmosoma Round & C.G. Alexander, 2002: 324 | ||||||
| 101. Licmosoma squamosum Round & C.G. Alexander, 2002 (Fig. 30) | ● | |||||
| Order: Rhaphoneidales Round, 1990 | ||||||
| Family: Psammodiscaceae Round et D.G. Mann, 1990 | ||||||
| Psammodiscus Round et D.G. Mann, 1980 | ||||||
| 102. Psammodiscus calceatus T. Watanabe, T. Nagumo et J. Tanaka, 2013. | ♦ | ● | ||||
| 103. Psammodiscus nitidus (Gregory) Round et D.G. Mann, 1980 | ● | |||||
| Order: Rhabdonematales Round et R.M. Crawford, 1990 | ||||||
| Family: Rhabdonemataceae Round et R.M. Crawford, 1990 | ||||||
| Rhabdonema Kützing 1844 | ||||||
| 104. Rhabdonema adriaticum Kützing, 1844 (Figs. 10 and 11) | * | ■ | ||||
| 105. Rhabdonema arcuatum (Lyngbye) Kützing, 1844 (Figs. 12 and 13) | * | |||||
| Order: Striatellales Round, 1990 | ||||||
| Family: Striatellaceae Kützing, 1844 | ||||||
| Grammatophora Ehrenberg, 1840 | ||||||
| 106. Grammatophora angulosa Ehrenberg, 1841 | ■ | |||||
| 107. Grammatophora hamulifera Kützing, 1844 | * | |||||
| 108. Grammatophora marina (Lyngbye) Kützing, 1844 (Fig. 21) | * | ■ | ● | |||
| 109. Grammatophora oceanica Ehrenberg, 1840 | * | ■ | ● | |||
| 110. Grammatophora oceanica var. macilenta (W. Smith) Grunow, 1862 | * | ■ | ▴ | |||
| 111. Grammatophora undulata Ehrenberg, 1840 (Fig. 22) | * | ■ | ● | ▴ | ||
| Striatella C. Agardh, 1832 | ||||||
| 112. Striatella interrupta (Ehrenb.) Heiberg, 1863 | * | |||||
| 113. Striatella unipunctata (Lyngbye) C. Agardh, 1832 (Fig. 36) | * | |||||
| Order: Surirellales D.G. Mann, 1990 | ||||||
| Family: Surirellaceae Kützing, 1844 | ||||||
| Plagiodiscus Grunow et Eulenstein, 1867 | ||||||
| 114. Plagiodiscus nervatus Grunow, 1867 (Fig. 27) | ● | |||||
| Surirella Turpin, 1828 | ||||||
| 115. Surirella armoricana H. Peragallo et M. Peragallo, 1899 | ● | |||||
| 116. Surirella fastuosa (Ehrenb.) Ehrenberg, 1843 (Fig. 9) | ■ | ● | ▴ | |||
| Order: Thalassionematales Round, 1990 | ||||||
| Family: Thalassionemataceae Round, 1990 | ||||||
| Thalassionema (Grunow) Grunow, 1881 | ||||||
| 117. Thalassionema frauenfeldii (Grunow) Tempère et Peragallo, 1910 | ■ | |||||
| 118. Thalassionema nitzschioides (Grunow) Mereschkowsky, 1902 | ■ | |||||
| Order: Toxariales Round, 1990 | ||||||
| Family: Toxariaceae Round, 1990 | ||||||
| Toxarium Bailey, 1854 | ||||||
| 119. Toxarium undulatum J.W. Bailey, 1854 | ■ |
Peer Review under the responsibility of Universidad Nacional Autónoma de México.