Mortality is one of the most important demographic features. Measures of size- or sex-specific mortality can help to explain local selective pressures and can also be used to construct demographic models that estimate population trends. This study estimated mortality rates for 2 populations of a viviparous freshwater fish, endemic to western Mexico (Poeciliopsis baenschi). We found that mortality was size- and sex-dependent and different between both populations. We compared our findings with mortality rates previously estimated for other populations of this species.
La mortalidad representa uno de los parámetros demográficos más importantes. Las medidas de mortalidad específicas por tamaño y por sexo pueden ayudar a explicar presiones selectivas locales y pueden también utilizarse para construir modelos demográficos que estiman tendencias poblacionales. Estimamos las tasas de mortalidad en 2 poblaciones de un pez vivíparo dulceacuícola endémico del oeste de México (Poeciliopsis baenschi). Encontramos que la mortalidad depende del tamaño y del sexo y que es diferente entre ambas poblaciones. Comparamos nuestros resultados con tasas de mortalidad estimadas previamente para otras poblaciones de esta especie.
The study of demographic parameters is necessary to fully understand ecological and evolutionary processes (Franco and Silvertown, 1996, 2004; Metcalf and Pavard, 2007). Mortality is one of the most important demographic features for explaining the strength and direction of selective pressures (Reznick et al., 1996; Johnson and Zúñiga-Vega, 2009). In fact, extrinsic mortality caused by biotic factors such as predation or population density, can be the most important agent of natural selection in some populations (Day et al., 2002; Reznick et al., 2002). Interestingly, differences in mortality rates among populations of the same species might indicate that the strength and direction of natural selection differs among populations, which in turn leads to intraspecific phenotypic divergence (Langerhans, 2006). Furthermore, estimates of mortality rates can be used to construct robust demographic models that project population trends over time (Caswell, 2001). These population projections can guide and justify conservation efforts (Mills, 2007). Therefore, studies focused on estimating mortality rates are crucial for understanding the ecology and evolution of a particular species (Caswell, 2001; Williams et al., 2002).
Despite the great importance of this demographic trait, few studies have estimated mortality rates for Neotropical freshwater fish populations (Reznick and Bryant, 2007; Johnson and Zúñiga-Vega, 2009), and are especially scarce for those endemic to Mexico (cf. Arce-Uribe, 2006; Canto-Maza and Vega-Cendejas, 2007). Poeciliopsis baenschi (family Poeciliidae) is one of the few species for which mortality rates have been estimated (Zúñiga-Vega et al., 2012a). The results of this previous study on P. baenschi were as follows: 1) mortality rates were similar between sexes despite a marked sexual dimorphism in size, 2) mortality differed among populations, 3) the intensity of mortality varied depending on size, with individuals of intermediate sizes experiencing different mortality rates compared to individuals of smaller and larger sizes, and 4) the effect of size on mortality rates differed markedly among populations. Thus, all these results could explain, at least partially, the phenotypic differences that have been observed among populations of P. baenschi (Scott and Johnson, 2010).
The main objective of the present study is to estimate and analyze mortality rates of 2 populations of P. baenschi. We also aim to compare mortalities in these 2 populations with those previously estimated by Zúñiga-Vega et al. (2012a) in 4 other populations of this species. Our results can lead to a better understanding of intraspecific differences in mortality among populations of freshwater fishes and to a broader knowledge of the ecology of this species.
Poeciliopsis baenschi is a viviparous fish endemic to a few fluvial systems in the states of Jalisco and Colima, Mexico (Miller et al., 2005). We selected 2 populations of this species that represent 2 independent fluvial systems without connection with those sites studied by Zúñiga-Vega et al. (2012a). Both populations are located in the State of Colima. Site 1 is located at the Río Coahuayana (18°54.6’ N, 103°39.8’ W) and site 2 is located at the Río Armería (19°10.4’ N, 103°49.7’ W). Other freshwater fishes coexisting with P. baenschi at both sites are Poeciliopsis turrubarensis, Poecilia butleri, as well as other members of the family Goodeidae. The blue tilapia, Oreochromis aureus, an exotic cichlid fish that preys on poeciliids (Jiménez-Badillo and Nepita-Villanueva, 2000), also occurs at site 1.
We conducted capture-mark-recapture experiments on P. baenschi during October and November of 2010. For this purpose, in the 2 rivers studied we selected one or 2 focal pools that kept the fish partially isolated from the main current of the rivers in order to avoid migration. Each population was sampled 5 times (one sampling event per week). On each sampling event we captured as many fish as possible using hand-held seine nets (1.3m depth × 5m length, 8mm mesh size). During every sampling event 4 persons searched, captured, and marked fish from 9:00 until 17:00hrs. Thus, the sampling effort was the same in all sampling events. Once captured, each fish was anesthetized using 3-amenobenzoic acid ethyl ester (MS-222). Fish were then sexed (males were identified by the presence of the gonopodium), measured, and marked individually (only on the initial capture) with visible implant elastomer (VIE) tags (Northwest Marine Technology Inc.) injected in the caudal peduncle. In all 5 sampling events, fish that were previously marked were registered as recaptures. These recaptured fish were also once again sexed and measured. Weekly sampling allowed us to obtain individualized recapture (encounter) histories for each marked fish (Lebreton et al., 1992; Johnson and Zúñiga-Vega, 2009).
We used the encounter histories to estimate mortality rates (1-estimated survival rates) of adult individuals (see Scott and Johnson, 2010; Zúñiga-Vega et al., 2012a). Survival rates were calculated by means of maximum likelihood procedures implemented in the program Mark (White and Burnham, 1999). This software uses the individual encounter histories to estimate survival (φ) and recapture (p) probabilities. To estimate these parameters we used a Cormack-Jolly-Seber framework, which has the following main assumptions (Amstrup, 2005): 1) every marked animal present in the population at sampling period i has the same probability of survival until sampling period i+1; 2) marks are neither lost nor overlooked and are recorded correctly; 3) all emigration from the sampled area is permanent, and 4) the fate of each animal with respect to capture and survival probability is independent of the fate of any other animal. In general, these assumptions were met by our data. However, we recognize that even though we selected partially isolated pools to avoid in- and-out migration, some temporary emigration might have occurred. Therefore, our survival estimates may be slightly underestimated.
We fitted a total of 32 different models to our mark-recapture data. Through these models we tested 3 different effects (size, sex and population) and their interactions on both parameters (except for size which only affected the survival probability). Constant models (“null” models that indicate no effect of size, sex, population or their interactions) were also tested on both φ and p. To identify the best-fitting model, we used an adjusted version of the Akaike's information criterion that is appropriate for small sample sizes (AICc; Akaike, 1973; Burnham and Anderson, 2002). A difference between 2 models in their AICc scores (ΔAICc) larger than 2 indicates considerable support for a real difference in the fit of such 2 models (Johnson and Omland, 2004). We also calculated AICc weights (wi), which are measures of the relative support in the data for each model (Burnham and Anderson, 2002). Based on these AICc weights we calculated model-weighted average estimates of φ and p as per Burnham and Anderson (2002). These weighted estimates of weekly φ and p (for each sex and for each population) take into account the weight of evidence for each competing model and, therefore, are more robust than estimates derived from any single model alone (Johnson and Omland, 2004). We also report the estimated relationships between size and survival for each sex and for each population.
We marked a total of 704 individuals (315 in site 1 and 389 in site 2). Of these, 545 were females (265 in site 1 and 280 in site 2) and 159 were males (50 in site 1 and 109 in site 2). Recapture probabilities varied between sites and sexes. Females from site 1 had the highest recapture probability (females from site 1 [mean ± SE]=0.325±0.08, males from site 1=0.255±0.18, females from site 2=0.168±0.09, males from site 2=0.145±0.09).
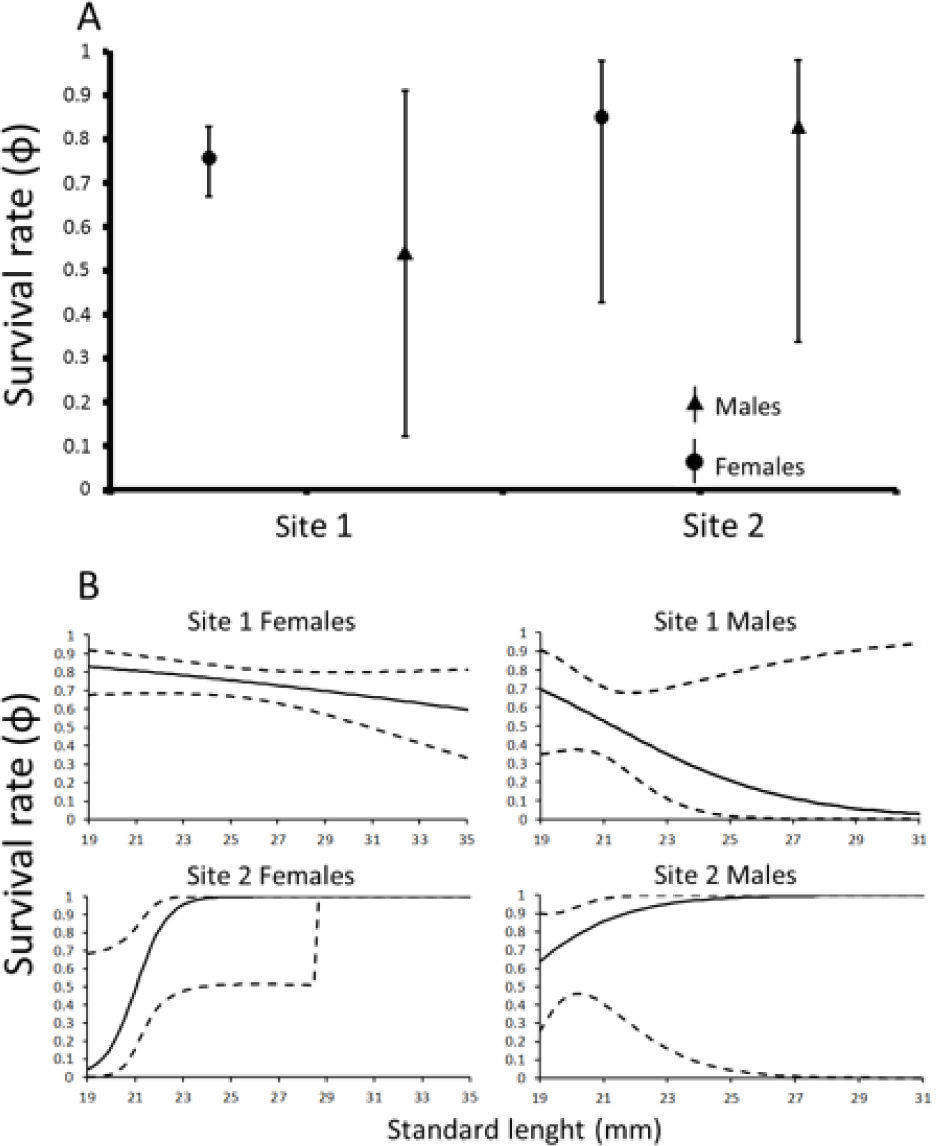
The best-fitting model (Table 1) indicated an interaction among sex, site and size affecting φ and an effect of site on p. According to this model, survival in site 1 decreases in larger sizes whereas in site 2 survival increases in larger sizes. The relative support for this model was 32% (wi=0.32). However, another model resulted in ΔAICc>2 compared to the best-fitting model, indicating model uncertainty. This model corresponded to a null (constant) model for φ with an interaction between site and sex affecting p. All other models had ΔAICc<2 when compared to the best-fitting model. Model-weighted estimates of weekly survival do not allow to detect differences between sexes or between populations because of the wide confidence intervals for 3 of the 4 estimated survival rates (Fig. 1A).
Model selection results for 2 populations of Poeciliopsis baenschi. Each model represents a biological hypothesis about variation in survival (φ) and recapture (p) probabilities. We tested 3 different effects (size, sex and population) and their interactions on both parameters (except size which only affected survival); (constant) represents the null model. We show the Akaike information criterion (AICc), the difference in the AICc scores between each model and the best-fitting model (ΔAICc) and the relative support for each competing model (wi). We only show here models with wi<0.05
| Model | AICc | ΔAICc | wi |
|---|---|---|---|
| φ(site × sex × size) p(site) | 1210.77 | 0.00 | 0.32 |
| φ(constant) p(site × sex) | 1212.53 | 1.76 | 0.13 |
| φ(sex) p(site) | 1212.96 | 2.19 | 0.11 |
| φ(site × size) p(site × sex) | 1213.76 | 2.99 | 0.07 |
| φ(size) p(site × sex) | 1214.42 | 3.65 | 0.05 |
A), model-weighted estimates of weekly survival rates (φ) for each site and sex. Error bars represent 95% confidence intervals. B), relationships between size of individuals (standard length) and weekly survival rate for each site and sex. Dotted lines represent 95% confidence intervals.
Our results revealed an important difference compared to the mortality patterns reported by Zúñiga-Vega et al. (2012a) for 4 populations of P. baenschi. This interesting difference is the effect of sex on survival that is strongly supported by our mark-recapture data (Table 1). In contrast, Zúñiga-Vega et al. (2012a) failed to detect a statistical effect of sex on survival. In study site 1, males appeared to suffer lower survival compared to females, whereas in site 2 no differences between sexes in survival were evident (just as in all other 4 populations studied by Zúñiga-Vega et al., 2012a). In Poeciliopsis species males harass females to gain copulations (Macías-Garcia and González-Zuarth, 2005). This behavior may increase their mortality risk since they become more conspicuous to predators (Magurran and Nowak, 1991). Further research is needed to test whether some additional factors occurring exclusively in site 1, such as increased mating attempts, brighter coloration, or increased activity of predators are causing higher than expected mortality rates in males of P. baenschi from this population.
In addition, our estimated survival rates, particularly those for females in site 1 and for both sexes in site 2 (Fig. 1A) are relatively high (< 0.7) and similar to those observed in the Purificación and Chandiablo rivers (Zúñiga-Vega et al., 2012a). However, we recognize that the precision of our estimates is low (excepting for female survival in site 1).
Our best fitting model indicates an interaction between size and site affecting φ (Table 1). In other words, we found that the effect of the size on φ differs between populations. In site 1, the larger individuals (both males and females) had a lower survival probability, whereas in site 2, the larger individuals (both males and females) had a higher survival probability. These different relationships between size and mortality might reflect different selective pressures occurring in these 2 populations. A potential explanation focuses on differences between these 2 sites in the size and abundance of predatory fishes. In site 1, the water volume of the river is larger than that in site 2 (see Zúñiga-Vega et al., 2012b for a complete description of the study sites). Therefore, larger individuals of the cichlids Cichlasoma istlanum and Oreochromis aureus are present at this site 1. These larger individuals might prey selectively on larger P. baenschi causing the observed lower survival probability for larger fish at site 1. Additionally, higher mortality in smaller fish at site 2 might result from increased intraspecific competition affecting young or small individuals as population density in this site 2 is notably higher than that in site 1 (148.1 individuals/m3 and 23.8 individuals/m3, respectively; Zúñiga-Vega et al., 2012b). The derived prediction is that smaller individuals are being favored in site 1, whereas larger individuals are being favored in site 2. These different selective patterns should result in phenotypic divergence between these 2 populations, with smaller individuals in site 1 and larger individuals in site 2. This prediction deserves future research.
We thank Pedro Mendoza-Hernández for field assistance. Fieldwork was conducted under permit no. FAUT-0240 issued by the Semarnat-México. Funding for this research was provided by the DGAPA-UNAM through the project PAPIIT IN206309-3 as well as by the Conacyt through the project 129675. We also thank all the students from UNAM that provided field assistance.