Mites of the families Pygmephoridae and Neopygmephoridae have been poorly studied in Mexico. Previous records from this group are: Acinogaster (A.) kansensis from Chiapas, A. (A.) marianae from Veracruz, Pygmephorus americanus from Mexico, some species of Siteroptes from Puebla and Pediculaster thailandensis from Quintana Roo. In this study we provide additional data, including specimens of Neopygmephoridae such as Kerdabania inconspicua, Bakerdania exigua, Pseudopygmephorus agarici, and P. shangaiensis; and of Pygmephoridae: Mahunkania secunda, Pediculaster ignotus and P. gracilis. Abundant specimens of Pediculaster ignotus were present in garlic crop soil.
Los ácaros de las familias Pygmephoridae y Neopygmephoridae de México han sido poco estudiados. Registros previos de este grupo son: Acinogaster (A.) kansensis de Chiapas, A. (A.) marianae de Veracruz, Pygmephorus americanus de México y especies de Siteroptes en muestras de Puebla, así como Pediculaster thailandensis del estado de Quintana Roo. En este estudio damos a conocer nuevos datos que incluyen a ejemplares de Neopygmephoridae como Kerdabania inconspicua, Bakerdania exigua, Pseudopygmephorus agarici and P. shangaiensis; también de la familia Pygmephoridae a Mahunkania secunda, Pediculaster ignotus y P. gracilis. La mayor abundancia de Pediculaster ignotus estuvo presente en suelos cultivados con ajo.
The family Pygmephoridae contains 30 genera and 350 species (Krantz & Walter, 2009). These figures have changed since the work by Kethley (1982), mainly due to recent reviews of this heterogeneous group. One of these changes is the re-establishment of the family Neopygmephoridae and the revision of generic characters to define them (Camerick, 2005; Khaustov, 2004). The most diverse genera of the families Pygmephoridae and Neopygmephoridae are Bakerdania Sasa, 1962 (about 100 species), Pygmephorus Kramer, 1877 (about 43 species) and Pediculaster Vitzthum, 1931 (about 95 species) (Camerick, 2010; Khaustov, 2004, 2008).
Pygmephorid mites from Mexico cited by Hoffmann and López-Campos (2000) are Acinogaster (Acinogaster) kansensis Ross & Cross, 1979 (USA, Mexico, Costa Rica, Panama, Ecuador, and Brazil) from Chiapas State; A. (A.) marianaeCross, 1965 (Mexico, Costa Rica, Panama, Ecuador, Guyana, and Trinidad) from Veracruz State; Pediculaster americanus (Banks, 1904) (USA, Mexico, and Haiti) from Mexico without more data. Species of Siteroptes Amerling, 1861 in soil samples from Puebla are also cited without any other data (Hoffmann & López-Campos, 2000). Pediculaster thailandensisCamerick, 2005 on Chloropidae flies, from Playa del Carmen, Quintana Roo and from a rocky seashore on Tethinidae flies, from Tulum, Quintana Roo, Mexico, are the most recent records (Camerick, 2005).
We have been studying Prostigmata mites found in cultivated soils since 2005. Some results have already been published, particularly those about Ereynetidae from cultivated soils with a garlic (Allium sativum L.) crop in Guanajuato, Mexico (Vázquez-Rojas & Estrada-Venegas, 2010). Here, we publish data on the families of Pygmephoridae and Neopygmephoridae found in garlic crop soil as well as, other natural, cultivated soils and compost. We consider the genera Mahunkania and Pediculaster as members of the family Pygmephoridae and the genera Bakerdania, Kerdabania and Pseudopygmephorus as members of the family Neopygmephoridae, following Khaustov (2004, 2009).
Materials and methodsAll samples were collected by E. Estrada and A. Equihua from different parcels at the states of Guanajuato, Mexico, Veracruz and D. F. One kilogram of soil was processed by a Berlese Funnel by E. Estrada and her team along 1 year. The following arrangement of locality data was used: Guanajuato municipalities (in italics), parcel names and 2 letters as key of that name. Salamanca: El Fuerte (EF), El Tajo (ET), La Cuadrilla (LC), Pozo Félix (PF), San Isidro (SI) and San Juan (SJ); Comonfort: La Huerta (LH); Los Rodríguez: Mina 5 (M5); San Luis de La Paz: El Zorrillo (EZ) and El Nacimiento (EN). Same arrangement was used for other states and habitats. Compost samples at Texcoco (Montecillo), Mexico State (MTx); soil samples from Rio Tuxtla, Veracruz state (RTV); and soil samples from crops such as corn (Zea maiz L. 1753), pumpkin (Cucurbita pepo L. 1753), coriander (Coriandrum sativum L. 1753), and goosefoot (Chenopodium nuttalliae); from parcels P.J. Capultitla in Xochimilco, Distrito Federal (PJCX). A total of 167 specimens of Pygmephoridae and Neopygmephoridae were mounted on slides and studied; these will be deposited in the collection of the second author.
Drawings were made with a Zeiss compound microscope equipped with a camera lucida and then processed with Photoshop CS5. All measurements are expressed in μm and were made following the procedure by Camerick, 1996.
Description| NeopygmephoridaeCross, 1965 |
| Pseudopygmephorus Cross, 1965 |
| Type species: Pygmephorus tarsalis Hirst, 1921;Cross, 1965: 221 |
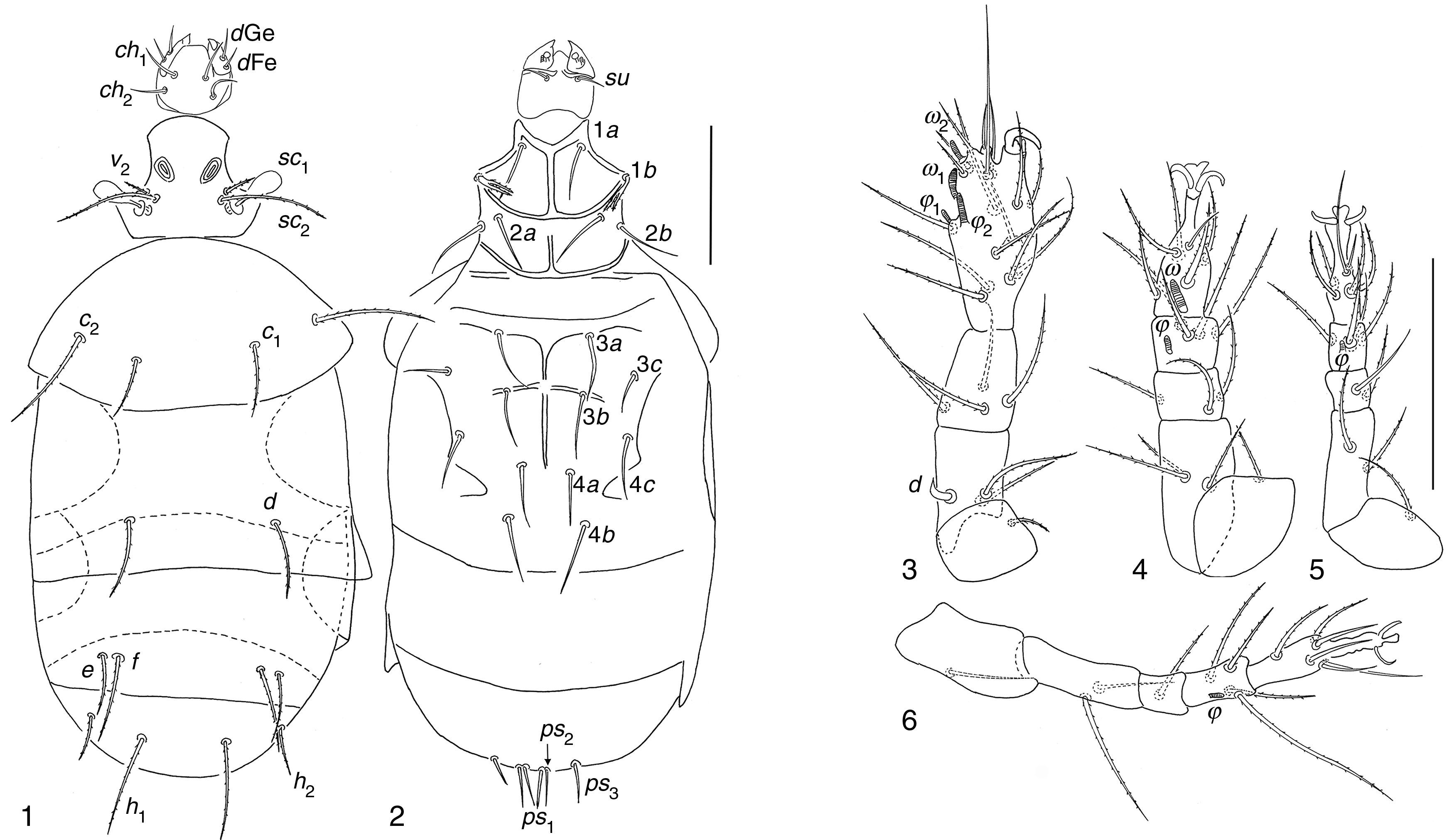
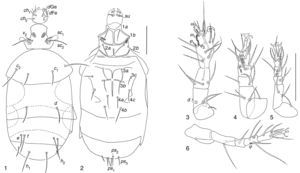
| Pseudopygmephorus agarici Zou, Gao & Ma, 1990: 373 (Figs. 1–6) |
Phoretomorph females in soil of garlic crop in Guanajuato. 10, M5, 11/04/2002; 15, LH, 16/08/2001; 4, PF, 26/07/2000; 1, EN, 13/11/2001; 1, RTV, 01/10/2004; 1, ET, 17/07/2001; 1, PF, 15/05/2002. Soil 1, PJCX, 11/04/2007.
Distribution and habitats of the species described by Zou, Jian-Rong, and En-Pei (1990)Holotype and allotype of P. agarici were found in mushroom compost as well as manure in Shangai, China. Paratypes were found in mushroom compost, compost being pasteurized and on straw in greenhouses (Zou et al., 1990).
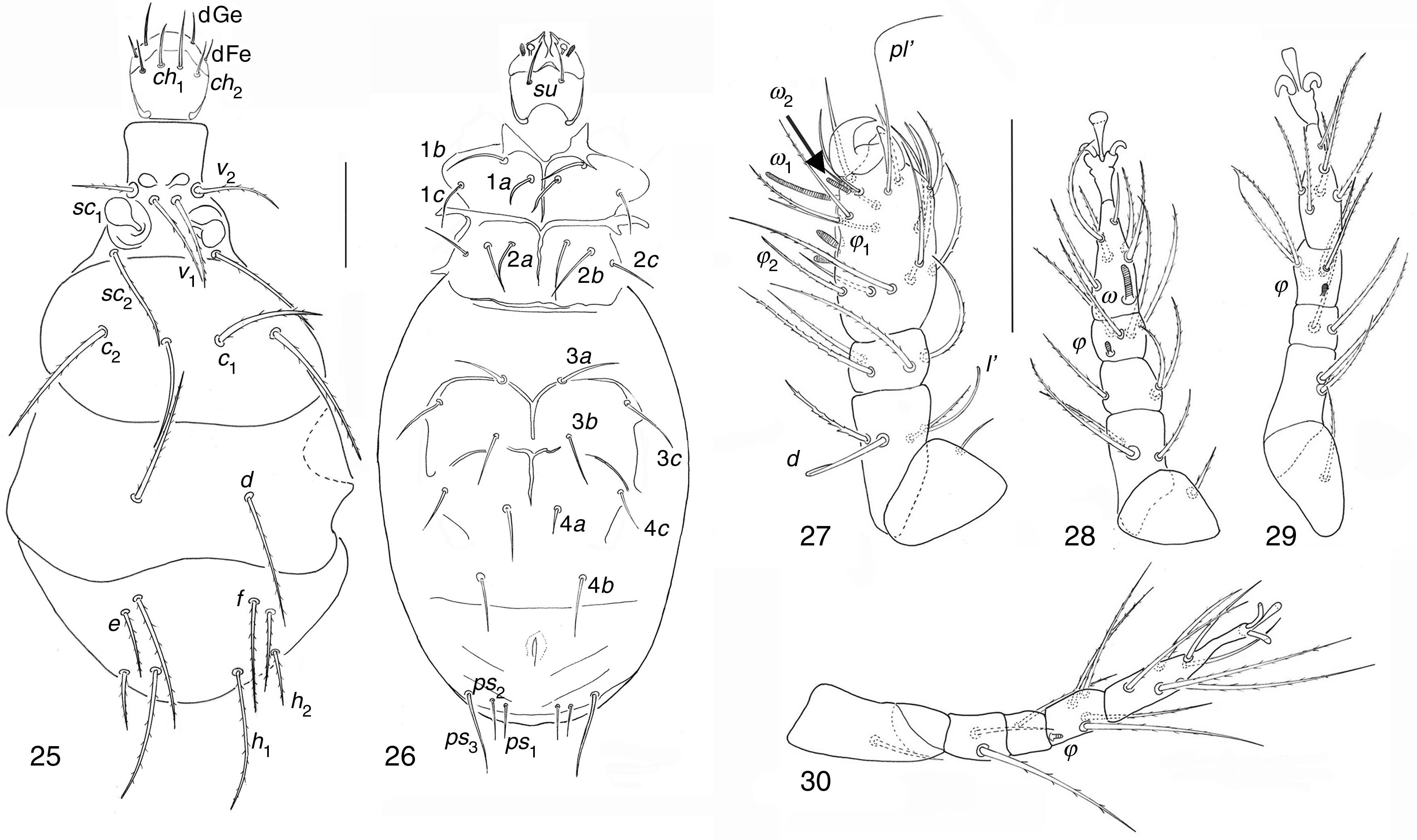
Pseudopygmephorus agariciSize intervals from 6 studied specimens. Body 81–131 wide; 195–320 long. Length of dorsal setae of 6 specimens: v2 8–10, sc2 28–47, c1 23–30, c2 39–60, d 21–40, e 17–33, f 24–44; h1 25–50, h2 16–20. Distances between dorsal setae: v2–v2 21–27, sc2–sc2 17–24, c1–c1 35–47, c2–c2 80–111, d–d 41–60, f–f 42–61, e–e 56–78, h1–h1 24–42, h2–h2 58–82. Length of ventral setae: 1a 20–28, 1b 9–19, 2a 23–34, 2b 24–36, 3a 23–36, 3b 24–34, 3c 12–24, 4a 23–30, 4b 29–41, 4c 21–30, ps1 11–18, ps2 11–17, ps3 11–20. Length of tibiotarsus solenidia: ω1 7–9, ω2 5–6, φ1 3–5, φ2 6–8.
Other species of P. aphodiiKhaustov, 2010 (Ukraine), have been found on dung beetles Aphodius fimetarius (L.), and P. smileyiHill & Deahl, 1978 (USA), on horse manure for commercial mushroom production (Hill & Deahl, 1978; Khaustov, 2010).
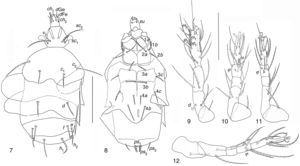
Pseudopygmephorus shangaiensis Zou, Gao, & Ma, 1990: 375 (Figs. 7–12)Phoretomorph females collected in compost. 8, MTx, 19/07/2005.
Distribution and habitat of the species described by Zou et al. (1990)Holotype, allotype and paratypes were found on manure and mushroom compost in Shangai, China (Zou et al., 1990).
Pseudopygmephorus shangaiensisSize intervals from 5 specimens. Body, 89–126 wide; 170–310 long. Length of dorsal setae of 5 specimens: v2 8–10, sc2 36–52, c1 22–30, c2 39–60, d 22–30, e 14–31, f 23–31; h1 27–38, h2 13–21. Distances between dorsal setae: v2–v2 24–32, sc2–sc2 18–24, c1–c1 38–51, c2–c2 80–119, d–d 43–69, f–f 44–68, e–e 47–86, h1–h1 22–35, h2–h2 54–83. Length of ventral setae: 1a 17–24, 1b 13–20, 2a 23–35, 2b 15–24, 3a 15–29, 3b 18–29, 3c 15–23, 4a 14–24, 4b 22–35, 4c 16–26, ps1 10–18, ps2 10–17, ps3 16–20. Length of tibiotarsus solenidia: ω1 7–9, ω2 3–5, φ1 4–5, φ2 6–7.
| Kerdabania Khaustov, 2009 |
| Type species: Kerdabania magnifica, Khaustov, 2009: 171 |
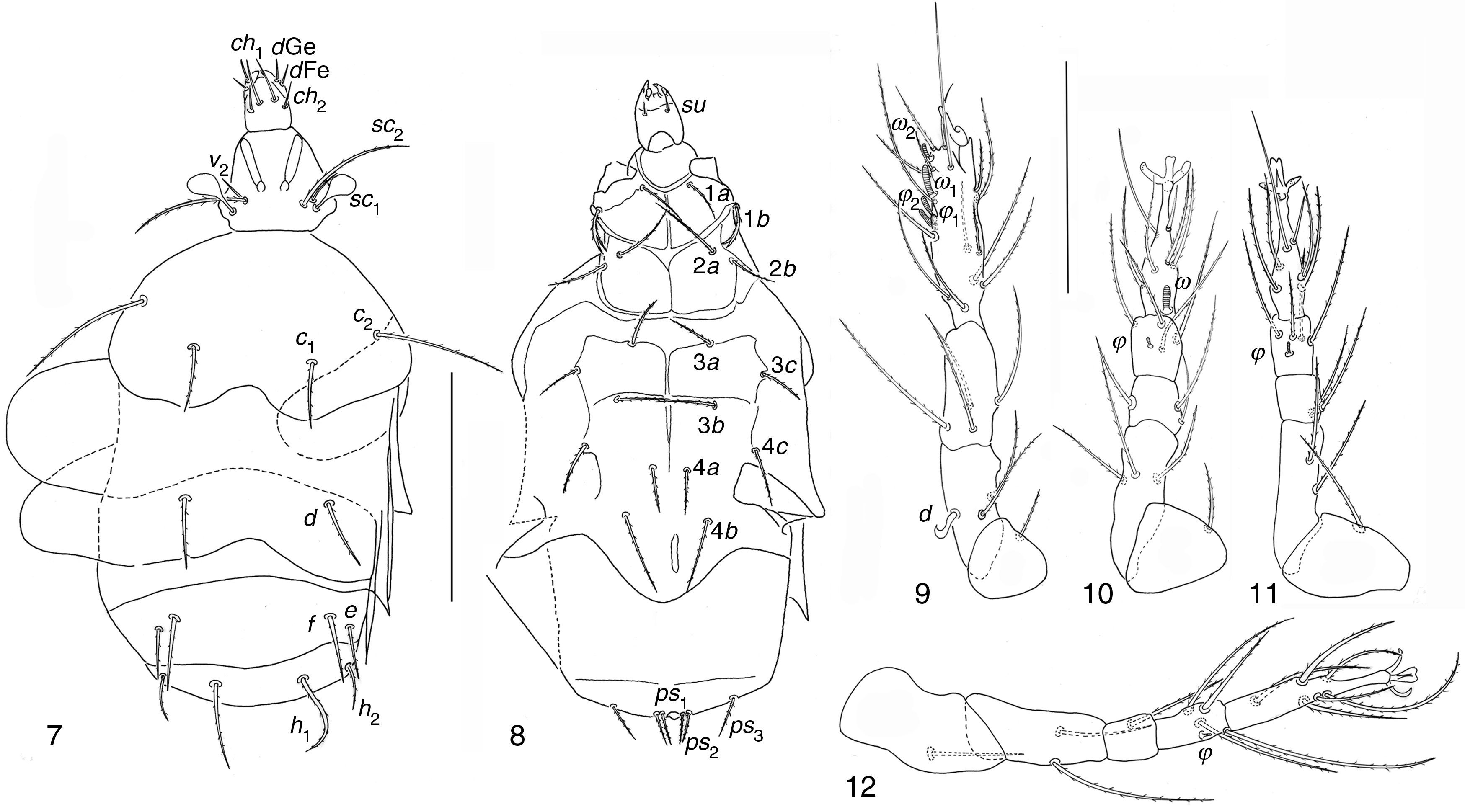
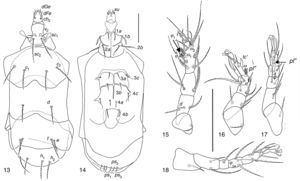
| Kerdabania inconspicua (Berlese, 1904) (Figs. 13–18) |
| Pigmephorus inconspicuus Berlese, 1904: 12 |
| Scutacarus centriger Cooreman, 1951 |
| Pygmephorus sellnickiKrczal, 1958: 69 |
| Kerdabania inconspicuus (Berlese, 1904)Khaustov, 2009:183 comb. n. |
Females in soil of garlic crop in Guanajuato State. 3, M5, 01/06/2002; 1, SI, 29/07/2002, 1; SI, 10/04/2002, 1, SI, 22/08/2002; 1, EN, 13/01/2001; 1, LC, 23/04/2003; 5, EZ, 29/07/2006. Females in cultivated soil in Xochimilco, D. F.: 2, PJCX, 23/05/2007, Coriandrum; 1, PJCX, 27/06/2007, corn; 1, PJCX, 11/04/2007, pumpkin; 1, PJCX, 28/03/2007, goosefoot.
Distribution and habitat of the species renamed by Khaustov (2009)The genus has worldwide distribution, except Antarctica. Forest litter and nests of small mammals and ants are known habitats. Phoresy unknown. K. inconspicua from Ukraine, vicinity of Poltava, soil under straw (Khaustov, 2009).
Kerdabania inconspicuaBody size interval, 232–282 wide, 107–116 long. Length of dorsal setae: v2 9–11, sc2 42–47, c1 38–42, c2 42–48, d 33–40, e 11–22, f 40–44, h1 35–41, h2 36–41. Distances between dorsal setae: v2–v2 14–17, sc2–sc2 12–19, c1–c1 37–45, c2–c2 76–93, d–d 43–58, f–f 43–60, e–e 57–72, h1–h1 20–27, h2–h2 43–55. Length of ventral setae: 1a 15–20, 1b 16–20, 2a 24–28, 2b 25–32, 3a 12–15, 3b 12–14, 3c 13–16, 4a 10–12, 4b 13–20, 4c 14–19. Length of tibiotarsus solenidia: ω1 6–9, ω2 4–6, φ1 3–4, φ2 6–9.
| Bakerdania Sasa, 1961 |
| Type species: Pygmephorus cultratus Berlese, 1904; (1970) |
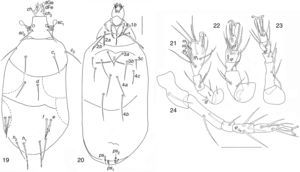
| Bakerdania exigua (Mahunka, 1969) (Figs. 19–24) |
| Neopygmephorus exiguus Mahunka, 1969: 533. |
| Bakerdania exiguus Mahunka, 1969; Mahunka, 1970: 348 |
| Bakerdania exigua (Mahunka, 1969): Rack, 1972: 284. |
Females in garlic crop soil in Guanajuato State. 2, EN, 16/01/2002; 1, PF, 13/02/2002; 1, 17/01/2002; 1, 14/03/2002; 1, SJ, 15/03/2002; 1, EF, 18/02/2002.
Distribution and habitat of species by Mahunka (1970)Probably cosmopolitan, known from Europe and South America (Mahunka, 1970).
Bakerdania exiguaBody chaetotaxy agrees with B. exigua (Mahunka, 1969) except ventral setae 4b (posesternals externals) which does not reach the vulva. Body 129–141 wide; 279–344 long. Length of dorsal setae: v2 7–8, sc2 36–42, c1 36–40, c2 45–52, d 33–40, e 34–40, f 44–50; h1 53–62, h2 48–53. Distances between dorsal setae: v2–v2 37–49, sc2–sc2 32–37, c1–c1 49–64, c2–c2 93–134, d–d 26–31, f–f 69–86, e–e 87–104, h1–h1 26–36, h2–h2 56–65. Length of ventral setae: 1a 24–32, 1b 26–28, 2a 30–36, 2b 41–53, 3a 40–44, 3b 41–51, 3c 24–27, 4a 39–61, 4b 57–67, 4c 23–38, ps1 12–13, ps2 7–9, ps3 11–13. Length of tibiotarsus solenidia: ω1 17–22, ω2 8–9, φ1 9–10, φ2 9–10.
| PygmephoridaeCross, 1965 |
| Pediculaster Vitzthum, 1931 |
| Type species: Pigmephorus mesembrinae Canestrini, 1880 |
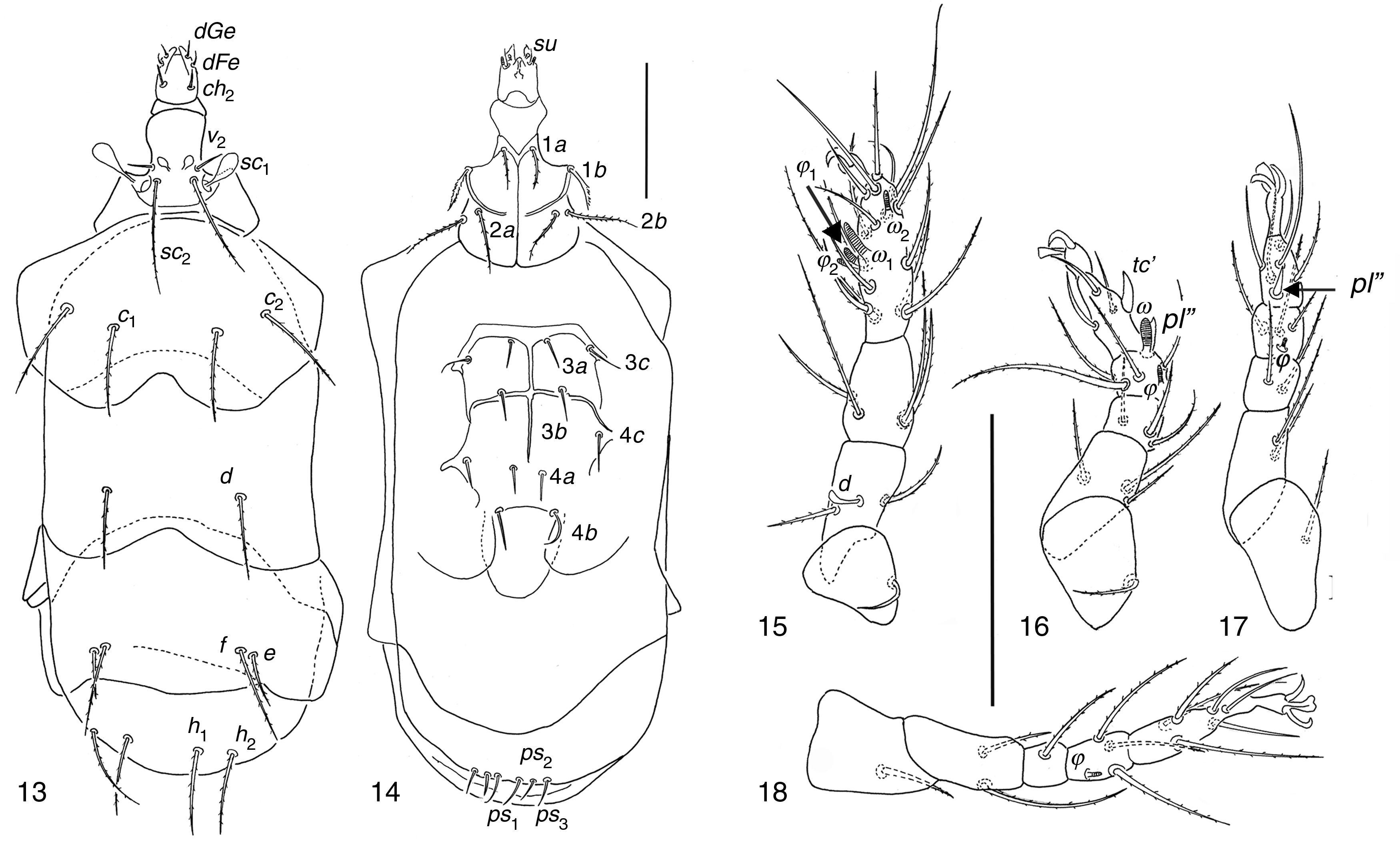
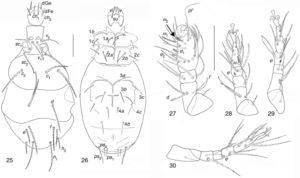
| Pediculaster ignotus Krczal, 1959 (Figs. 25–30) |
Phoretomorph females in soil of garlic crop in Guanajuato State. 6, PF, 15/03/2002; 36, PF, 15/05/2002; 1, EZ, 29/07/2002; 1, EN, 13/11/2001.
Distribution and habitat of species by Rack (1965) and Camerick and Coetzee (1997)The genus Pediculaster is cosmopolitan, with some species inhabit dung and fungi. Common habitat is cattle dung and compost; the mites are phoretic on Diptera (Camerick & Coetzee, 1997). This genus is also known to inhabit soil, litter, mosses, and mammal droppings, mammal nests, plants and fruits (Camerick, 1996). Pediculaster ignotus was recorded from Hamburg-Langenhorn North, in organic material of 2–3 years of age (Rack, 1965).
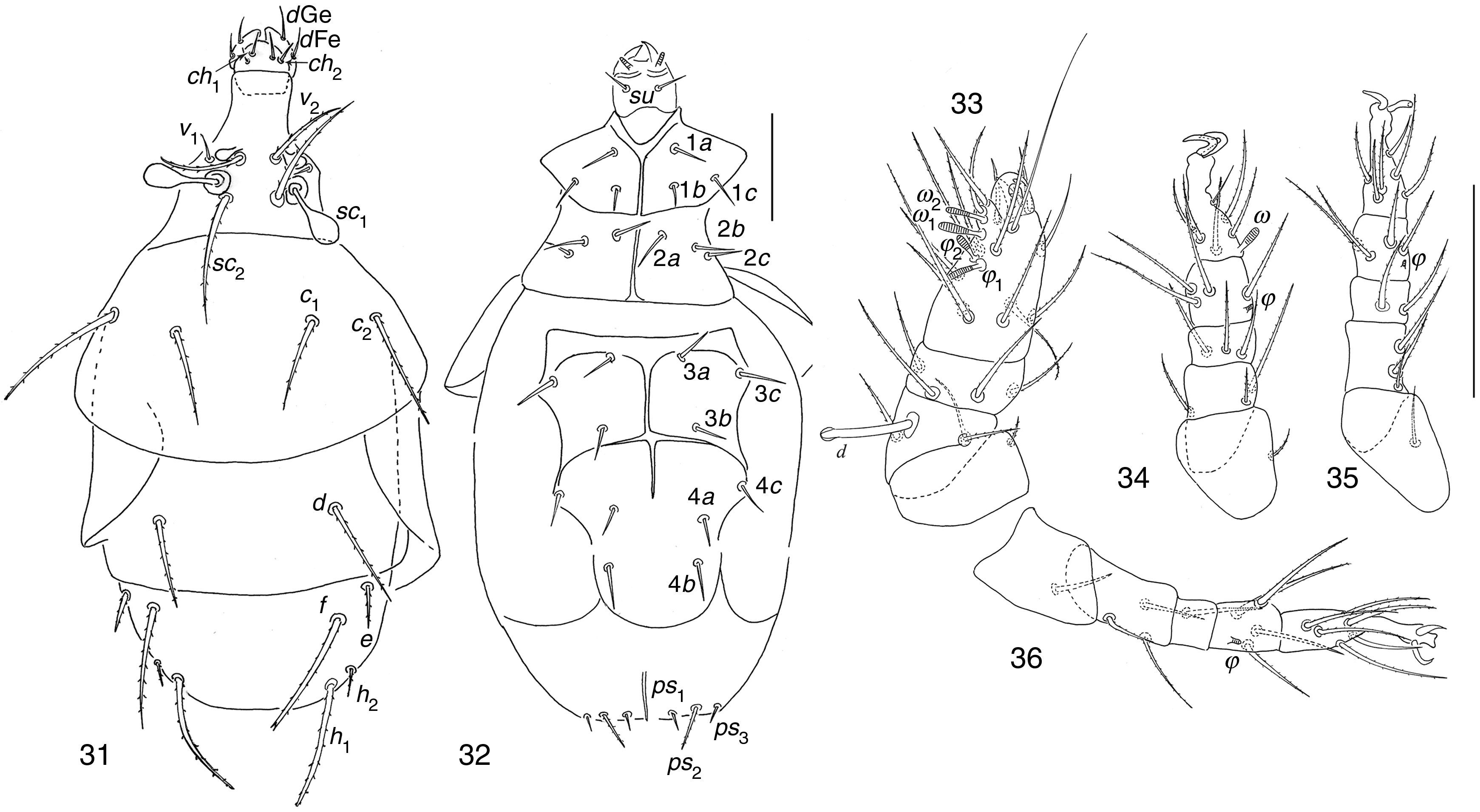
Pediculaster ignotusCharacters in general agree with the genus and the species but ps2 is different from other species in the genus because it is the shortest seta between ps setae.
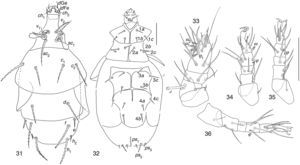
Pediculaster gracilis Camerick & Ueckermann, 1995. (Figs. 31–36)Phoretomorph females in soil of garlic crop in Guanajuato State. 2, EN, 13/12/2001; 1, EN, 13/09/2001; 2, EZ, 29/06/2002.
Distribution and habitat of species described by Camerick and Ueckermann (1995)Type locality. Republic of South Africa, Johannesburg, Sandton, Innesfree Farm; habitat: horse and cow dung. Specimens also on Cynipidae (Hymenoptera) indeterminate (Camerick & Ueckermann, 1995).
Pediculaster gracilisVentral side: Apodeme 3 not interrupted between setae 3a.
| Mahunkania Rack, 1972 |
| Type species: Mahunkania hallensis Rack, 1972: 278 |
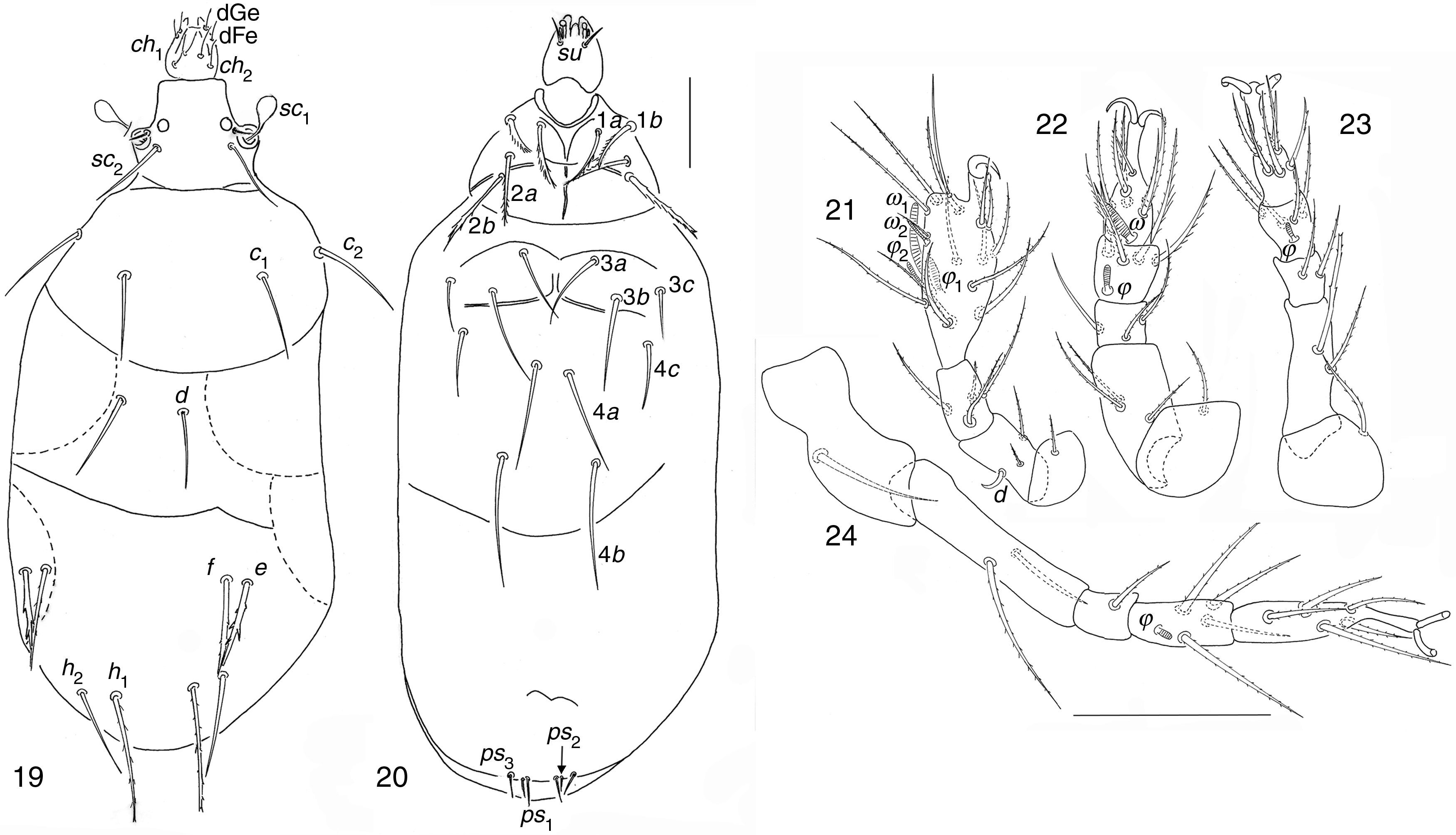
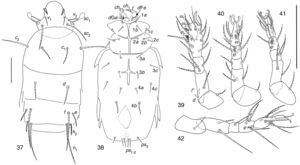
| Mahunkania secundaRack, 1975(Figs. 37–42) |
Phoretomorph females in soil of garlic crop in Guanajuato State. 3, PF, 15/05/2002.
Distribution and habitat of species described byRack (1972)Mahunkania secunda was collected in Florida, USA from Fragaria sp. (Rack, 1972).
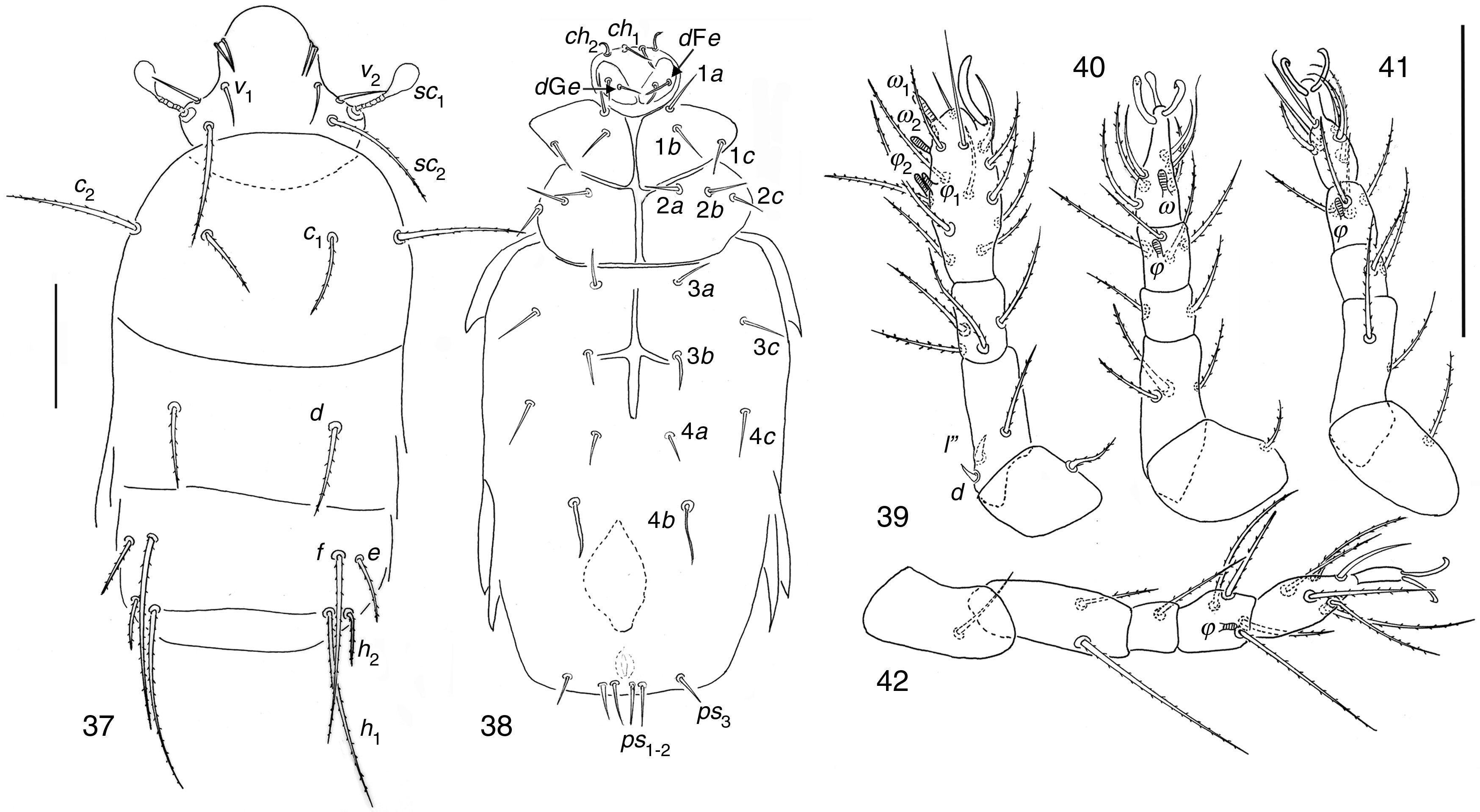
Mahunkania secundaStudied specimens share with M. secunda the following characters after Kurosa (2002). Stigmata elongate-rectangular; prodorsal setae v1 subequal in length to v2; setae e about ½ as long as f; coxal setae 1b directly posterior to 1a, not reaching apodeme 2 when directed backward; coxal 1c neither longer nor thicker than 1a and 1b; solenidia ω1 just apical in position, well apart from, and somewhat larger than ω2; seta d on femur IV reaching apex of tibia; setae d (3.35) and l″ (3.78) on femur I subequal in size.
DiscussionThe biology and behavior of Pygmephorid mites have been poorly studied, and they usually have been found associated phoretically to insects but the soil relationships are not fully understood. These mites have been collected in soils with high levels of organic matter, feeding on fungi (Kurosa, 1999), in different ecosystems as soil crust in desert habitat (Villarreal-Rosas, Palacios-Vargas, & Maya, 2014), termite nests (Wang, Powell, & O’Connor, 2002) and Arctic deserts (Khaustov & Makarova, 2005). Xochimilco soils are constantly improved with organic matter from the base of the lake, where the Chinampas zone is established. Garlic crop is usually associated with pathogenic fungi in the Guanajuato fields, so these conditions favor the Pygmephorid species. These mites are also vectors of fungal pathogens of plants and may feed preferentially on these phytopathogen fungi (Krantz & Lindquist, 1979).
The genus Bakerdania is one of the largest genera in the Neopygmephoridae family, and includes about 100 species (Khaustov, 2008). They are found in all continents except Antarctica. These mites are piercing-sucking fungi with different feeding habits (Walter & Proctor, 2013). They inhabit litter and eutrophic habitats, where they arrive by means of phoresy on insects (Kurosa, 1999).
The genus Pediculaster was abundant in samples, especially in garlic crop soil, with less abundant genera such as Bakerdania and Mahunkania, yet the latter remain very rare. Specimens of Pediculaster appear to be common in garlic crop soil, especially P. ignotus. With regard to this species, the difference in size of ps2 is notorious with respect to other Pediculaster species; nevertheless, this seta is similar to ps2 of P. ignotus shown by Rack (1965). In the drawing 14 (page 26) of Rack's paper the ps2 is the shorter seta and the ps3 is the largest, as was found in the specimens of this study. All specimens of P. ignotus are phoretic females; we believe that their insect hosts may be the visitors in or near the garlic crop. It is clearly necessary to collect insects related to the crop searching for mites to confirm this relationship (Camerick, 1996).
Pediculaster thailandensis was only found in compost. We assume that this species may be associated with some dipteran that dwells compost. As previously, the female phoretomorph leads us to believe that the presence of these mites is linked to the presence of host insects in the crop (Camerick, 2005).
All the records presented here are new for Mexico and for the substrata where they were collected.
We thank Anne Camerick, University of Witwatersrand, Johannesburg, South Africa, for identifying specimens of Pediculaster and for submitting reprints on the subject. We thank also A. Khaustov from Tyumen State University, Tyumen, Russia, for helpful advice on the systematics of Neopygmephoridae and Heterostigmata, thanks for sending reprints of papers and help to the first author to identify species of Kerdabania and Bakerdania.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.