Between May and November 2015, 8 specimens of Didelphis marsupialis Linnaeus, 1758 (Didelphimorphia: Didelphidae) collected in San Martín, Peru were examined for the presence of helminths. A total of 582 helminths representing 11 taxa were identified (2 digeneans and 9 nematodes). Five new host records and 4 species of nematodes [Gongylonemoides marsupialis (Vaz & Pereira, 1934) Freitas & Lent, 1937, Trichuris didelphis Babero, 1960, Viannaia hamata Travassos, 1914 and Viannaia viannaia Travassos, 1914] are added to the composition of the helminth fauna of the marsupials in this country. Further, a checklist of all available published accounts of helminth parasites reported from Peru is provided. To date, a total of 38 helminth parasites have been recorded. Digeneans have the highest species richness in number and percentage (n=19, 50%), followed by nematodes (n=17, 45%) and acanthocephalans (n=2, 5%). The parasites with the highest number of records were the digeneans Plagiorchis didelphidis (Parona, 1896) Stossich, 1904 (n=4) and Rhopalias coronatus Kifune & Uyema, 1982 (n=4) and the nematode Aspidodera sp. (n=4). Additional sampling in this country will probably increase the richness of the helminthological inventory of this group of mammals.
Entre mayo y noviembre del 2015, 8 ejemplares de Didelphis marsupialis Linnaeus, 1758 (Didelphimorphia: Didelphidae) recolectados en San Martín, Perú, fueron examinados en busca de helmintos. Un total de 582 helmintos representando 11 taxones fueron identificados (2 digéneos y 9 nemátodos). Cinco registros nuevos y 4 especies de nemátodos [Gongylonemoides marsupialis (Vaz y Pereira, 1934) Freitas y Lent, 1937, Trichuris didelphis Babero, 1960, Viannaia hamata Travassos, 1914 y Viannaia viannaia Travassos, 1914] se agregan a la composición de la fauna de helmintos de marsupiales de Perú. Además, se proporciona una lista de todos los registros publicados de este grupo de parásitos para marsupiales en dicho país. Hasta la fecha, se ha registrado un total de 38 helmintos; los digéneos tienen la mayor riqueza de especies en número y porcentaje (n=19, 50%), seguido por los nemátodos (n=17, 45%) y acantocéfalos (n=2, 5%). Los parásitos con mayor número de registros fueron los digéneos Plagiorchis didelphidis (Parona, 1896) Stossich, 1904 (n=4) y Rhopalias coronatus Kifune y Uyema, 1982 (n=4) y el nemátodo Aspidodera sp. (n=4). Nuevos muestreos en este país probablemente aumentarán la riqueza del inventario helmintológico de este grupo de mamíferos.
With 508 species of native mammals, Peru is the third most diverse country in the New World, after Brazil and Mexico, and the fifth most diverse for mammals in the world (Pacheco, Cadenillas, Salas, Tello, & Zeballos, 2009). In Peru, the order Didelphimorphia is represented by the family Didelphidae with 40 species in 13 recognized genera (Pacheco et al., 2009). However, despite this great diversity, the information on its helminth parasites is still very scarce (Tantaleán, Díaz, Sánchez, & Portocarrero, 2010).
The common opossum, Didelphis marsupialis Linnaeus, 1758, is a marsupial species in the family Didelphidae, living in rainforest and subtropical forest, secondary forest, and near human settlements. This species is widely distributed from Mexico, south to Peru, Bolivia, Paraguay and northeastern Argentina, including Trinidad and the Lesser Antilles (Aponte, 2013; Emmons & Feer, 1997; Rueda, Ramírez, & Osorio, 2013). It is listed as presenting Least Concern status by the International Union for Conservation of Nature and Natural Resources (IUCN Red List). Information about parasite diversity of this species in different countries along its geographical distribution is scarce (Acosta-Virgen, López-Caballero, García-Prieto, & Mata-López, 2015; Fernandes, Justo, & Cárdenas, 2015; García-Prieto, Falcón-Ordaz, & Guzmán-Cornejo, 2012; Jiménez, Catzeflis, & Gardner, 2011; Rodríguez-Ortiz, García-Prieto, & Pérez-Ponce de León, 2004). Although there are some reports of digeneans (Kifune & Uyema, 1982; Miyazaki, Kifune, Habe, & Uyema, 1978; Tantaleán, Sarmiento, & Huiza, 1992), nematodes (Arrojo, 2002; Sarmiento, Tantaleán, & Huiza, 1999; Tantaleán et al., 2010) and acanthocephalans (Tantaleán, Sánchez, Gómez, & Huiza, 2005) from D. marsupialis in Peru, the knowledge of the helminth richness associated with this host species is still incomplete due to the wide distribution of this host in Peru.
In the present study, we report new records of helminth species parasitizing D. marsupialis in Peru. In addition, a checklist of helminth parasites of Peruvian marsupials is presented.
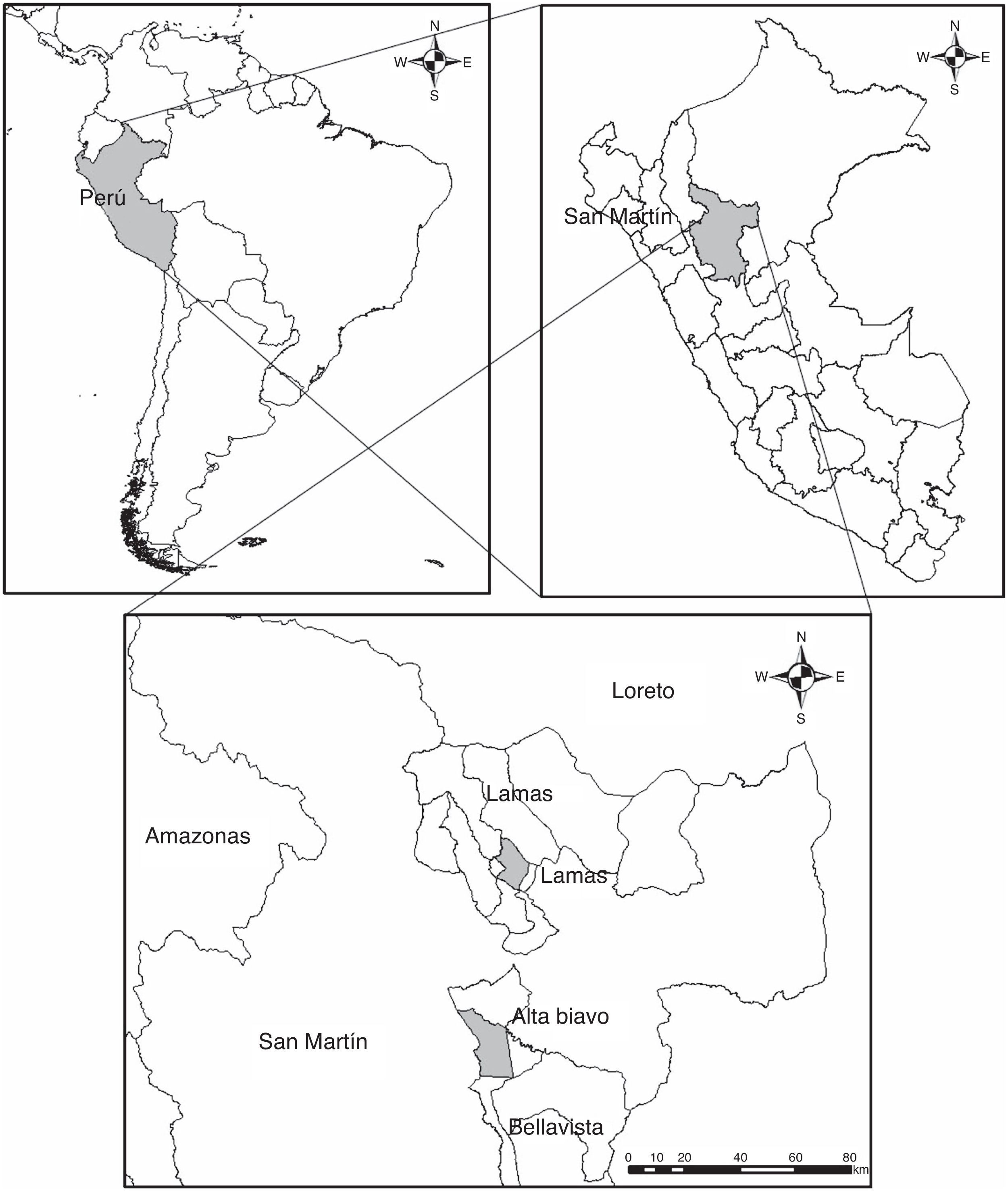
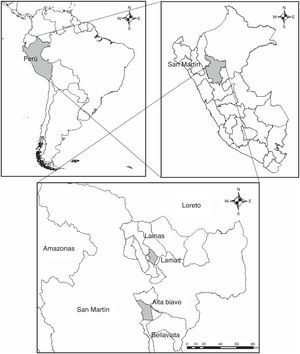
Materials and methodsBetween May and November 2015, 8 specimens of D. marsupialis (Didelphidae) were found dead in the jungle of San Martín, Peru (Fig. 1). The specimens were collected and transferred to the laboratory of Clinical Analysis Morales Lab. for the respective necropsy. During the necropsy of hosts, trematodes and nematodes were extracted from the gastrointestinal tract, placed in Petri dishes with tap water, fixed in 4% hot formaldehyde and preserved in 70% ethanol. For morphological study, trematodes were stained with Semichon's carmine, dehydrated in successive series of ethanol (up to absolute ethanol), cleared in Eugenol and mounted in Canada balsam. Nematodes were cleared in Amman's lactophenol and temporarily mounted for morphological study (Lamothe-Argumedo, 1997). The parasites were analyzed and measured using a Leica-DM500 microscope with LEICA-ICC50 HD camera and Software LAS (Leica Application Suite), EZ versión 1.80, 2009, Switzerland. Measurements are in millimeters (mm). The taxonomic determination of the parasites was in accordance with the diagnosis proposed by Gibson, Jones, and Bray (2002) and Haverkost and Gardner (2008) for trematodes, and Chagas-Moutinho, Oliveira-Menezes, Cárdenas, and Lanfredi (2007), Lent and Freitas (1937a,b), Santos, Lent, and Gomes (1990), Travassos (1922), Vicente (1966), and Vicente, Rodrigues, Gomes, and Pinto (1997) for nematodes. The terms prevalence and mean intensity were used according to Bush, Lafferty, Lotz, and Shostak (1997).
Vouchers of all helminth species were deposited in the Helminthological and Minor Invertebrates Collection of the Museum of Natural History at the San Marcos University (MUSM), Peru.
Furthermore, a checklist was compiled based on the new and previous reports of helminths parasites of marsupials from Peru. Undergraduate theses and scientific meetings do not constitute formal publications, and thus were not considered. Records published until April 2016 were included. The checklist consists of 2 sections: the first part is the list of helminth parasites of marsupials, indicating host, site of infection, locality and reference. The taxonomy of helminths follows Amin (2013) for Acanthocephala, Anderson, Chabaud, and Willmott (2009) for Nematoda, and Gibson et al. (2002) for Trematoda. The second part includes the list of hosts and their respective parasites. Marsupial species are in alphabetical order. The updated name of marsupials follows Pacheco et al. (2009).
In this work, all applicable institutional, national and international guidelines for the care and use of wild animals were followed. Furthermore, all individuals of the host D. marsupialis were found dead and are not considered as Critically Endangered by IUCN.
ResultsA total of 582 endoparasites were collected in the study specimens and the total prevalence was 100%. Morphological analyses of parasites permitted the identification of 11 taxa (2 digeneans and 9 nematodes) belonging to 9 genera and 8 families.
Phylum Platyhelminthes Gegenbaur, 1959
Class Trematoda Rudolphi, 1808
Family Plagiorchiidae Ward, 1917
Plagiorchis didelphidis (Parona, 1869) Stossich, 1904
Description: based on 5 adult specimens. Body 4.754–6.455 (5.452) long, maximum width 1.383–1.724 (1.583). Tegument covered with numerous small spines. Oral sucker subterminal, 0.420–0.564 (0.518) long by 0.537–0.708 (0.649) wide. Pharynx 0.239–0.333 (0.303) long by 0.260–0.393 (0.348) wide. Acetabulum 0.676–0.956 (0.837) long by 0.708–1.026 (0.872) wide. Anterior testis 0.421–0.611 (0.513) long by 0.475–0.703 (0.554) wide. Posterior testis 0.444–0.640 (0.528) long by 0.455–0.655 (0.535). Ovary 0.268–0.328 (0.289) long by 0.213–0.337 (0.289) wide. Eggs numerous, 0.042–0.046 long by 0.019–0.021 wide.
Taxonomic summaryInfection site: small intestine.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W).
Specimens deposited: MUSM 3400.
Prevalence: 25% (2 infected marsupials of 8).
Mean intensity: 8.
Mean abundance: 2.
Species of Plagiorchis Luehe, 1899 are intestinal trematodes of amphibians, reptiles, birds and mammals (Rodrigues, 1994). In Peru, 2 species are currently known: P. didelphidis from D. marsupialis, Didelphis albiventris Lund, 1840, Metachirus nudicaudatus (É. Geoffroy, 1803) and Philander opossum (Linnaeus, 1758) and Plagiorchis sp. from Leucophaeus pipixcan (Wagler, 1831) (Tantaleán et al., 1992). This species is also described for Brazil and Paraguay (Fernandes et al., 2015).
Family Rhopaliasidae (Looss, 1899) Yamaguti, 1958
Rhopalias caballeroi Kifune & Uyema, 1982
Description: based on 5 adult specimens. Body 3.489–4.090 (3.79) long, maximum width 0.820–1.074 (0.95). Oral sucker 0.058–0.254 (0.130) long by 0.070–0.283 (0.130) wide. Pharynx 0.053–0.223 (0.121) long by 0.030–0.149 (0.077) wide. Esophagus 0.097 long. Acetabulum 0.319–0.370(0.345) long by 0.289–0.341 (0.315) wide. Cirrus sac 0.230–0.938 (0.512) long. Anterior testis 0.488–0.541 (0.515) long by 0.515–0.547 (0.531) wide. Posterior testis 0.582–0.924 (0.750) long by 0.438–0.448 (0.440) wide. Ovary 0.120–0.229 (0.169) long by 0.233–0.238 (0.236) wide. Eggs 0.093–0.099 (0.096) long by 0.057–0.060 (0.058) wide.
Taxonomic summaryInfection site: small intestine and tongue.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W); Lamas, San Martín, Peru (6°25′S, 76°30′W).
Specimens deposited: MUSM 3401.
Prevalence: 50% (4 infected marsupials of 8).
Mean intensity: 2.5.
Mean abundance: 0.625.
Species of Rhopalias (Rudolphi, 1819) are parasites of the small intestines of marsupials from the Nearctic and Neotropical regions (Haverkost & Gardner, 2008; Fernandes et al., 2015). Five species, namely R. baculifer Braun, 1900, R. caballeroi, R. coronatus Kifune & Uyema, 1982, R. horridus (Diesing, 1850) and Rhopalias sp. have been reported in Peru (Miyazaki et al., 1978; Morales, Sarmiento, Sánchez, Floríndez, & Lamas, 2005; Tantaleán & Chávez, 2004; Tantaleán et al., 1992). According to Haverkost and Gardner (2008), R. caballeroi is distinguished by the absence of oral and flanking spines, and because it has between 4 and 11 spines visible within tentacle sacs. This species has also been reported for Argentina, Bolivia, Brazil, Colombia, Mexico, and Paraguay (Acosta-Virgen et al., 2015; Fernandes et al., 2015).
Rhopalias coronatus (Rudolphi, 1819) Stiles & Hassall, 1898
Description: based on 5 adult specimens. Body 2.452–9.352(4.436) long, maximum width 0.212–1.581(0.733). Oral sucker 0.120–0.344 (0.183) long by 0.088–0.325 (0.178) wide. Pharynx 0.111–0.421 (0.201) long by 0.042–0.244 (0.121) wide. Acetabulum 0.149–0.821 (0.376) long by 0.156–0.800 (0.352) wide. Cirrus sac 0.562–2.198 (0.966) long. Anterior testis 0.158–0.624 (0.336) long by 0.102–0.263 (0.160) wide. Posterior testis 0.249–0.900 (0.500) long by 0.075–0.276 (0.149) wide. Ovary 0.070–0.345 (0.164) long by 0.089–0.351 (0.177) wide. Eggs 0.069–0.105 (0.092) long by 0.031–0.068 (0.048) wide.
Taxonomic summaryInfection site: small intestine and tongue.
Locality: Lamas, San Martín, Peru (6°25′S, 76°30′W).
Specimens deposited: MUSM 3402.
Prevalence: 25% (2 infected marsupials of 8).
Mean intensity: 11.
Mean abundance: 2.75.
This species is a common intestinal parasite of marsupials from North, Central and South America (Fernandes et al., 2015; Haverkost & Gardner, 2008; Rivillas, Caro, Carvajal, & Vélez, 2004). According to Haverkost and Gardner (2008), R. coronatus is distinguished by the presence of flanking and oral spines, between 3 and 11 spines visible within tentacle sacs, which extend far beyond the posterior margin of the pharynx. In Peru, R. coronatus has been registered in four marsupial hosts. This species has also been recorded from Argentina, Bolivia, Brazil, Costa Rica, Ecuador, Mexico, Paraguay, and Venezuela (Acosta-Virgen et al., 2015; Fernandes et al., 2015; Rodríguez-Ortiz et al., 2004).
Phylum Nematoda Rudolphi, 1808
Family Aspidoderidae Skrjabin & Schikhobalova, 1947
Aspidodera raillietiTravassos, 1913
Description: based on 12 adult specimens (6 male and 6 female). Male: Body 5.78–7.91 (7.10) long by 0.22–0.32 (0.28) wide. Cephalic expansion 0.12–0.16 (0.14) long. Esophagus 0.75–0.89 (0.84) long, bulb 0.22–0.27 (0.25) long by 0.17–0.21 (0.19) wide. Excretory pore at 0.60–0.76 (0.68) from anterior end. Spicules 0.84–1.07 (0.94) long. Gubernaculum 0.20–0.23 (0.21) long. Cloaca at 0.41–0.51 (0.47) from posterior end. Female: Body 7.60–8.67 (8.05) long by 0.41–0.49 (0.45) wide. Cephalic expansion 0.15–0.18 (0.16) long. Esophagus 0.85–1.06 (0.98) long, bulb 0.25–0.30 (0.27) long by 0.22–0.25 (0.24) wide. Excretory pore at 0.61–0.73 (0.68) from anterior end. Vulva located at the middle of the body 2.93–3.09 (3.02) from the anterior end. The anal opening at 1.03–1.13 (1.08) from the posterior end. Eggs 0.06–0.07 (0.068) long by 0.04–0.05 (0.043) wide.
Taxonomic summaryInfection site: small intestine.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W); Lamas, San Martín, Peru (6°25′S, 76°30′W).
Specimens deposited: MUSM 3251.
Prevalence: 63% (5 infected marsupials of 8).
Mean intensity: 18.2.
Mean abundance: 11.37.
Nematodes of the family Aspidoderidae are widely distributed in the Americas, in countries such as Brazil, Mexico, Panama, Paraguay, Trinidad, Argentina, USA, Peru, Bolivia, Guatemala, Venezuela, and Suriname (Chagas-Moutinho et al., 2007). These nematodes are parasites of mammals of the orders Edentata, Marsupialia, and Rodentia (Chagas-Moutinho et al., 2007; Jiménez-Ruiz, Gardner, & Varela-Stokes, 2006; Santos et al., 1990). Santos et al. (1990) reviewed the genus and established some characters to identify these nematodes to the level of species, which include the cephalic cordons, the shape and length of the spicules, the shape of the spinneret, and the number of caudal papillae (Gomes, da Cruz, Vicente, & Pinto, 2003; Jiménez-Ruiz et al., 2006). Aspidodera raillieti was described by Travassos (1913) on the basis of specimens collected in the caecum from the Brazilian Common Opossum Didelphis aurita (Wied-Neuwi, 1826) from Brazil. The taxonomic characters were performed by Chagas-Moutinho et al. (2007) and Santos et al. (1990). According to Sarmiento et al. (1999) specimens of Aspidodera harwoodi Chandler, 1932 have been recorded from D. marsupialis in Peru. However, Santos et al. (1990) considered A. harwoodi synonymous of A. raillieti. Other hosts of A. raillieti include Caluromys lanatus (Olfers, 1818); Didelphis virginiana Allen, 1900; D. albiventris; D. aurita; Chironectes minimus (Zimmermann, 1780); Chiropotes satanas (Hoffmannsegg, 1807); P. opossum and Nectomys squamipes (Brants, 1827) and Tolypeutes tricinctus (Linnaeus, 1758); T. apereoides in Brazil and D. virginiana in Mexico (Acosta-Virgen et al., 2015; Pinto, Knoff, Gomes, & Noronha, 2011; Vicente et al., 1997). This species is also listed in Brazil and Mexico, in D. marsupialis (Chagas-Moutinho et al., 2007; Jiménez et al., 2011).
Family Kathlaniidae Travassos, 1918
Cruzia tentaculata (Rudolphi, 1819) Travassos, 1917
Description: based on 10 adult specimens (5 male and 5 female). Male: Body 11.78–12.91 (12.30) long by 0.54–0.71 (0.68) wide. Esophagus 2.75–2.89 (2.84) long, bulb 0.29–0.30 (0.25) long by 0.27–0.30 (0.28) wide. Excretory pore at 1.18–1.30 (1.20) from anterior end. Spicules 0.80–0.93 (0.89) long. Gubernaculum 0.16–0.20 (0.19) long. Cloaca at 0.15–0.20 (0.18) from posterior end. Female: Body 11.60–12.67 (10.05) long by 0.50–0.58 (0.51) wide. Oral capsule 0.18–0.26 (0.22) long. Esophagus 1.85–2.06 (1.98) long, bulb 0.25–0.28 (0.27) long by 0.26–0.29 (0.27) wide. Excretory pore at 1.18–1.30 (1.23) from anterior end. Vulva located at the middle of the body 5.24–5.34 (5.26) from the anterior end. The anal opening at 0.70–1.09 (0.98) from the posterior end. Eggs 0.1–0.12 (0.11) long by 0.04–0.05 (0.04) wide.
Taxonomic summaryInfection site: small intestine.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W); Lamas, San Martín, Peru (6°25′S, 76°30′W).
Specimens deposited: MUSM 3253.
Prevalence: 63% (5 infected marsupials of 8).
Mean intensity: 15.2.
Mean abundance: 9.5.
Species of Cruzia are parasites of the large intestine of marsupials, reptiles, amphibians, and mammals (Adnet, Anjos, Menezes-Oliveira, & Lanfredi, 2009). The specimens collected from the small intestine of D. marsupialis belonging to the genus CruziaTravassos, 1917 by having a mouth with three well-developed triangular lips, pharynx with three longitudinal rows of hooks and three structures truncated like tooth at the base, esophagus with well-developed bulb and with anterior blind intestine (Vicente et al., 1997). Morphometric characteristics of the specimens studied in the present paper fit with the aforementioned by Travassos (1917) and Vicente et al. (1997) for Cruzia tentaculata (Rudolphi, 1819) Travassos, 1917. Cruzia tentaculata is a common parasite of marsupials in South and Central America (Gomes et al., 2003; Pinto et al., 2011). In Peru, C. tentaculata has been found parasitizing the intestine of D. marsupialis and M. nudicaudatus (Geoffroy, 1803) from Cajamarca and Iquitos, respectively (Sarmiento et al., 1999; Tantaleán et al., 2010).
Family Gongylonematidae Sobolev, 1949
Gongylonemoides marsupialis (Vaz & Pereira, 1934) Freitas & Lent, 1937
Description: based on 10 adult specimens (6 male and 4 female). Male: Body 20.64–22.32 (21.10) long by 0.22–0.27 (0.24) wide. Muscular and glandular esophagus 0.50–0.64 (0.52) and 3.60–3.7 (3.65) long, respectively. Excretory pore at 0.60–0.68 (0.66) from anterior end. Right and left spicules 0.28–0.30 (0.29) and 0.154–0.180 (0.16) long, respectively. Cloaca at 0.205–0.208 (0.207) from posterior end. Female: Body 45.60–46.67 (46.05) long by 0.30–0.32 (0.31) wide. Muscular and glandular esophagus 0.60–0.71 (0.68) and 6.48–6.72 (6.65) long, respectively. Excretory pore at 0.65–0.72 (0.66) from anterior end. Vulva located at the middle of the body 5.60–6.09 (5.80) from the posterior end. The anal opening at 0.237–0.263 (0.240) from the posterior end. Eggs 0.04–0.05 (0.04) long by 0.01–0.02 (0.01) wide.
Taxonomic summaryInfection site: trachea.
Locality: Lamas, San Martín, Peru (6°25′S, 76°30′W).
Specimens deposited: MUSM 3254.
Prevalence: 25% (2 infected marsupials of 8).
Mean intensity: 15.
Mean abundance: 3.75.
The species was described as Gongylonema marsupialis by Vaz and Pereira (1934) based on a female specimen collected from D. aurita from Brazil. Freitas and Lent (1937) created the genus Gongylonemoides to include this species, considering the absence of the gubernaculum, the most outstanding character. The specimens studied herein were closer to these referred by Freitas and Lent (1937). Gongylonemoides marsupialis has been registered in D. aurita, D. marsupialis, and M. opossum from Brazil (Gomes et al., 2003; Vicente et al., 1997). This paper constitutes the first register of this nematode species in Peru.
Family Physalopteridae Leiper, 1908
Physaloptera mirandai Lent & Freitas, 1937
Description: based on 10 adult specimens (5 male and 5 female). Male: Body 29.75–37.55 (32.05) long by 0.45–0.99 (0.77) wide. Muscular and glandular esophagus 064–0.89 (0.78) and 4.33–7.08 (5.84) long, respectively. Excretory pore at 1.01–1.21 (1.13) from anterior end. Spicules, 0.46–0.62 (0.55) long. Cloacal aperture at 1.47–2.04 (1.71) from posterior end. Female: Body 30.87–46.31 (37.80) long by 0.64–1.03 (0.84) wide. Muscular and glandular esophagus 0.67–0.94 (0.76) and 7.49–8.40 (7.74) long, respectively. Excretory pore at 1.07–1.37 (1.20) from anterior end. Vulva located at 10.31–11.29 (10.83) from anterior end. Cloacal aperture at 1.47–2.04 (1.71) from posterior end. Anal aperture at 0.78–0.96 (0.84) from the posterior end.
Taxonomic summaryInfection site: stomach.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W).
Specimens deposited: MUSM 3252.
Prevalence: 25% (2 infected marsupials of 8).
Mean intensity: 11.5.
Mean abundance: 2.87.
Lent and Freitas (1937a) described Physaloptera mirandai recovered from the brown four-eyed opossum M. nudicaudatus in Brazil. Our specimens show morphometric characteristics similar to those indicated in the original description of P. mirandai by Lent and Freitas (1937a). This species has been previously registered in Peru by Tantaleán et al. (2010) in M. nudicaudatus. This paper constitutes the first record of P. mirandai parasitizing to D. marsupialis (Vicente et al., 1997).
Turgida turgida (Rudolphi, 1819) Travassos, 1919
Taxonomic summaryInfection site: stomach.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W).
Specimens deposited: MUSM 3403.
Prevalence: 13% (1 infected marsupial of 8).
Mean intensity: 2.
Mean abundance: 0.25.
Turgida turgida (Rudolphi, 1819) Travassos, 1919, is a common parasite of marsupials from North and South America (Gomes et al., 2003). Adult worms are found parasitizing the stomach, mainly the greater curvature, producing a large fibrous ulceration at the point of attachment (Alden, 1995; Gray & Anderson, 1982; Humberg et al., 2011). The taxonomic characters used by us to identify our specimens follow Matey, Kuperman, and Kinsella (2001) and Travassos (1920). According to Gomes et al. (2003), Humberg et al. (2011), Pinto et al. (2011) and Vicente et al. (1997), T. turgida is parasitie of D. albiventris, D. aurita, D. marsupialis, D. virginiana, Caluromys philander, Ch. minimus, and M. nudicaudatus from Brazil. In Peru, Tantaleán et al. (2010) recorded this species from P. opossum.
Family Trichuridae Railliet, 1915
Trichuris marsupialis (Rud, 1819) Hall, 1916
Description: based on 10 adult specimens (5 male and 5 female). Male: Body 12.5–15.5 (14.65) long. Spicules 0.90–1.30 (0.97) long. Cloaca at 1.26–1.50 (1.30) from posterior end. Female: Body 13.6–20.23 (17.45) long. Eggs 0.062–0.068 (0.066) long by 0.031–0.032 (0.031) wide.
Taxonomic summaryInfection site: stomach.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W); Lamas, San Martín, Peru (6°25′S, 76°30′W).
Specimens deposited: MUSM 3256.
Prevalence: 50% (4 infected marsupials of 8).
Mean intensity: 12.
Mean abundance: 6.
Trichuris marsupialis has been found parasitizing the intestine of D. virginiana in the United States of America by Babero (1960); D. marsupialis, Didelphis sp., D. virginiana, and P. opossum from Mexico by Acosta-Virgen et al. (2015) and Monet-Mendoza, Osorio-Sarabia, and García-Prieto (2005), and in D. albiventris from Brazil by Pinto et al. (2011). In Peru, Trichuris sp. has been registered in the intestine of four marsupial hosts (Tantaleán et al., 2010). Thus, T. marsupialis represents the first species recorded from marsupials of Peru.
Family Viannaiidae Durette-Desset & Chabaud, 1981
Viannaia hamataTravassos, 1914
Description: based on 10 adult specimens (5 male and 5 female). Male: Body 2.65–2.82 (2.79) long by 0.130–0.172 (0.154) wide. Cephalic expansion 0.045–0.047 (0.046) long. Esophagus 0.305–0.320 (0.316) long. Excretory pore at 0.312–0.340 (0.320) from anterior end. Spicules 0.133–0.140 (0.138) long. Gubernaculum 0.019–0.023 (0.022) long. Female: Body 2.42–3.80 (3.29) long by 0.175–0.220 (0.200) wide. Cephalic expansion 0.040–0.047 (0.046) long. Esophagus 0.250–0.352 (0.322) long. Excretory pore at 0.282–0.354 (0.334) from anterior end. Vulva located at the middle of the body 0.073–0.103 (0.099) from the anterior end. Eggs 0.054–0.062 (0.059) long by 0.04–0.06 (0.05) wide.
Taxonomic summaryInfection site: stomach.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W); Lamas, San Martín, Peru (6°25′S, 76°30′W).
Specimens deposited: MUSM 3404.
Prevalence: 38% (3 infected marsupials of 8).
Mean intensity: 41.3.
Mean abundance: 15.5.
Our specimens show morphometric characteristics similar to those indicated in the redescription of V. hamata by Guerrero (1985). Viannaia hamata had been recorded only in D. marsupialis and D. aurita from Brazil (Gomes et al., 2003; Pinto & Gomes, 1980; Pinto et al., 2011); therefore, Viannaia hamata represents a new record in Peru.
Viannaia viannaiaTravassos, 1914
Description: based on 10 adult specimens (5 male and 5 female). Male: Body 1.650–2.720 (2.360) long by 0.077–0.16 (0.106) wide. Cephalic expansion 0.035–0.050 (0.047) long. Esophagus 0.221–0.290 (0.278) long. Excretory pore at 0.270–0.355 (0.320) from anterior end. Spicules 0.097–0.117 (0.108) long. Female: Body 2.30–3.13 (3.09) long by 0.140–0.210 (0.180) wide. Cephalic expansion 0.030–0.048 (0.044) long. Esophagus 0.251–0.352 (0.320) long. Excretory pore at 0.284–0.350 (0.330) from anterior end. Vulva located at the middle of the body 0.072–0.102 (0.097) from the anterior end. Eggs 0.052–0.062 (0.057) long by 0.04–0.06 (0.05) wide.
Taxonomic summaryInfection site: stomach.
Locality: Bella Vista, San Martín, Peru (7°15′S, 76°28′W).
Specimens deposited: MUSM 3255.
Prevalence: 25% (2 infected marsupials of 8).
Mean intensity: 72.5.
Mean abundance: 18.2.
The genus Viannaia was erected by Travassos (1914) to accommodate those species of neotropical Trichostrongylidae with short spicules (Guerrero, 1985). Our specimens completely correspond with the original description given by Travassos (1914) and the redescription made by Guerrero (1985) for Viannaia viannaia. This nematode was recorded in D. marsupialis, D. aurita, P. opossum, and Akodon cursor (Winge, 1887) from Brazil; D. virginiana in USA, D. marsupialis and P. opossum in Venezuela and D. marsupialis and P. opossum in Mexico (Jiménez et al., 2011; Vicente et al., 1997). This paper constitutes the first record of V. viannaia in Peru.
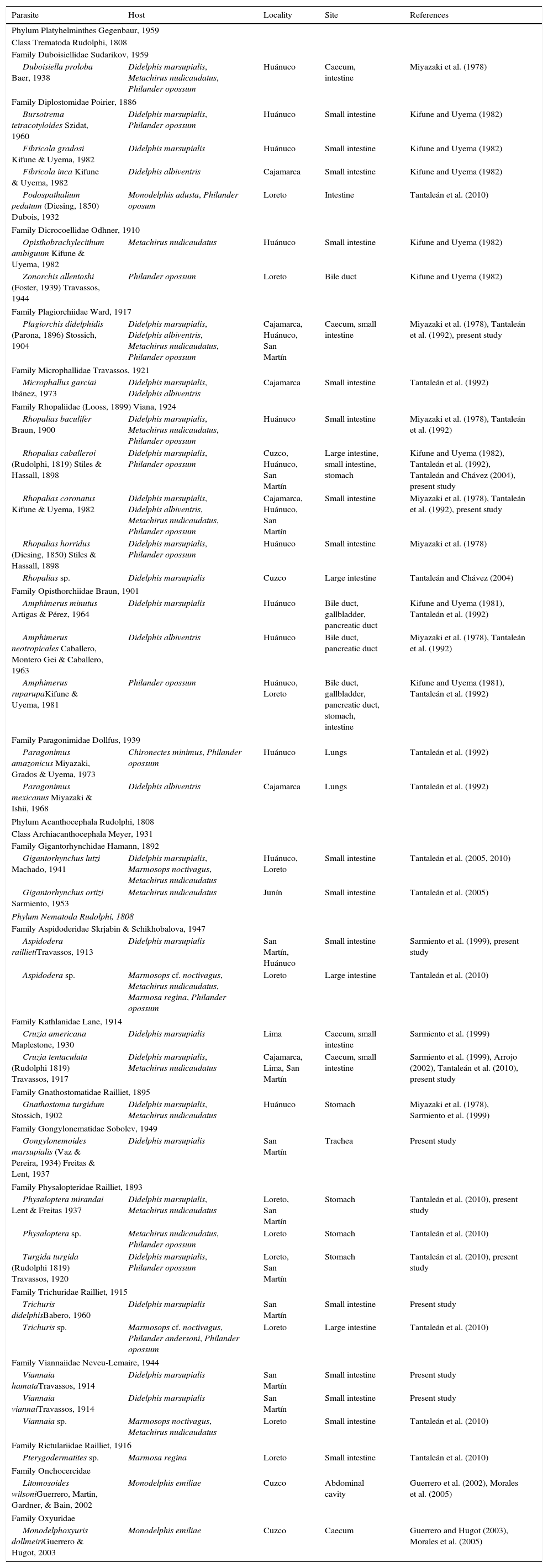
To date, a total of 38 helminth parasites have been recorded in Peruvian marsupials (Table 1), all in adult stage. Digeneans have the highest species richness in number and percentage (n=19, 50%), followed by nematodes (n=17, 45%) and acanthocephalans (n=2, 5%). The parasites with the highest numbers of records were the digeneans Plagiorchis didelphidis (Parona, 1896) Stossich, 1904 (n=4) and Rhopalias coronatus Kifune & Uyema, 1982 (n=4), and the nematode Aspidodera sp. (n=4). For Peru information exists on the parasites of 10 marsupials, distributed in 7 genera: Chironectes (1 sp.), Didelphis (2 spp.), Marmosa (1 sp.), Marmosops (1 sp.), Metachirus (1 sp.), Monodelphis (2 spp.) and Philander (2 spp.) (Table 2). Marsupials that harbored the highest number of parasites were D. marsupialis (n=22) followed by P. opossum (n=15), M. nudicaudatus (n=13) and D. albiventris (n=6) (Table 2). The localities with the highest number of records were Huánuco (28%, n=15), Loreto (21%, n=11) and San Martín (21%, n=11) (Table 1).
List of records of helminth species from marsupials in Peru, with their hosts, locality, site and data-sources.
| Parasite | Host | Locality | Site | References |
|---|---|---|---|---|
| Phylum Platyhelminthes Gegenbaur, 1959 | ||||
| Class Trematoda Rudolphi, 1808 | ||||
| Family Duboisiellidae Sudarikov, 1959 | ||||
| Duboisiella proloba Baer, 1938 | Didelphis marsupialis, Metachirus nudicaudatus, Philander opossum | Huánuco | Caecum, intestine | Miyazaki et al. (1978) |
| Family Diplostomidae Poirier, 1886 | ||||
| Bursotrema tetracotyloides Szidat, 1960 | Didelphis marsupialis, Philander opossum | Huánuco | Small intestine | Kifune and Uyema (1982) |
| Fibricola gradosi Kifune & Uyema, 1982 | Didelphis marsupialis | Huánuco | Small intestine | Kifune and Uyema (1982) |
| Fibricola inca Kifune & Uyema, 1982 | Didelphis albiventris | Cajamarca | Small intestine | Kifune and Uyema (1982) |
| Podospathalium pedatum (Diesing, 1850) Dubois, 1932 | Monodelphis adusta, Philander oposum | Loreto | Intestine | Tantaleán et al. (2010) |
| Family Dicrocoellidae Odhner, 1910 | ||||
| Opisthobrachylecithum ambiguum Kifune & Uyema, 1982 | Metachirus nudicaudatus | Huánuco | Small intestine | Kifune and Uyema (1982) |
| Zonorchis allentoshi (Foster, 1939) Travassos, 1944 | Philander opossum | Loreto | Bile duct | Kifune and Uyema (1982) |
| Family Plagiorchiidae Ward, 1917 | ||||
| Plagiorchis didelphidis (Parona, 1896) Stossich, 1904 | Didelphis marsupialis, Didelphis albiventris, Metachirus nudicaudatus, Philander opossum | Cajamarca, Huánuco, San Martín | Caecum, small intestine | Miyazaki et al. (1978), Tantaleán et al. (1992), present study |
| Family Microphallidae Travassos, 1921 | ||||
| Microphallus garciai Ibánez, 1973 | Didelphis marsupialis, Didelphis albiventris | Cajamarca | Small intestine | Tantaleán et al. (1992) |
| Family Rhopaliidae (Looss, 1899) Viana, 1924 | ||||
| Rhopalias baculifer Braun, 1900 | Didelphis marsupialis, Metachirus nudicaudatus, Philander opossum | Huánuco | Small intestine | Miyazaki et al. (1978), Tantaleán et al. (1992) |
| Rhopalias caballeroi (Rudolphi, 1819) Stiles & Hassall, 1898 | Didelphis marsupialis, Philander opossum | Cuzco, Huánuco, San Martín | Large intestine, small intestine, stomach | Kifune and Uyema (1982), Tantaleán et al. (1992), Tantaleán and Chávez (2004), present study |
| Rhopalias coronatus Kifune & Uyema, 1982 | Didelphis marsupialis, Didelphis albiventris, Metachirus nudicaudatus, Philander opossum | Cajamarca, Huánuco, San Martín | Small intestine | Miyazaki et al. (1978), Tantaleán et al. (1992), present study |
| Rhopalias horridus (Diesing, 1850) Stiles & Hassall, 1898 | Didelphis marsupialis, Philander opossum | Huánuco | Small intestine | Miyazaki et al. (1978) |
| Rhopalias sp. | Didelphis marsupialis | Cuzco | Large intestine | Tantaleán and Chávez (2004) |
| Family Opisthorchiidae Braun, 1901 | ||||
| Amphimerus minutus Artigas & Pérez, 1964 | Didelphis marsupialis | Huánuco | Bile duct, gallbladder, pancreatic duct | Kifune and Uyema (1981), Tantaleán et al. (1992) |
| Amphimerus neotropicales Caballero, Montero Gei & Caballero, 1963 | Didelphis albiventris | Huánuco | Bile duct, pancreatic duct | Miyazaki et al. (1978), Tantaleán et al. (1992) |
| Amphimerus ruparupaKifune & Uyema, 1981 | Philander opossum | Huánuco, Loreto | Bile duct, gallbladder, pancreatic duct, stomach, intestine | Kifune and Uyema (1981), Tantaleán et al. (1992) |
| Family Paragonimidae Dollfus, 1939 | ||||
| Paragonimus amazonicus Miyazaki, Grados & Uyema, 1973 | Chironectes minimus, Philander opossum | Huánuco | Lungs | Tantaleán et al. (1992) |
| Paragonimus mexicanus Miyazaki & Ishii, 1968 | Didelphis albiventris | Cajamarca | Lungs | Tantaleán et al. (1992) |
| Phylum Acanthocephala Rudolphi, 1808 | ||||
| Class Archiacanthocephala Meyer, 1931 | ||||
| Family Gigantorhynchidae Hamann, 1892 | ||||
| Gigantorhynchus lutzi Machado, 1941 | Didelphis marsupialis, Marmosops noctivagus, Metachirus nudicaudatus | Huánuco, Loreto | Small intestine | Tantaleán et al. (2005, 2010) |
| Gigantorhynchus ortizi Sarmiento, 1953 | Metachirus nudicaudatus | Junín | Small intestine | Tantaleán et al. (2005) |
| Phylum Nematoda Rudolphi, 1808 | ||||
| Family Aspidoderidae Skrjabin & Schikhobalova, 1947 | ||||
| Aspidodera raillietiTravassos, 1913 | Didelphis marsupialis | San Martín, Huánuco | Small intestine | Sarmiento et al. (1999), present study |
| Aspidodera sp. | Marmosops cf. noctivagus, Metachirus nudicaudatus, Marmosa regina, Philander opossum | Loreto | Large intestine | Tantaleán et al. (2010) |
| Family Kathlanidae Lane, 1914 | ||||
| Cruzia americana Maplestone, 1930 | Didelphis marsupialis | Lima | Caecum, small intestine | Sarmiento et al. (1999) |
| Cruzia tentaculata (Rudolphi 1819) Travassos, 1917 | Didelphis marsupialis, Metachirus nudicaudatus | Cajamarca, Lima, San Martín | Caecum, small intestine | Sarmiento et al. (1999), Arrojo (2002), Tantaleán et al. (2010), present study |
| Family Gnathostomatidae Railliet, 1895 | ||||
| Gnathostoma turgidum Stossich, 1902 | Didelphis marsupialis, Metachirus nudicaudatus | Huánuco | Stomach | Miyazaki et al. (1978), Sarmiento et al. (1999) |
| Family Gongylonematidae Sobolev, 1949 | ||||
| Gongylonemoides marsupialis (Vaz & Pereira, 1934) Freitas & Lent, 1937 | Didelphis marsupialis | San Martín | Trachea | Present study |
| Family Physalopteridae Railliet, 1893 | ||||
| Physaloptera mirandai Lent & Freitas 1937 | Didelphis marsupialis, Metachirus nudicaudatus | Loreto, San Martín | Stomach | Tantaleán et al. (2010), present study |
| Physaloptera sp. | Metachirus nudicaudatus, Philander opossum | Loreto | Stomach | Tantaleán et al. (2010) |
| Turgida turgida (Rudolphi 1819) Travassos, 1920 | Didelphis marsupialis, Philander opossum | Loreto, San Martín | Stomach | Tantaleán et al. (2010), present study |
| Family Trichuridae Railliet, 1915 | ||||
| Trichuris didelphisBabero, 1960 | Didelphis marsupialis | San Martín | Small intestine | Present study |
| Trichuris sp. | Marmosops cf. noctivagus, Philander andersoni, Philander opossum | Loreto | Large intestine | Tantaleán et al. (2010) |
| Family Viannaiidae Neveu-Lemaire, 1944 | ||||
| Viannaia hamataTravassos, 1914 | Didelphis marsupialis | San Martín | Small intestine | Present study |
| Viannaia viannaiTravassos, 1914 | Didelphis marsupialis | San Martín | Small intestine | Present study |
| Viannaia sp. | Marmosops noctivagus, Metachirus nudicaudatus | Loreto | Small intestine | Tantaleán et al. (2010) |
| Family Rictulariidae Railliet, 1916 | ||||
| Pterygodermatites sp. | Marmosa regina | Loreto | Small intestine | Tantaleán et al. (2010) |
| Family Onchocercidae | ||||
| Litomosoides wilsoniGuerrero, Martin, Gardner, & Bain, 2002 | Monodelphis emiliae | Cuzco | Abdominal cavity | Guerrero et al. (2002), Morales et al. (2005) |
| Family Oxyuridae | ||||
| Monodelphoxyuris dollmeiriGuerrero & Hugot, 2003 | Monodelphis emiliae | Cuzco | Caecum | Guerrero and Hugot (2003), Morales et al. (2005) |
Host–parasite list of Peruvian marsupials with their helminth species.
| Host | Parasite |
|---|---|
| Order Didelphimorphia (Gill, 1872) | |
| Family Didelphidae Gray, 1821 | |
| Chironectes minimus (Zimmermann, 1780) | Paragonimus amazonicus (TE) |
| Didelphis albiventris Lund, 1840 | Amphimerus neotropicalis (TE) |
| Fibricola gradosi (TE) | |
| Microphallus garciai (TE) | |
| Paragonimus mexicanus (TE) | |
| Plagiorchis didelphidis (TE) | |
| Rhopalias coronatus (TE) | |
| Didelphis marsupialis Linnaeus, 1758 | Amphimerus minutes (TE) |
| Bursotrema tetracotyloides (TE) | |
| Duboisiella proloba (TE) | |
| Fibricola gradosi (TE) | |
| Microphallus garciai (TE) | |
| Plagiorchis didelphidis (TE) | |
| Rhopalias baculifer (TE) | |
| Rhopalias caballeroi (TE) | |
| Rhopalias coronatus (TE) | |
| Rhopalias horridus (TE) | |
| Rhopalias sp. (TE) | |
| Gigantorhynchus lutzi (AC) | |
| Aspidodera raillieti (NE) | |
| Cruzia americana (NE) | |
| Cruzia tentaculata (NE) | |
| Gnathostoma turgidum (NE) | |
| Gongylonemoides marsupialis (NE) | |
| Physaloptera mirandai (NE) | |
| Trichuris didelphis (NE) | |
| Turgida turgida (NE) | |
| Viannaia hamata (NE) | |
| Viannaia viannai (NE) | |
| Marmosa (Micoureus) regina Thomas, 1898 | Aspidodera sp. (NE) |
| Pterygodermatites sp. (NE) | |
| Marmosops noctivagus (Tschudi, 1844) | Gigantorhynchus lutzi (AC) |
| Aspidodera sp. (NE) | |
| Trichuris sp. (NE) | |
| Viannaia sp. (NE) | |
| Metachirus nudicaudatus (É. Geoffroy, 1803) | Duboisiella proloba (TE) |
| Opisthobrachylecithum ambiguum (TE) | |
| Plagiorchis didelphidis (TE) | |
| Rhopalias baculifer (TE) | |
| Rhopalias coronatus (TE) | |
| Gigantorhynchus lutzi (AC) | |
| Gigantorhynchus ortizi (AC) | |
| Aspidodera sp. (NE) | |
| Cruzia tentaculata (NE) | |
| Gnathostoma turgidum (NE) | |
| Physaloptera mirandai (NE) | |
| Physaloptera sp. (NE) | |
| Viannaia sp. (NE) | |
| Monodelphis adusta (Thomas, 1897) | Podospathalium pedatum (NE) |
| Monodelphis emiliae (Thomas, 1912) | Litomosoides wilsoni (NE) |
| Monodelphoxyuris dollmeiri (NE) | |
| Philander andersoni (Osgood, 1913) | Trichuris sp. (NE) |
| Philander opossum (Linnaeus, 1758) | Amphimerus ruparupa (TE) |
| Bursotrema tetracotyloides (TE) | |
| Duboisiella proloba (TE) | |
| Paragonimus amazonicus (TE) | |
| Plagiorchis didelphidis (TE) | |
| Podospathalium pedatum (TE) | |
| Rhopalias baculifer (TE) | |
| Rhopalias caballeroi (TE) | |
| Rhopalias coronatus (TE) | |
| Rhopalias horridus (TE) | |
| Zonorchis allentoshi (TE) | |
| Aspidodera sp. (NE) | |
| Physaloptera sp. (NE) | |
| Trichuris sp. (NE) | |
| Turgida turgida (NE) | |
AC: Acanthocephala, NE: Nematoda, TE: Trematoda.
As a result of this study, we report 11 helminth species (3 digeneans and 9 nematodes) in the helminth communities of D. marsupialis from San Martín region, Peru. Species of parasites that represent new records for the country include V. viannaia, V. hamate, and G. marsupialis. All parasites recorded in this study are reported for the first time from the San Martín region.
In the present study, nematodes represented 82% of the total species in the component community of D. marsupialis. The composition of the majority of parasite communities of didelphid marsupials show a higher number of nematode species (Acosta-Virgen et al., 2015; Alden, 1995; Grover & Harkema, 1970; Jiménez et al., 2011; Monet-Mendoza et al., 2005). According to Acosta-Virgen et al. (2015), the structuring factor of the helminthofauna in marsupials is the diet; most of the nematode species infect these host species through ingestion of eggs, larvae or intermediate hosts.
In the present work, 6 of 11 helminth species collected in D. marsupialis have indirect patterns of transmission by intermediate host ingestion (G. marsupialis, P. didelphidis, R. caballeroi, R. coronatus, P. mirandai, and T. turgida) and 5 are transmitted directly by ingestion of eggs (A. raillieti, C. tentaculata, T. marsupialis, V. hamate, and V. viannaia). The helminthfauna of D. marsupialis in San Martín seemed to be dominated by helminths with direct patterns of transmission. This is indicated by the highest values of prevalence of A. raillieti, C. tentaculata, and T. marsupialis, which exceeded 49%. In contrast, helminths with indirect patterns of transmission had low values of prevalence. Jiménez et al. (2011) mention 2 hypotheses to explain the low prevalence values in helminths with indirect patterns of transmission, including as the main reason the wide spectrum of food items used by marsupials such as D. marsupialis.
The checklist presented herein includes 32 named and 6 undetermined species of helminths associated with 10 marsupial host species from Peru; these come from 7 of the 26 Peruvian regions. The greatest richness of parasites was registered among digeneans (19 spp.) and nematodes (17 spp.), in contrast to what was observed by Jiménez, Scheibel, Byles, and Gardner (2013) in the parasites of marsupials in Bolivia, where nematodes and cestodes are the dominant group of parasites, while digeneans barely are represented by 2 species; this situation also was observed by Pérez-Ponce de León, García-Prieto, and Mendoza-Garfias (2007) in digeneans of marsupials from Mexico, where digeneans showed a low species richness. The large number of species being represented by digeneans in this work could be a result of the extraordinary diversification of sites of infection, life-cycles, and in the modes of feeding (Pérez-Ponce de León et al., 2007).
The second species-richest taxon was Nematoda, represented by 17 species, similar to compiled by García-Prieto et al. (2012), who listed 14 named and 7 unidentified nematode species in marsupials from Mexico, but it is a different number to that reported from Bolivia (21 nematode species) (Jiménez et al., 2013). According to Garrido-Olvera, García-Prieto, and Pérez-Ponce de León (2006), nematodes are probably the second largest taxon in the Animal kingdom after arthropods, when considering the number of described and estimated unknown species.
The other group of parasites, Acanthocephala, was represented by only a few species, which corresponds to the situation in Mexico (García-Prieto, García-Varela, Mendoza-Garfias, & Pérez-Ponce de León, 2010).
The digeneans Amphimerus neotropicales Caballero, Montero, Gei & Caballero, 1963, Duboisiella proloba Baer, 1938, P. didelphidis, Rhopalias baculifer Braun, 1900, R. coronatus and Rhopalias horridus (Diesing, 1850) Stiles & Hassall, 1898 and the nematode Gnathostoma turgidum Stossich, 1902 were the first species of helminths recorded from Peruvian marsupials (Miyazaki et al., 1978).
In Peru, there are 40 known marsupial species (Pacheco et al., 2009), of which only 25% (10 hosts of one family) have parasitological records; this information likely corresponds to only a small proportion of the true richness of parasites with a high amount of diversity remaining untouched. The marsupial species D. marsupialis, P. opossum, M. nudicaudatus, and D. albiventris presented the highest richness of parasites. This is similar to that reported from Mexico, where D. marsupialis and P. opossum are the hosts with the highest number of helminth parasites after D. virginiana (Pérez-Ponce de León & García-Prieto, 2001).
To Ney Saavedra Grandez and Pol Martan Huayama Alverca for their invaluable help collecting opossums. Our appreciation is extended to Blanca Saldaña Arevalo and Alex Ruser Vela Noriega for assistance in the parasitological examinations. We are grateful to Eric Wetzel for useful comments on an early draft of the manuscript.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.