Eighty-six specimens representing 7 species of rodents from 9 localities in the state Zacatecas were examined for helminths. Only 5 host species were found to harbor at least 1 species: Chaetodipus sp., Dipodomys merriami atronasus, Neotoma mexicana, Otospermophilus variegatus, and Peromyscus sp.; in contrast Mus musculus and Reithrodontomys sp., were not parasitized. Three species of cestodes (Catenotaeniidae gen. sp., Hymenolepis sp., and Raillietina sp.) and 7 nematodes (Gongylonema sp., Heteromyoxyuris longejector, Lamotheoxyuris cf. ackerti, Mastophorus dipodomis, Rauschtineria sp., Pterygodermatites dipodomis, and Trichuris dipodomis) were recorded. The intestine is the most parasitized (7 taxa) and trematodes were not found. The highest species richness was recorded in D. merriami atronasus from Rancho La Barranca (6 taxa), which also had the most sampling effort. H. longejector in Chaetodipus sp. from El Gordillo, showed the greatest mean abundance, and mean intensity values, followed by Rauschtineria sp. in O. variegatus from Morelos-Zacatecas Road. All species are new locality records, 14 new host records, and 2 taxa recorded for the first time in Mexico: Rauschtineria sp. and M. dipodomis. This is the first study of helminth parasites of rodents from Zacatecas.
Fueron examinados 86 especímenes de helmintos representando 7 especies de roedores, de 9 localidades en el estado de Zacatecas. Solo 5 especies de hospederos se encontraron con al menos 1 especie: Chaetodipus sp., Dipodomys merriami atronasus, Neotoma mexicana, Otospermophilus variegatus y Peromyscus sp.; en contraste, Mus musculus and Reithrodontomys sp., no resultaron parasitados. Se registraron 3 especies de céstodos (Catenotaeniidae gen. sp., Raillietina sp., e Hymenolepis sp.) y 7 nemátodos (Gongylonema sp., Heteromyoxyuris longejector, Lamotheoxyuris cf. ackerti, Mastophorus dipodomis, Rauschtineria sp., Pterygodermatites dipodomis y Trichuris dipodomis). El intestino es el más parasitado (7 taxa) y no se encontraron tremátodos. La mayor riqueza de especies y el mayor esfuerzo de muestreo se registró en D. merriami atronasus del rancho La Barranca (6 taxa). H. longejector en Chaetodipus sp. de El Gordillo, mostró la mayor abundancia e intensidad promedio, seguido de Rauschtineria sp. en O. variegatus de Morelos-Zacatecas. Todos son registros nuevos de localidad, 14 registros nuevos de hospedero y 2 taxones registrados por primera vez en México: Rauschtineria sp. y M. dipodomis. Este es el primer estudio de helmintos parásitos de roedores de Zacatecas.
The vegetation cover of Mexico has been transformed, losing up to 50% of its original coverage, 22% of these areas are now composed of secondary coverage and 27% have been transformed into agricultural and livestock areas. As a consequence of this dramatic shift, mammals in Mexico have become much more endangered (López-Ortega, Ballesteros-Barrera, Acosta, & Cervantes-Reza, 2012; Martínez-Meyer, Sosa-Escalante, & Álvarez, 2014), along with the helminth parasites these species harbor. Despite the efforts made to expand our knowledge on the biodiversity and natural history of mammals distributed in Mexico (Sánchez-Cordero et al., 2014), only 24% of the species richness has been examined for helminth parasites, and few studies have been conducted in north-central Mexico (e.g., Caspeta-Mandujano, Jiménez, Peralta-Rodríguez, & Guerrero, 2013; Falcón-Ordaz, Monks, & Pulido-Flores, 2013; García-Prieto, Falcón-Ordaz, & Guzmán-Cornejo, 2012; Guzmán-Cornejo, García-Prieto, Acosta-Gutiérrez, Falcón-Ordaz, & León-Paniagua, 2012; Jiménez-Ruiz, Rosas-Valdez, & Gardner, 2013; Jiménez-Ruiz, Peralta-Rodríguez, Caspeta-Mandujano, & Ramírez-Díaz, 2014; Pulido-Flores, Monks, & Falcón-Ordaz, 2013).
The advances in faunal surveys and inventories of helminth parasites in Mexico are still modest, they have focused only on some host species and are geographically limited (Pérez-Ponce de León, García-Prieto, & Mendoza-Garfias, 2011). As a result, there are undoubtedly many species waiting to be discovered, described and identified for this group of invertebrates. The inventory of the helminth parasite fauna of Rodentia (Bowdich, 1821), which is the most species-rich order in Mexico, comprises 3 phyla: Acanthocephala, Platyhelminthes, and Nematoda, which has the highest percentage of species diversity (e.g., García-Prieto et al., 2012). The geographical distribution of these parasites include localities in 18 states, with most of them located in Hidalgo, San Luis Potosí, Ciudad de México, and Nuevo León (Falcón-Ordaz et al., 2013; García-Prieto et al., 2012; García-Prieto, Mendoza-Garfias, & Pérez-Ponce de León, 2014; García-Prieto, Osorio-Sarabia, & Lamothe-Argumedo, 2014; Pulido-Flores, Moreno-Flores, & Monks, 2005; Pulido-Flores et al., 2013).
Zacatecas is located in north-central Mexico (=Central Mexican Plateau). Its territorial surface encompasses 3.8% of the total area of the country (=75,275km2), with climate conditions including dry and semi-dry (79% the coverage territory of the state) and temperate sub-humid areas (Inegi, 2013, 2014). One hundred and fifteen species of mammals have been reported in Zacatecas, of these 66% are rodents (López-Ortega et al., 2012; Sánchez-Cordero et al., 2014). To the best of our knowledge, there are only 2 published works related to the helminth fauna of 1 species of mammal, the bat Tadarida brasiliensis mexicana Saussure, 1860 (Chiroptera) from Zacatecas (Falcón-Ordaz, Guzmán-Cornejo, García-Prieto, & Gardner, 2006; Guzmán-Cornejo, García-Prieto, Pérez-Ponce de León, & Morales-Malacara, 2003), but there is no further information on the helminth parasites of rodent wildlife from this state. Loss and changes of vegetation coverage in the state have affected at least half of the territory, which considerably reduces the area available for the establishment of populations of wild mammals (López-Ortega et al., 2012), and their helminth parasites. For this reason, it is important to carry out biodiversity studies in the state for this biological system. Here, a survey of the helminth parasite fauna of rodents from Zacatecas, Mexico is presented for the first time.
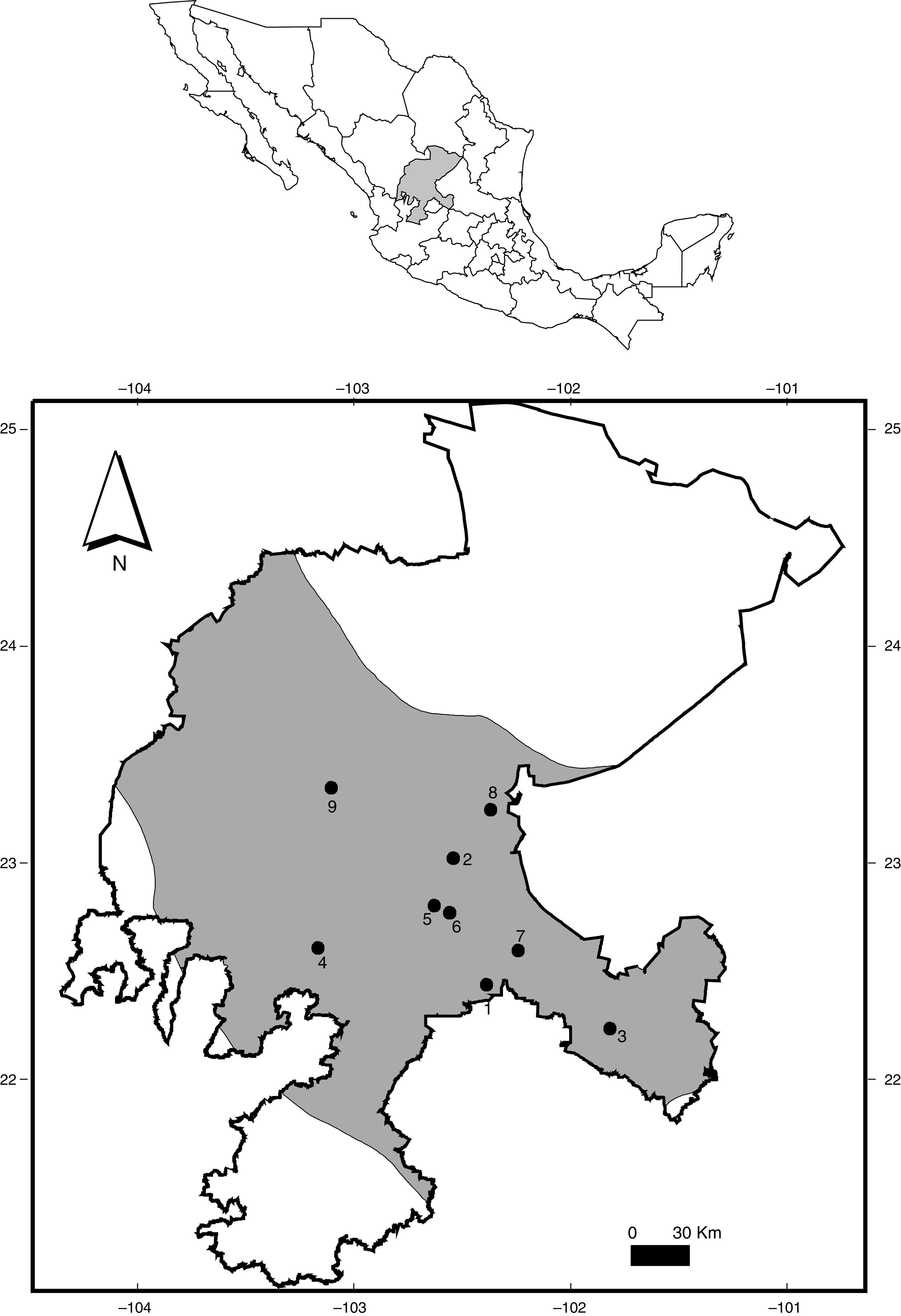
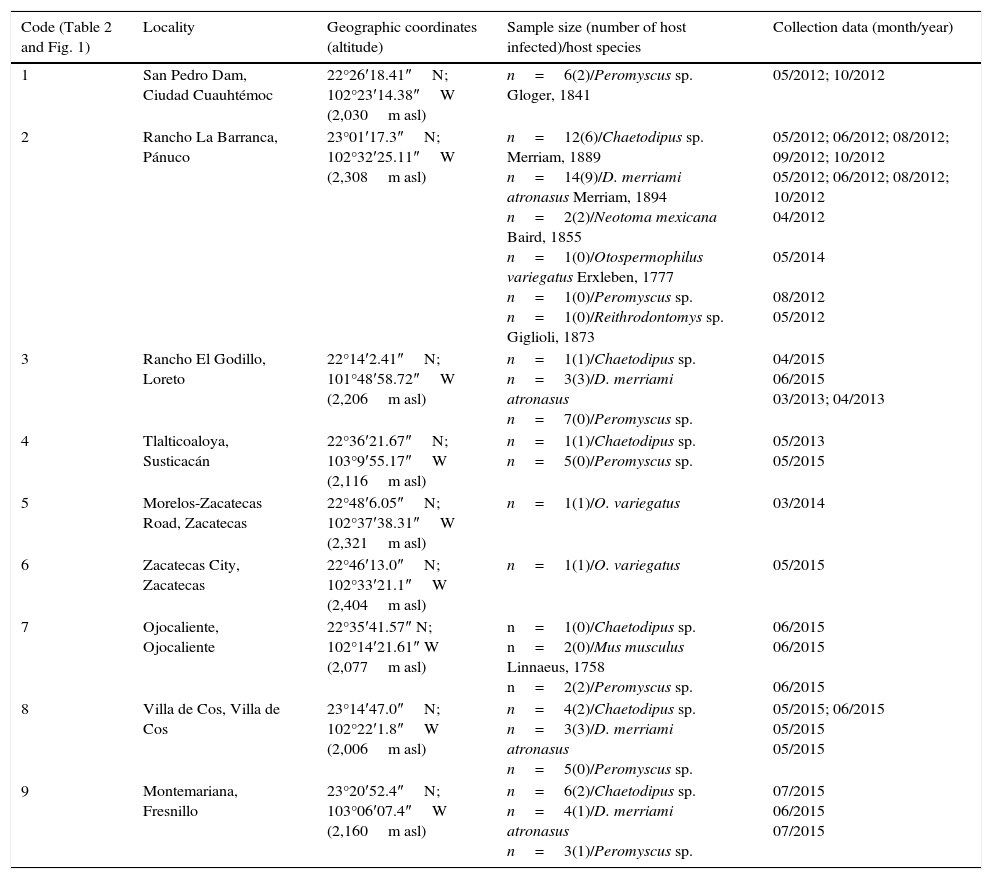
Materials and methodsEighty-six specimens of rodents were collected using live-traps baited with oats from 9 localities within Zacatecas between May 2012 to July 2015 (Table 1; Fig. 1). Four transects were sampled simultaneously for 2 nights at each locality in Spring, Summer and Autumn (15 traps×4 transects×2 nights=120 trap-nights in each locality) (collection data in Table 1). Most specimens were transported live to the laboratory, and euthanized following the standard procedures and techniques of Gannon and Sikes (2007), and were examined for helminths by standard methods under a stereoscope. Parasites were placed in 0.85% w/v saline solution, then fixed by sudden immersion in hot (steaming) 70% ethanol, and stored in 70% ethanol to preserve morphological traits for further identification. Nematodes were cleared for morphological study with Ammans's Lactophenol and with a mixture of ethanol–glycerin (2:8). Cestodes were stained with Delafield's Hematoxylin and Mayer's hydrochloric carmine, and whole-mounted in Canada balsam. Taxonomic identification was conducted by comparing morphological traits using taxonomic keys and descriptions from the specialized literature (e.g., Anderson, 2000; Falcón-Ordaz, Fernández, & García-Prieto, 2010; García-Prieto, Falcón-Ordaz, Lira-Guerrero, & Mendoza-Garfias, 2008; Khalil, Jones, & Bray, 1994; Kruidenier & Peebles, 1958; Lichtenfels, 1970; Quentin, 1970, 1973; Read & Millemann, 1953; Read, 1956; Schmidt, 1986; Smith, 1954; Tiner, 1948; Yamaguti, 1961). Parasites identified were counted to obtain the infection parameters according with the definitions suggested by Bush, Lafferty, Lotz, and Shostak (1997).
| Code (Table 2 and Fig. 1) | Locality | Geographic coordinates (altitude) | Sample size (number of host infected)/host species | Collection data (month/year) |
|---|---|---|---|---|
| 1 | San Pedro Dam, Ciudad Cuauhtémoc | 22°26′18.41″N; 102°23′14.38″W (2,030m asl) | n=6(2)/Peromyscus sp. Gloger, 1841 | 05/2012; 10/2012 |
| 2 | Rancho La Barranca, Pánuco | 23°01′17.3″N; 102°32′25.11″W (2,308m asl) | n=12(6)/Chaetodipus sp. Merriam, 1889 n=14(9)/D. merriami atronasus Merriam, 1894 n=2(2)/Neotoma mexicana Baird, 1855 n=1(0)/Otospermophilus variegatus Erxleben, 1777 n=1(0)/Peromyscus sp. n=1(0)/Reithrodontomys sp. Giglioli, 1873 | 05/2012; 06/2012; 08/2012; 09/2012; 10/2012 05/2012; 06/2012; 08/2012; 10/2012 04/2012 05/2014 08/2012 05/2012 |
| 3 | Rancho El Godillo, Loreto | 22°14′2.41″N; 101°48′58.72″W (2,206m asl) | n=1(1)/Chaetodipus sp. n=3(3)/D. merriami atronasus n=7(0)/Peromyscus sp. | 04/2015 06/2015 03/2013; 04/2013 |
| 4 | Tlalticoaloya, Susticacán | 22°36′21.67″N; 103°9′55.17″W (2,116m asl) | n=1(1)/Chaetodipus sp. n=5(0)/Peromyscus sp. | 05/2013 05/2015 |
| 5 | Morelos-Zacatecas Road, Zacatecas | 22°48′6.05″N; 102°37′38.31″W (2,321m asl) | n=1(1)/O. variegatus | 03/2014 |
| 6 | Zacatecas City, Zacatecas | 22°46′13.0″N; 102°33′21.1″W (2,404m asl) | n=1(1)/O. variegatus | 05/2015 |
| 7 | Ojocaliente, Ojocaliente | 22°35′41.57″ N; 102°14′21.61″ W (2,077m asl) | n=1(0)/Chaetodipus sp. n=2(0)/Mus musculus Linnaeus, 1758 n=2(2)/Peromyscus sp. | 06/2015 06/2015 06/2015 |
| 8 | Villa de Cos, Villa de Cos | 23°14′47.0″N; 102°22′1.8″W (2,006m asl) | n=4(2)/Chaetodipus sp. n=3(3)/D. merriami atronasus n=5(0)/Peromyscus sp. | 05/2015; 06/2015 05/2015 05/2015 |
| 9 | Montemariana, Fresnillo | 23°20′52.4″N; 103°06′07.4″W (2,160m asl) | n=6(2)/Chaetodipus sp. n=4(1)/D. merriami atronasus n=3(1)/Peromyscus sp. | 07/2015 06/2015 07/2015 |
Map of Zacatecas indicating the localities where specimens of some rodents (Cricetidae, Heteromyidae, and Sciuridae) have been examined for helminth parasites (codes in Table 1; gray=Central Mexican Plateau).
Voucher specimens of host and parasites were deposited in the Colecciones Biológicas, Unidad Académica de Ciencias Biológicas (UACB), Universidad Autónoma de Zacatecas (UAZ), Zacatecas, Zacatecas, Mexico: Colección de Vertebrados (CVZ) (mammals section) (CVZM 0016–0021, 0036–0042, 0054–0057, 0067–0090, 0153–0173); and Colección de Invertebrados no Artrópodos (CINZ) (CINZ 130–147, 175, 186–202), respectively. Additional vouchers were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City, Mexico (CNHE 9826–9827, 8195, 9996–10004).
ResultsEighty-six rodents were collected and examined for parasites from 7 host species and 9 localities in Zacatecas (44% of all hosts examined were parasitized). Only Chaetodipus sp, Dipodomys merriami atronasus, Neotoma mexicana, Otospermophilus variegatus, and Peromyscus sp. were infected with at least 1 species of helminth. No parasites were found in Reithrodontomys sp., Peromyscus sp. and O. variegatus from La Barranca; Peromyscus sp. from near Tlalticoaloya, El Godillo, and Villa de Cos; and none from Chaetodipus sp. and Mus musculus from Ojocaliente.
The helminth inventory of sampled hosts included 9 families and 10 species, as follows: (1) Platyhelminthes; 3 cestodes [Catenotaeniidae gen. sp. Spassky, 1950 (Catenotaeniidae), Hymenolepis sp. Weinland, 1858 (Hymenolepididae) and Raillietina sp. Fuhrmann, 1920 (Davaineidae)], and (2) Nematoda; 6 species [Heteromyoxyuris longejector Hannum, 1934 and RauschtineriaHugot, 1980 (Oxyuridae), Lamotheoxyuris cf. ackertiFalcón-Ordaz et al., 2010 (Heteroxynematidae), Pterygodermatites dipodomisTiner, 1948 (Rictulariidae), Gongylonema sp. Molin, 1857 (Gongylonematidae), Mastophorus dipodomisRead and Millemann, 1953 (Spirocercidae), and Trichuris dipodomisRead, 1956 (Trichuridae)]. No Trematoda were collected in the study.
Prevalence, mean abundance, mean intensity, intensity range, site of infection, hosts and localities are given in Table 2. Nematoda is the group with the highest species richness. Eight of the 10 helminth species inhabit the intestine (Catenotaeniidae gen. sp., Raillietina sp., Hymenolepis sp., H. longejector, L. cf. ackerti, Rauschtineria sp., P. dipodomis, and T. dipodomis), with an intensity ranging from 1 to 22 helminths per infected rodent. D. merriami atronasus presented the highest parasite species richness with 6 helminth species, including 1 cestode (Hymenolepis sp.) and 5 nematodes (Gongylonema sp., H. longejector, P. dipodomis, M. dipodomis, and T. dipodomis) (Table 2). All of the parasite taxa were recovered in the adult stage, with the exception of L. cf. ackerti, which was recovered as a single adult female and 2 larvae (found with the primordium of the vulva). The majority of helminth life cycles sampled here are unknown, however, they could be inferred based on information from the literature based upon closely related species (Anderson, 2000; Falcón-Ordaz et al., 2010; García-Prieto et al., 2008; Joyeux & Baer, 1945; Khalil et al., 1994; Kruidenier & Peebles, 1958; Lichtenfels, 1970; Quentin, 1970, 1973; Read & Millemann, 1953; Read, 1956; Schmidt, 1986; Smith, 1954; Tiner, 1948; Yamaguti, 1961) (Table 2).
Characterization of the infection parameters of helminths within rodents form 9 localities from Zacatecas, Mexico. Infection sites: I=intestine, S=stomach. Life cycle: CD=direct; CI=indirect. Host: Cricetidae: Nm. Neotoma mexicana; P. Peromyscus sp.; Heteromyidae: C. Chaetodipus sp.; Dm. Dipodomys merriami atronasus; Sciuridae: O. Otospermophilus variegatus. Host diet¶: G=primarily granivore; H=herbivore. HIP=host infected for parasite; N=total collected parasites; Pre (%)=prevalence; MA=mean abundance; MI=mean intensity; IR=intensity range.
| Helminth species | Localities (code Table 1) | Host/diet¶ | HIP | N | Pre (%) (HI) | MA | MI | IR | Accession Number° |
|---|---|---|---|---|---|---|---|---|---|
| Platyhelminthes: Cestoda | |||||||||
| Catenotaeniidae gen. sp I,CI | 1 | PG | 1 | 1 | 16.66 (1) | 0.166 | 1 | 1 | CINZ 137–138 |
| Hymenolepis sp.I,CI | 2 | DmG‡ | 1 | 1 | 7.14 (1) | 0.071 | 1 | 1 | CINZ 130–131 |
| Railletina sp.I,CI | 9 | CG‡ | 2 | 16 | 33.33 (2) | 2.66 | 8 | 1–14 | CINZ 196 CNHE 8195 |
| Nematoda | |||||||||
| Gongylonema sp.S,CI | 2 | DmG‡ | 1 | 1 | 7.14 (1) | 0.071 | 1 | 1 | CINZ 132 |
| Heteromyoxyuris longejectorI,CD | 2 | DmG‡ | 2 | 19 | 14.28 (2) | 1.35 | 9.50 | 9–10 | CINZ 145 CNHE 9996 |
| 2 | CG | 4 | 49 | 33.33 (4) | 4.083 | 12.25 | 3–22 | CINZ 139,146–147 CNHE 9997 | |
| 3 | CG | 1 | 21 | 100 (1) | 21 | 21 | 21 | CINZ 188 CNHE 9998 | |
| 7 | PG‡ | 2 | 10 | 100 (2) | 5 | 5 | 1–9 | CINZ 189 | |
| 8 | DmG‡ | 2 | 7 | 67 (2) | 2.333 | 3.5 | 1–4 | CINZ 190 | |
| 8 | CG | 2 | 22 | 50 (2) | 5.5 | 11 | 1–14 | CINZ 192 CNHE 9999 | |
| Rauschtineria sp.I,CD† | 5 | OH‡ | 1 | 14 | 100 (1) | 14 | 14 | 14 | CINZ 186 CNHE 9827 |
| 6 | OH‡ | 1 | 8 | 100 (1) | 8 | 8 | 8 | CINZ 187 CNHE 9826 | |
| Pterygodermatites dipodomisI,CI | 1 | PG‡ | 1 | 1 | 16.66 (1) | 0.166 | 1 | 1 | Ø |
| 2 | DmG | 1 | 7 | 7.14 (1) | 0.5 | 7 | 7 | CINZ 144 CNHE 10000 | |
| 3 | DmG | 2 | 7 | 67 (2) | 2.33 | 3.5 | 3–4 | CINZ 199 | |
| 8 | DmG | 1 | 5 | 33.33 (1) | 1.667 | 5 | 5 | CINZ 194 | |
| 8 | CG‡ | 1 | 5 | 25 (1) | 1.25 | 5 | 5 | CINZ 191 CNHE 10001 | |
| Lamotheoxyuris cf. ackertiI,CD | 2 | NmH‡ | 2 | 5 | 100 (2) | 2.5 | 2.5 | 1–4 | CINZ 136 |
| Masthophorus dipodomisS,CI† | 2 | DmG‡ | 7 | 15 | 50.00 (7) | 2.143 | 1.07 | 1–6 | CINZ 133, 140–142 CNHE 10002 |
| 3 | DmG‡ | 1 | 2 | 33.33 (1) | 0.67 | 2 | 2 | CINZ 198 | |
| 2 | CG‡ | 2 | 3 | 16.66 (2) | 0.25 | 1.5 | 1–2 | CINZ 143 | |
| 8 | CG‡ | 1 | 4 | 25 (1) | 1 | 4 | 4 | CINZ 193 | |
| Trichuris dipodomisI,CD | 2 | DmG‡ | 5 | 9 | 35.71 (5) | 0.642 | 1.08 | 1–5 | CINZ 134,135 CNHE 10003 |
| 3 | DmG‡ | 1 | 6 | 33.33 (1) | 2 | 6 | 6 | CINZ 200 | |
| 4 | CG‡ | 1 | 7 | 100 (1) | 7 | 7 | 7 | CINZ 175 CNHE 10004 | |
| 8 | CG‡ | 1 | 4 | 25 (1) | 1 | 4 | 6 | CINZ 201 | |
| 9 | DmG‡ | 1 | 3 | 24 (1) | 0.75 | 3 | 3 | CINZ 197 | |
| 9 | PG‡ | 1 | 1 | 33.33 (1) | 0.33 | 1 | 1 | CINZ 202 |
H. longejector from El Gordillo and Ojocaliente, T. dipodomis from Tlalticoaloya, Rauschtineria sp. from Morelos-Zacatecas Road and Zacatecas City, and L. cf. ackerti from Rancho La Barranca, had the highest prevalence (100%), followed by Hymenolepis sp. (80%) in D. merriami atronasus from Ojocaliente. H. longejector had the highest mean abundance (21), whereas Chaetodipus sp. from El Gordillo had the highest mean intensity (21). The nematode M. dipodomis infected 2 species of rodents (D. merriami atronasus and Chaetodipus sp.) from the same locality (Rancho La Barranca), whereas the nematode T. dipodomis was present in 2 host species (D. merriami atronasus and Chaetodipus sp.) at different localities, Rancho La Barranca and Tlalticoaloya, respectively (Table 2). H. longejector was present in 3 localities, and infected 2 species of rodents (D. merriami atronasus, and Chaetodipus sp.), and P. dipodomis was present in 3 localities also infecting 3 different host (D. merriami atronasus, Chaetodipus and Peromyscus sp.) (Table 2). Finally, Catenotaeniidae gen. sp. and P. dipodomis from San Pedro Dam, were found at the same prevalence, mean abundance, mean intensity, and intensity range, these parameters are equivalent to Hymenolepis sp., and Gongylonema sp. from Rancho La Barranca (Table 2).
DiscussionThe class Mammalia is a widespread and charismatic group of animals in Mexico (Sánchez-Cordero et al., 2014). There are very few studies on the diversity of helminths of wild mammals in Mexico, which contrasts with the number of mammalian species present in this country. Moreover, 67% of terrestrial mammals from Zacatecas are rodents (López-Ortega et al., 2012; Sánchez-Cordero et al., 2014), and have not been examined for helminths (see Falcón-Ordaz et al., 2013; García-Prieto et al., 2012; Pulido-Flores et al., 2013).
In this paper, we present new locality records for 10 taxa of helminths that are recorded in Zacatecas for the first time, and 2 species are reported for the first time in Mexico (Rauschtineria sp. and M. dipodomis). Nine species of helminths were found that result in 21 new host records, 5 taxa were identified to species, 4 to genus, 1 only to family, and 5 taxa have indirect life cycles (Table 2). Of these helminthes, 30% were cestodes (3 taxa) and the rest were nematodes (7). Nematodes showed the highest species richness, a pattern previously observed for mammal hosts in North America (e.g., Decker, Duszynski, & Patrick, 2001; García-Prieto, Mendoza-Garfias et al., 2014; García-Prieto, Osorio-Sarabia et al., 2014; King & Babero, 1974; Munger & Slichter, 1995; Read & Millemann, 1953) (Table 2).
Studies on the life cycles of the helminths of mammals are relatively few (Georgiev, Bray, & Littlewood, 2006; Morand, Bouamer, & Hugot, 2006). We present information of congener species or species of the same family or order and extrapolate them to the present infections only when they are constant at the relevant taxonomic level. This includes an arthropod as the intermediate host for tapeworms and the direct pattern of transmission for most pinworms (i.e., Anderson, 2000; Joyeux & Baer, 1945; Lichtenfels, 1970; Schmidt, 1986; Yamaguti, 1961). This allow us to understand the relationships among a variety of host-related and environmental-related factors in semi-arid and arid environmental (Bienek & Klikoff, 1974; Reichman, 1975).
The cestodes observed here include 3 taxa, Catenotaeniidae gen. sp., Hymenolepis sp. and Railletina sp. Regarding their life cycles, it is known that members of Catenotaeniidae (whose host range is restricted to rodents) (Table 2), use mites (Siphonaptera) as intermediate hosts (Joyeux & Baer, 1945), while hymenolepidids use beetles (Coleoptera) (Cunningham & Olson, 2010). Therefore, it is assumed that arthropods are included in the diet of the rodents studied. However, the cestodes have a relative low prevalence (Table 2), suggesting that arthropods represent a low proportion of the diet reported in the literature (Decker et al., 2001).
For identification to species level, more specimens of cestodes will be required, allowing us to confirm if these specimens correspond to other species recorded in the country. For example, Catenotaeniids are represented in Mexico by 1 species from a Central Mexican locality (Carmona-Huerta, 1994). Hymenolepids include 3 species, Hymenolepis diminuta Rudolphi, 1819, Hymenolepis horrida Linstow, 1910 and Hymenolepis sp. from 3 different hosts in the Transmexican Volcanic Belt (Carmona-Huerta, 1994; García-Prieto et al., 2012) and hosts of Raillietina sp. from Mexico include 7 rodent species from the Mexican Plateau and Mexican Transvolcanic Belt (García-Prieto et al., 2012; Underwood, Owen, & Engstrom, 1986). The distribution previously reported for cestodes and the typical occurrence of these in rodents, is supported by the data found in the current study. It should be noted that hymenolepid cestodes are typically parasites of heteromyids from North America (Gardner, 1985; Gardner & Schmidt, 1987).
The nematode species and localities reported herein represent 12 new hosts and 25 new locality records (Table 2). Specifically, within these host mammals Gongylonema sp. and Rauschtineria sp., are recorded for the first time in D. merriami atronasus and O. variegatus, respectively for Mexico. It is noteworthy that N. mexicana represents a new host record for L. cf. ackerti, a species recorded exclusively from the genus Neotoma, Neotoma nelsoni Goldman, 1905 in North America (Falcón-Ordaz et al., 2010). M. dipodomis is recorded for the first time in Mexico. Identification to species level was possible with the exception of Gongylonema sp., whose individual were all females, as male specimens are required for proper identification.
Two life cycle groups of nematodes are recognized. Gongylonema sp., M. dipodomis and P. dipodomis have indirect life cycle (heteroxenic parasites species), where the individuals of the 3 genera require an arthropod as an intermediate host (Coleoptera, Lepidoptera, Orthoptera or Siphonaptera) to complete their life cycles (Dyer & Olsen, 1967; King & Babero, 1974; Ransom & Hall, 1915). The other 4 taxa have direct life cycles (monoxenic parasite species) with no intermediate hosts (Anderson, 2000).
The nematode Rauschtineria sp. is known to parasitize terrestrial Sciuridae by habiting the intestinal caecum (Hugot, 1980; Hugot, Feliu, Douangboupha, & Ribas, 2013), and may develop through a direct life cycle as other oxyurids (Anderson, 2000; Gibbons, 2010; Hugot et al., 2013), and this species has not been previously reported in Mexico. There are other known Oxyuridae parasites of squirrels in México, namely Syphatineria sp., a parasite of aerial Sicuridae, Sciurus aureogaster Cuvier, 1829 from México City, and Citellina abdita (Caballero-y Caballero, 1937), parasitizing the terrestrial Sciuridae, O. variegatus (=reported originally as Spermophilus variegatus) from Guanajuato and Hidalgo (García-Prieto et al., 2012). This is the first record of the species and a new host record for the genus in Mexico.
No trematodes were found in this study; however, 6 species of trematode parasites in rodents from Mexico have previously been reported (García-Prieto et al., 2012). One species, Caballerolecythus ibunamiLamothe-Argumedo, Falcón-Ordaz, García-Prieto, and Fernández-Fernández (2005), has been previously reported in other heteromyid host (Liomys irroratus) from the Transmexican Volcanic Belt (Falcón-Ordaz, Acosta-Gutiérrez, Fernández, & Lira-Guerrero, 2012; Lamothe-Argumedo et al., 2005). For localities where trematode species were reported from semiarid zones with similar conditions as the localities from Zacatecas, however, the prevalence of trematodes was low. A possible explanation for this phenomenon is that arid ecosystems generate a lower exposure rate of arthropods (second intermediate host) and to snails (first intermediate host). Conversely, species of Brachylaima spp. are parasites of heteromyids (i.e. Liomys pictus) and Crisetids (i.e. Peromyscus guatemalensis and P. difficilis), from tropical zones in Mexico (Caballero-Deloya, 1970; Ubelaker & Dailey, 1966), where habitats are optimal for the survival of snails and exposure to secondary intermediate hosts should be more frequent (Yamaguti, 1971, 1975).
Rodent diet studies associated with semi-arid and arid regions which has been scarcely documented (e.g., Bienek & Klikoff, 1974; Reichman, 1975). Decker et al. (2001) suggested that rodents in dry environments consume variable quantities of arthropods (15.5% of diet) depending upon availability in the ecosystem, while green vegetation made up only 6.1% of the diet (see Reichman, 1975). In contrast, in higher moisture environments, a granivorous diet is preferred. About half of the diversity we sampled is represented by helminths with indirect life cycles (Table 2), which parasitize host species that are primarily considered as “granivores” (González-Salazar, Martínez-Meyer, & López-Santiago, 2014), which suggests that the diet of these hosts is variable (excluding N. mexicana). Even though indirect life cycles are predominant in the species reported here, the nematode H. longejector (direct life cycle) is the most abundant species and is present in all of the seasons analyzed (Flores-Rodríguez & Martínez-Salazar, Pers. Obs.).
Parasites can be indicative of food-web structure and environmental conditions (Decker et al., 2001; Marcogliese & Cone, 1997), and based upon the presence of helminth parasites with both types of life cycles, we suggest that the localities analyzed still represent an optimal ecological environment for them. Unfortunately, not much is known about the life cycles of these particular species since most of the information is known at the supra-specific level; such is the case of the cestodes, Catenotaeniidae gen sp., Hymenolepis sp., and the nematodes P. dipodomis, Gongylonema sp. and M. dipodomis. All of these species are thought to require arthropods as an intermediate host to complete their life cycles (Cunningham & Olson, 2010; King & Babero, 1974; Luong & Hudson, 2012; Lyons, 1978). Systematic studies of the hosts that characterize the diet would help to elucidate the life cycles of their parasites, and may help to answer the question as to why helminths with indirect life cycles are prevalent in these rodents from arid and semi-arid environments (Bienek & Klikoff, 1974; Decker et al., 2001; Garner, Richardson, & Felts, 1976; Reichman, 1975).
As for the geographical distribution of our results, no apparent pattern is observed in terms of the closeness of the locations and parasites. For example, sites 2, 3 and 8, have 4 species of nematodes in common (T. dipodomis, H. longejector, P. dipodomis and M. dipodomis), however even though localities 2 and 8 are geographically close, locality 3 is located away far away from them. Furthermore, the presence of parasite species appears to be more related to the rodent family they occur within; we observed that there is host specificity by parasites for the families Heteromydae and Cricetidae. This assertion is supported by the fact although species of both families were found in locations 2, 3, 7, 8 and 9, no parasite species are shared by members of both families. Furthermore, only one locality has a shared parasite (T. dipodomis) between Peromyscus sp. (Cricetidae) and D. merriami atronasus (Heteromydae). Species richness could be underestimated based on the sampling effort in our study (Tables 1 and 2); therefore, a greater sampling effort is required at specific localities (e.g. Morelos-Zacatecas Road, San Pedro Dam, Ciudad Cuauhtémoc or Zacatecas city). Although the number of hosts collected was asymmetric, we found fauna typical of D. merriami from North America (i.e., H. longejector, P. dipodomis, Gongylonema sp. and M. dipodomis) (Decker et al., 2001; King & Babero, 1974; Voge, 1956). The helminth fauna found in D. merriami of Zacatecas is similar to other localities of the Mexican Plateau (Durango, San Luis Potosí and Guanajuato, Falcón-Ordaz, Pers. Obs.). The presence of these species of nematodes in D. merriami atronasus provides evidence that the environments where the host is similar throughout their distribution. Records for helminth parasites and their geographical distribution was not enough to demonstrate a geographical pattern in Mexico, but nevertheless, Nearctic affinities within helminth fauna reported here in the Central Mexican Plateau is evident.
The geographic distribution and helminth species richness was heterogeneous in this study. Most of the sampling effort during these years was focused on Rancho La Barranca primarily due to the accessibility of the site (Fig. 1, Table 2). This site is next to anthropogenic activities which includes livestock and pastureland, but the landscape is relatively conserved, as well as the other collecting sites. All sites sampled presented almost 1 species of parasite for each (Tables 1 and 2), which may reflect a relatively healthy ecosystem.
It is necessary to increase our knowledge of the dietary preferences of rodents associated with arid and semi-arid desert environments, as well as information on the life cycles of parasitic helminths as an indicator of the food-web structure and trophic interactions. Likewise, monitoring the populations of these host-parasite systems associated with global climate change data and the correlation with anthropogenic landscape changes, could contribute to conservation in arid and semi-arid environments within Mexico in order to provide information for conservation. This is the first study in this region for these host-parasite systems and the beginning of a systematic inventory of parasites of wild mammals. This allows us to address future questions related to the evolution and biogeography of the parasite-host systems in Zacatecas.
To Alejandra Sandoval, Daniel Ochoa, Jeziel González, Fernando Córdova, Rodolfo Zacarías and Zaira Esparza for collecting and assisting the collection of some specimens from El Gordillo, near Tlalticoaloya, Morelos-Zacatecas Road, Zacatecas City, Montemariana and Villa de Cos. To Rosa Ma. Ramírez, Edith Varela, Ma. Elena Varela, Eloy Varela, and Alan Lara for hosting during fieldwork in Rancho la Barranca. To Cristina Raudales, Judith Medina, Lucero Landeros, Byanca Velázquez, Edith Esparza, and Oscar Romo for their assistance with fieldwork in Rancho la Barranca and San Predro Dam. To Aranxa Hernández, Jesús Sígala, Joane Delgado, Emmeth Rodríguez, Monica Díaz, Octavio Rodríguez, and Oscar Vázquez for their help collecting in San Pedro Dam. We would also like to thank Edgar Sánchez, Susana Trejo, and Anarosa Olmedo for their assistance in the laboratory. Special thanks go to Tyler Elliott and Nicholas W. Jeffery for the revision of the English language, and 2 anonymous referees whose comments greatly improved this manuscript. Scientific collecting license FAUT-268 (E.A. Martínez-Salazar). J. Falcón-Ordaz would like to thank PRODEP-SEP for the program “Apoyo a la Incorporación de Nuevos PTC” at UAEH. R. Rosas-Valdez thanks to PRODEP-SEP through the program “Apoyo a la Incorporación de Nuevos PTC” (UAZ-PTC-PTC-194). E.A. Martínez-Salazar is supported by Conacyt through the program “Apoyos Complementarios para la Consolidación Institucional de Grupos de Investigación (Repatriación 2010)”, FOMIX-ZAC-2011-01-C01-170798, and PRODEP-SEP through the program “Apoyo a la Incorporación de Nuevos PTC” (UAZ-PTC-169), and UAZ-2013-36452.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.