We report the results of the non-volant mammal survey at the Maracaju Mountains located in the central region of Mato Grosso do Sul State, southwestern Brazil. The sampling encompasses different vegetation types of the Cerrado domain that occur in the region. The mammal survey was conducted by the use of pitfalls (1804 bucket-nights), live traps (7508 trap-nights), direct observation and indirect evidence (624 hours of observations). Fifty-eight non-volant mammal species of 9 orders and 20 families were recorded, representing 12% of the Brazilian terrestrial mammal species. The non-volant mammal community of Maracaju Mountains is typical of the Cerrado, but some species found occur in other domains, especially from Pantanal, Atlantic and Amazonian Forests. Among the recorded species, 11 are threatened in Brazil or on a global scale. Therefore, the Maracaju Mountains have a high richness of mammals with endangered and rare species that are still present in the extensive natural areas which persist in the region. These areas are very important for conservation of mammals and should be considered relevant areas for the establishment of Conservation Units in Cerrado and in the Mato Grosso do Sul State.
Se presentan los resultados del inventario de mamíferos no voladores en la sierra de Maracaju, que se encuentra en la región central del estado de Mato Grosso do Sul, en el suroeste de Brasil. El muestreo abarca diferentes tipos de vegetación en la región del Cerrado. El inventario de mamíferos se llevó a cabo mediante el uso de trampas de caída (1804 trampas/noche) y trampas Sherman y Tomahawk (7508-trampas/noche); también se utilizaron datos de observación directa y rastros (624 horas de observaciones). Se registraron 58 especies de mamíferos no voladores de 9 órdenes y 20 familias, lo que representa el 12% de las especies de mamíferos terrestres brasileñas. La comunidad de mamíferos no voladores de la sierra de Maracaju es típica del Cerrado, pero contiene especies que se presentan en otros biomas, especialmente en el Pantanal, Mata Atlántica y Floresta Amazónica. Entre las especies registradas, 11 están amenazadas en Brasil o a una escala global. Así, la sierra de Maracaju tiene una alta riqueza de mamíferos con especies amenazadas y raras que todavía están presentes en los extensos espacios naturales que persisten en la región. Estas áreas son muy importantes para la conservación de los mamíferos y se deben considerar como pertinentes para la creación de unidades de conservación en el Cerrado y en el estado de Mato Grosso do Sul.
The Cerrado and Pantanal are included in the so-called diagonal of open formations or corridors of xeric vegetation, which includes the much drier Caatinga, in northeastern Brazil, and the Chaco domain, in parts of Paraguay-Bolivia-Argentina. This corridor runs between the 2 main domains of moist forest of tropical South America: Amazonian forest in the north and northwest and Atlantic Forest in the east and southeast (Oliveira-Filho & Ratter, 2002).
The Cerrado and Pantanal occupy most of the territory of Mato Grosso do Sul State, and so the most of the mammal fauna is composed of species typical of these domains (Cáceres, Carmignotto, Fischer, & Santos, 2008). However, Atlantic and Amazonian Forests also play an important role on the biogeography of Cerrado mammals, especially on forest formations (Carmignotto, 2005; Costa, 2003; Johnson, Saraiva, & Coelho, 1999). Also, in the Cerrado of Mato Grosso do Sul, occur Amazonian species, such as Oecomys paricola, O. mamorae and Proechimys longicaudatus, and Atlantic species, such as Akodon montensis, Oecomys catherinae and Cavia fulgida (Cáceres, Carmignotto, et al., 2008). Additionally, the Chaco and Chiquitano forests (Zanella, 2011) also influence the mammal composition in the state of Mato Grosso do Sul, especially on the western edge of the Pantanal (Rodrigues, Medri, Tomas, & Mourão, 2002), in the proximities of Paraguay and Bolivia countries. In Mato Grosso do Sul, the Chiquitanean species Marmosops ocellatus, Aotus azarae, Callicebus pallescens, Mico melanurus and Urosciurus spadiceus occur only in the western edges of Pantanal, while Cryptonanus chacoensis, Akondon toba and Oligoryzomys chacoensis, which are Chacoan species, and have broader distributions in the High Paraguay River Basin (Cáceres, Carmignotto, et al., 2008).
The state of Mato Grosso do Sul presents 151 species of mammals distributed in 10 orders and 29 families, with 90 species of non-volant mammals and 61 species of bats (Cáceres, Carmignotto, et al., 2008). Despite this high species richness, few studies on non-volant mammal communities have been conducted in the State (Bordignon, Cáceres, França, Casella, & Vargas, 2006; Cáceres, Bornschein, Lopes, & Percequillo, 2007; Cáceres, Carmignotto, et al., 2008; Cáceres, Nápoli, Casella, & Hannibal, 2010; Cáceres, Godoi, Hannibal, & Ferreira, 2011; Godoi, Cunha, & Cáceres, 2010; Mauro & Campos, 2000; Rodrigues, Medri, et al., 2002). Therefore, there is a gap in the knowledge on mammal species composition and distribution in this portion of southwestern Brazil (Eisenberg & Redford, 1999; Vieira & Palma, 2005). The majority of the species lists of non-volant mammals available for the state come from the Pantanal wetland (Rodrigues, Medri, et al., 2002) and some mountainous regions in the surroundings of Pantanal, like Urucum mountains (Cáceres et al., 2011; Godoi et al., 2010; Mauro & Campos, 2000), Bodoquena mountains (Cáceres, Bornschein, et al., 2007) and regions of Aporé and Sucuriú rivers, in the northeastern of the state (Bordignon et al., 2006). Beyond these areas, in Mato Grosso do Sul State, there another important region for mammal conservation, the Maracaju Mountains, an extensive mountainous region of Cerrado located in the eastern border of Pantanal. These mountains still present great extensions of preserved natural areas and despite their importance to mammal conservation in southwestern Brazil, their mammal diversity and composition are still poorly known. Therefore, the main objective of this paper is to present the composition of non-volant mammal communities of the Maracaju Mountains, and contribute to the knowledge of mammalian fauna in a biogeographically important and poorly known region of the Cerrado. Additionally, we provide data on the frequency of occurrence of species in the region and the role of adjacent phytogeographic domains on the regional species composition.
Materials and methodsStudy areaThe Maracaju Mountains (23°15'S, 55°31’W; 17°34'S, 54°45’W) are located in the central region of Mato Grosso do Sul State, dividing it in the north-south direction, acting as a watershed between High Paraguay River Basin, to the west, and High Paraná River Basin, to the east (Fig. 1). The mountains average 900 m in altitude. They are located in the core area of the Cerrado domain in the southern part, being covered by the physiognomies of cerradão (arboreal savannas), cerrado sensu stricto (shrubland savannas), seasonal forests, riparian forests and veredas, a riparian formation dominated by Buriti palms (Mauritia flexuosa) (Foster, Pott, & Salis, 2000). The vegetation of this region presents great anthropogenic influence due to cattle farms, Eucalyptus and sugar-cane plantations, and the natural remnants are fragmented and located in a matrix constituted of exotic grasses. However, in many regions there are portions that have continuous areas of preserved habitats, especially on the slopes of mountains (Harris et al., 2006; Sano, Rosa, Brito, & Ferreira, 2010). The climate of the region is tropical sub-humid (Aw), according to the Köppen classification, with wet (October to March) and dry (April to September) seasons well defined. The mean annual precipitation is 1,400mm, with 80% of rainfall during the wet season. The mean temperatures are 22 °C in July and 28 °C in January (Rego, 2008).
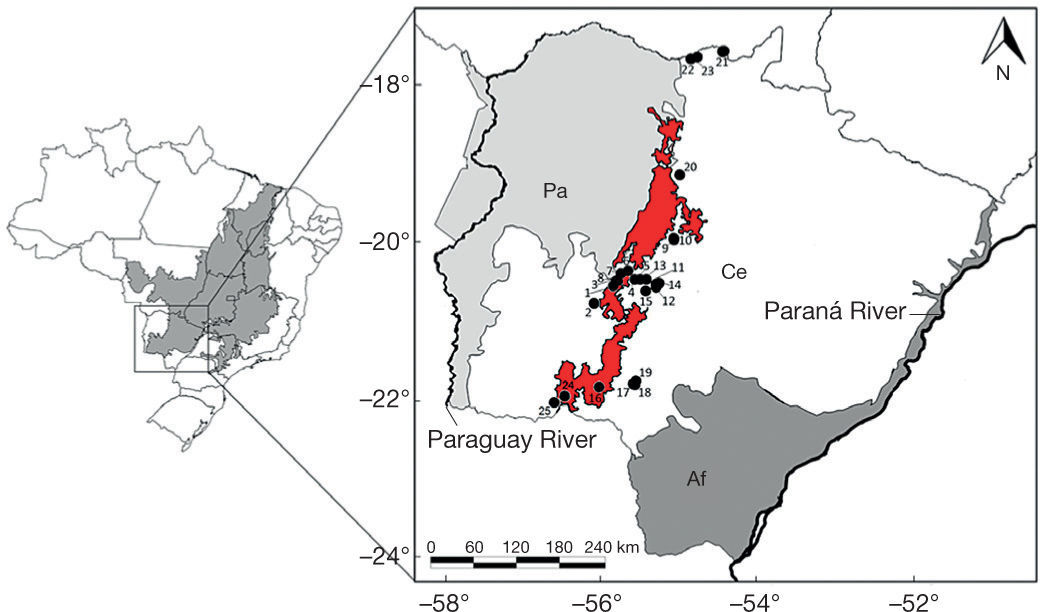
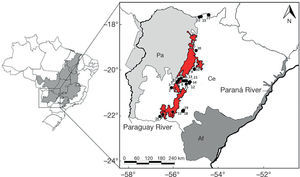
Map showing Brazil and Cerrado domain (shaded area) and the 25 localities sampled in the Maracaju Mountains and surroundings, state of Mato Grosso do Sul, southwestern Brazil, during the period of 2006 to 2010. In detail the limits of Atlantic Forest (Af), Cerrado (Ce) and Pantanal (Pa) in the state of Mato Grosso do Sul. Red delimits the Maracaju Mountains (according to MMA, 2007). For the localities number see Appendix 1.
The data were collected from 2006 to 2010 in 25 localities that covered important subsets of Maracaju Mountains and surroundings. The municipalities sampled were: Anastácio, Aquidauana, Bela Vista, Corguinho, Dois Irmãos do Buriti, Jardim, Maracaju, Rio Verde de Mato Grosso and Sonora (Fig. 1; Appendix 1). Localities 21, 22 and 23, although not marked in red area of the map (Fig. 1), occur in Sonora Mountains, an extension of Maracaju mountains (Tsilfidis & Soares-Filho, 2010). Accordingly, these localities were maintained in this study, adding important information about an area poorly studied in Cerrado domain.
List of all localities mentioned in this report, sampling effort and the types of habitats surveyed
| Point | Municipality | Locality | Latitude (S) | Longitude (W) | Sampling effort | Type of habitat |
|---|---|---|---|---|---|---|
| 1 | Anastácio | Fazenda Jatiúca | 20°32’% | 55°50’% | 26 sampling hours, 156 live traps and 100 pitfall traps | SF |
| 2 | Anastácio | Fazenda Boa Esperança | 20°45’% | 56°05’% | 26 sampling hours, 156 live traps and 100 pitfall traps | RF, SF, VE |
| 3 | Aquidauana | Campus da Universidade Estadual do Mato Grosso do Sul (UEMS) | 20°28’% | 55°47’% | 60 sampling hours, 1,016 live traps and 280 pitfall traps | RF, SF |
| 4 | Aquidauana | Córrego das Antas – Piraputanga | 20°27’% | 55°34’% | 20 sampling hours and 1,200 live traps | RF |
| 5 | Aquidauana | Acambapi - Piraputanga | 20°27’% | 55°29’% | 20 sampling hours and 600 live traps | RF |
| 6 | Aquidauana | Córrego João Dias Nascente | 20°20’% | 55°39’% | 40 sampling hours | RF |
| 7 | Aquidauana | Fazenda Rolândia | 20°22’% | 55°44’% | 40 sampling hours and 40 live traps | RF, SF |
| 8 | Aquidauana | Córrego João Dias Fóz | 20°28’% | 55°48’% | 40 sampling hours | RF |
| 9 | Corguinho | RPPN – Gavião de Penacho | 19°55’% | 55°04’% | 20 sampling hours and 72 live traps | SF |
| 10 | Corguinho | RPPN – Vale do Bugio | 19°57’% | 55°04’% | 20 sampling hours and 72 live traps | SF |
| 11 | Dois Irmãos do Buriti | Fazenda São Cristovão F-40 | 20°31’% | 55°17’% | 20 sampling hours and 600 live traps | SF |
| 12 | Dois Irmãos do Buriti | Fazenda São Cristovão F-400 | 20°33’% | 55°17’% | 20 sampling hours and 1,200 live traps | SF |
| 13 | Dois Irmãos do Buriti | Fazenda Correntes | 20°27’% | 55°25’% | 26 sampling hours, 156 live traps and 100 pitfall traps | SF, SS |
| 14 | Dois Irmãos do Buriti | Fazenda Taruanã | 20°30’% | 55°15’% | 26 sampling hours, 156 live traps and 100 pitfall traps | RF, SF, VE |
| 15 | Dois Irmãos do Buriti | Fazendo Vô Fiorindo | 20°36’% | 55°25’% | 26 sampling hours, 156 live traps and 108 pitfall traps | RF, VE |
| 16 | Jardim | RPPN Xodó do Vô Rui | 21°49’% | 56°01’% | 20 sampling hours and 72 live traps | Sf |
| 17 | Maracaju | Usina Vista Alegre – P1 | 21°47’% | 55°34’% | 15 sampling hours, 132 live traps and 60 pitfall traps | SF |
| 18 | Maracaju | Usina Vista Alegre – P2 | 21°47’% | 55°33’% | 15 sampling hours, 132 live traps and 60 pitfall traps | SF |
| 19 | Maracaju | Usina Vista Alegre – P3 | 21°44’% | 55°32’% | 15 sampling hours, 132 live traps and 60 pitfall traps | SF |
| 20 | Rio Verde | Fazenda Cruzeiro | 19°08’% | 55°02 | 15 sampling hours | SF, SS |
| 21 | Sonora | PCH – Santa Gabriela – P1 | 17°32’% | 54°26’% | 40 sampling hours, 740 live traps and 480 pitfall traps | RF, SF, SS |
| 22 | Sonora | Usina Sonora – P1 | 17°38’% | 54°51’% | 25 sampling hours, 360 live traps and 178 pitfall traps | RF |
| 23 | Sonora | Usina Sonora – P2 | 17°36’% | 54°46’% | 25 sampling hours, 360 live traps and 178 pitfall traps | RF, SF |
| 24 | Bela Vista | Fazenda Redomão | 22°05’% | 56°43’% | 12 sampling hours and 195 live traps | SF |
| 25 | Bela Vista | Exército | 22°07’% | 56°55’% | 12 sampling hours and 195 live traps | SF |
SF: seasonal semidecidual forests; RF: riparian forests; VE: veredas; Sampling effort: sampling hours, for medium and large mammals, and pitfall traps (bucket-night) and live traps (trap-night) for small mammals; SS: shrubland savannas (cerrado sensu stricto); RPPN (Reserva Particular do Patrimônio Natural): private reserves; PCH (Pequena Central Hidroelétrica): small hydroelectric projects.
Considering all localities, we sampled areas of shrubland savannas (cerrado sensu stricto), seasonal forests (semidecidual forests and cerradão), riparian forests (gallery forests), veredas of Buriti palms (M. flexuosa) and anthropogenic habitats, such as exotic grass pastures (Brachyaria pastures), sugar-cane plantations, soybean, Eucalyptus and peri-urban areas, like roads.
At each locality, which usually comprised an area with a 1km radius, the species of medium and large mammals were registered during 624 h of observations by: i) direct observations, ii) carcass and isolated body parts, such as teeth and horns, iii) tracks, iv) burrows (Order Cingulata), v) feces, and vi) vocalizations (Order Primates). For some groups, like Mazama spp., tracks and direct observations were used together for identification of species, when possible. The animal tracks and other signs were identified according to Borges and Tomas (2004).
Non-volant small mammals (<1kg) were sampled using pitfall and live traps. The pitfall traps were composed of 4 buckets of 60 or 108 l separated by 10 m and disposed in “Y” (1 in the center and other 3 buckets in the extremities of the Y) or in transects (with 4 buckets per transect). These were connected by a black plastic fence or plastic screen fence (50cm high) to guide animals into the buckets. These traps were active during 3 consecutive nights in each field expedition, totaling 1,804 bucket-nights for all localities surveyed. Tomahawk and Sherman live traps of different sizes were organized in transects with 5 to 10 traps that were active during 3 to 5 consecutive nights for each field expedition, totaling 7,508 trap-nights for all localities surveyed. The sampling methods and sampling effort utilized in each locality are described in Appendix 1. In all areas, the live traps were placed on the ground and in the understory (1.5-2 m high). The baits utilized were bananas, pumpkins and bacon in a mixture with sardine or peanut butter and/or cod-liver oil. The small mammals captured were photographed, identified and released at the same point of capture. The identification, when possible, was conducted based on morphological features of the species (according to Bonvicino, Oliveira, & D’andrea, 2008; Oliveira & Bonvicino, 2011; Rossi & Bianconi, 2011). Voucher specimens and available records of small mammals species were assessed primarily through analyses of specimens (skin and/or skull) deposited in the Mammals Collection of the Universidade Federal de Santa Maria (UFSM), in Brazil (Appendix 2 The captures were made with the collection licenses IBAMA n° 025/2009, Process 02014.000702/2009-14; n° 002/2013, process 02014.000829/2010-69 and SISBIO n° 17188-1, process 1925136. We follow Paglia et al. (2012) for the taxonomic classification of all mammal species surveyed. The specimens of Calomys were classified at the genus level because this group is difficult to identify without karyotypic analyses. The identification of 1 species of Oligoryzomys was not possible, and so this species was only classified at the genus level.
We used the index of frequency of occurrence to determine the status of occurrence of mammal species in the Maracaju Mountains. This index is based on the ratio between the numbers of locations where the species was recorded by the total number of sampling sites. The frequency of occurrence does not show how many mammal species are abundant in the study area in terms of absolute abundance or population density, but allowed us to identify which species are rare or which are more common in the survey area.
The species of non-volant mammals were classified as threatened according to the list of mammals threatened for extinction in Brazil (Chiarello et al., 2008) and the Red List of the Species Threatened of International Union for Conservation of Nature (IUCN, 2013).
To determine biogeographical influences on the mammal fauna of Maracaju Mountains, we listed the species in their phytogeographic domains of occurrence following the classification presented by Paglia et al. (2012). Thus, we calculated the contribution (in percentages) of each domain to the richness of non-volant mammals of Maracaju Mountains.
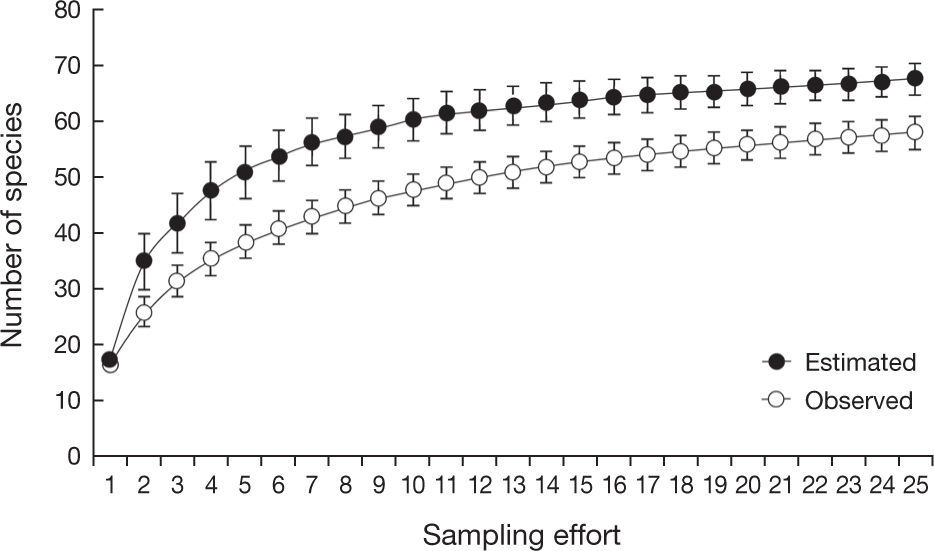
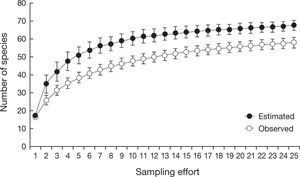
The species accumulation curve was provided to evaluate the efficiency of our sampling to record species of non-volant mammals. This analysis was performed using the observed and estimated species richness in the software EstimateS 8.2 (Colwell, 2005). The observed richness was based on the number of new species added as a function of the field sampling effort (number of localities), including all sampling methods. The estimated richness was calculated using Jacknife 1, considering only presence-absence data.
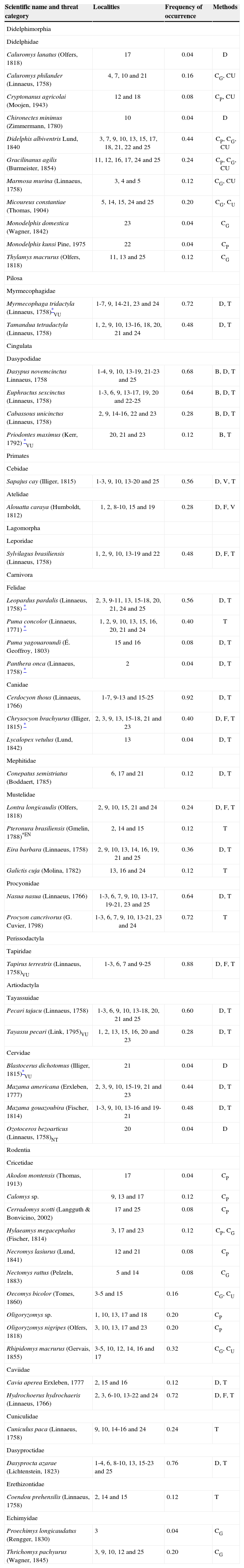
ResultsIn the Maracaju Mountains and surroundings we recorded 58 species of non-volant mammals representing 9 orders and 20 families, with 11 species that are considered as threatened (Table 1). The richest order were the Rodentia (17 species), followed by Carnivora (14 spp.), Didelphimorphia (11 spp.), Artiodactyla (6 spp.), and Cingulata (4 spp.). The richest families were the Didelphidae (11 species) and Cricetidae (10 spp.), which comprised the small non-volant mammalian fauna of the region (Table 1). The species accumulation curves for observed and estimated richness showed a tendency for stabilization (Fig. 2) suggesting that most of mammal species of the region were sampled in this study.
Species composition, threat category, distribution and frequency of occurrence of non-volant mammal species in the Maracaju Mountains and surroundings, Mato Grosso do Sul state, southwestern Brazil, during the period of 2006 to 2010
| Scientific name and threat category | Localities | Frequency of occurrence | Methods |
|---|---|---|---|
| Didelphimorphia | |||
| Didelphidae | |||
| Caluromys lanatus (Olfers, 1818) | 17 | 0.04 | D |
| Caluromys philander (Linnaeus, 1758) | 4, 7, 10 and 21 | 0.16 | CG, CU |
| Cryptonanus agricolai (Moojen, 1943) | 12 and 18 | 0.08 | CP, CU |
| Chironectes minimus (Zimmermann, 1780) | 10 | 0.04 | D |
| Didelphis albiventris Lund, 1840 | 3, 7, 9, 10, 13, 15, 17, 18, 21, 22 and 25 | 0.44 | CP, CG, CU |
| Gracilinanus agilis (Burmeister, 1854) | 11, 12, 16, 17, 24 and 25 | 0.24 | CP, CG, CU |
| Marmosa murina (Linnaeus, 1758) | 3, 4 and 5 | 0.12 | CG, CU |
| Micoureus constantiae (Thomas, 1904) | 5, 14, 15, 24 and 25 | 0.20 | CG, CU |
| Monodelphis domestica (Wagner, 1842) | 23 | 0.04 | CG |
| Monodelphis kunsi Pine, 1975 | 22 | 0.04 | CP |
| Thylamys macrurus (Olfers, 1818) | 11, 13 and 25 | 0.12 | CG |
| Pilosa | |||
| Myrmecophagidae | |||
| Myrmecophaga tridactyla (Linnaeus, 1758)*VU | 1-7, 9, 14-21, 23 and 24 | 0.72 | D, T |
| Tamandua tetradactyla (Linnaeus, 1758) | 1, 2, 9, 10, 13-16, 18, 20, 21 and 24 | 0.48 | D, T |
| Cingulata | |||
| Dasypodidae | |||
| Dasypus novemcinctus Linnaeus, 1758 | 1-4, 9, 10, 13-19, 21-23 and 25 | 0.68 | B, D, T |
| Euphractus sexcinctus (Linnaeus, 1758) | 1-3, 6, 9, 13-17, 19, 20 and 22-25 | 0.64 | B, D, T |
| Cabassous unicinctus (Linnaeus, 1758) | 2, 9, 14-16, 22 and 23 | 0.28 | B, D, T |
| Priodontes maximus (Kerr, 1792) *VU | 20, 21 and 23 | 0.12 | B, T |
| Primates | |||
| Cebidae | |||
| Sapajus cay (Illiger, 1815) | 1-3, 9, 10, 13-20 and 25 | 0.56 | D, V, T |
| Atelidae | |||
| Alouatta caraya (Humboldt, 1812) | 1, 2, 8-10, 15 and 19 | 0.28 | D, F, V |
| Lagomorpha | |||
| Leporidae | |||
| Sylvilagus brasiliensis (Linnaeus, 1758) | 1, 2, 9, 10, 13-19 and 22 | 0.48 | D, F, T |
| Carnivora | |||
| Felidae | |||
| Leopardus pardalis (Linnaeus, 1758) * | 2, 3, 9-11, 13, 15-18, 20, 21, 24 and 25 | 0.56 | D, T |
| Puma concolor (Linnaeus, 1771) * | 1, 2, 9, 10, 13, 15, 16, 20, 21 and 24 | 0.40 | T |
| Puma yagouaroundi (É. Geoffroy, 1803) | 15 and 16 | 0.08 | D, T |
| Panthera onca (Linnaeus, 1758) * | 2 | 0.04 | D, T |
| Canidae | |||
| Cerdocyon thous (Linnaeus, 1766) | 1-7, 9-13 and 15-25 | 0.92 | D, T |
| Chrysocyon brachyurus (Illiger, 1815) * | 2, 3, 9, 13, 15-18, 21 and 23 | 0.40 | D, F, T |
| Lycalopex vetulus (Lund, 1842) | 13 | 0.04 | D, T |
| Mephitidae | |||
| Conepatus semistriatus (Boddaert, 1785) | 6, 17 and 21 | 0.12 | D, T |
| Mustelidae | |||
| Lontra longicaudis (Olfers, 1818) | 2, 9, 10, 15, 21 and 24 | 0.24 | D, F, T |
| Pteronura brasiliensis (Gmelin, 1788)*EN | 2, 14 and 15 | 0.12 | T |
| Eira barbara (Linnaeus, 1758) | 2, 9, 10, 13, 14, 16, 19, 21 and 25 | 0.36 | D, T |
| Galictis cuja (Molina, 1782) | 13, 16 and 24 | 0.12 | T |
| Procyonidae | |||
| Nasua nasua (Linnaeus, 1766) | 1-3, 6, 7, 9, 10, 13-17, 19-21, 23 and 25 | 0.64 | D, T |
| Procyon cancrivorus (G. Cuvier, 1798) | 1-3, 6, 7, 9, 10, 13-21, 23 and 24 | 0.72 | T |
| Perissodactyla | |||
| Tapiridae | |||
| Tapirus terrestris (Linnaeus, 1758)VU | 1-3, 6, 7 and 9-25 | 0.88 | D, F, T |
| Artiodactyla | |||
| Tayassuidae | |||
| Pecari tajacu (Linnaeus, 1758) | 1-3, 6, 9, 10, 13-18, 20, 21 and 25 | 0.60 | D, T |
| Tayassu pecari (Link, 1795)VU | 1, 2, 13, 15, 16, 20 and 23 | 0.28 | D, T |
| Cervidae | |||
| Blastocerus dichotomus (Illiger, 1815)*VU | 21 | 0.04 | D |
| Mazama americana (Erxleben, 1777) | 2, 3, 9, 10, 15-19, 21 and 23 | 0.44 | D, T |
| Mazama gouazoubira (Fischer, 1814) | 1-3, 9, 10, 13-16 and 19-21 | 0.48 | D, T |
| Ozotoceros bezoarticus (Linnaeus, 1758)NT | 20 | 0.04 | D |
| Rodentia | |||
| Cricetidae | |||
| Akodon montensis (Thomas, 1913) | 17 | 0.04 | CP |
| Calomys sp. | 9, 13 and 17 | 0.12 | CP |
| Cerradomys scotti (Langguth & Bonvicino, 2002) | 17 and 25 | 0.08 | CP |
| Hylaeamys megacephalus (Fischer, 1814) | 3, 17 and 23 | 0.12 | CP, CG |
| Necromys lasiurus (Lund, 1841) | 12 and 21 | 0.08 | CP |
| Nectomys rattus (Pelzeln, 1883) | 5 and 14 | 0.08 | CG |
| Oecomys bicolor (Tomes, 1860) | 3-5 and 15 | 0.16 | CG, CU |
| Oligoryzomys sp. | 1, 10, 13, 17 and 18 | 0.20 | CP |
| Oligoryzomys nigripes (Olfers, 1818) | 3, 10, 13, 17 and 23 | 0.20 | CP |
| Rhipidomys macrurus (Gervais, 1855) | 3-5, 10, 12, 14, 16 and 17 | 0.32 | CG, CU |
| Caviidae | |||
| Cavia aperea Erxleben, 1777 | 2, 15 and 16 | 0.12 | D, T |
| Hydrochoerus hydrochaeris (Linnaeus, 1766) | 2, 3, 6-10, 13-22 and 24 | 0.72 | D, F, T |
| Cuniculidae | |||
| Cuniculus paca (Linnaeus, 1758) | 9, 10, 14-16 and 24 | 0.24 | T |
| Dasyproctidae | |||
| Dasyprocta azarae (Lichtenstein, 1823) | 1-4, 6, 8-10, 13, 15-23 and 25 | 0.76 | D, T |
| Erethizontidae | |||
| Coendou prehensilis (Linnaeus, 1758) | 2, 14 and 15 | 0.12 | T |
| Echimyidae | |||
| Proechimys longicaudatus (Rengger, 1830) | 3 | 0.04 | CG |
| Thrichomys pachyurus (Wagner, 1845) | 3, 9, 10, 12 and 25 | 0.20 | CG |
Species threat category according to IUCN (2013): (EN) endangered, (VU) vulnerable - and Chiarello et al. (2008) - (asterisk) vulnerable. Localities according to Appendix 1, methods: (B) burrow, (CP) pitfall traps, (CG) live traps on the ground, (CU) live traps in the understory, (D) direct observation, (F) feces, (V) vocalizations, (T) tracks.
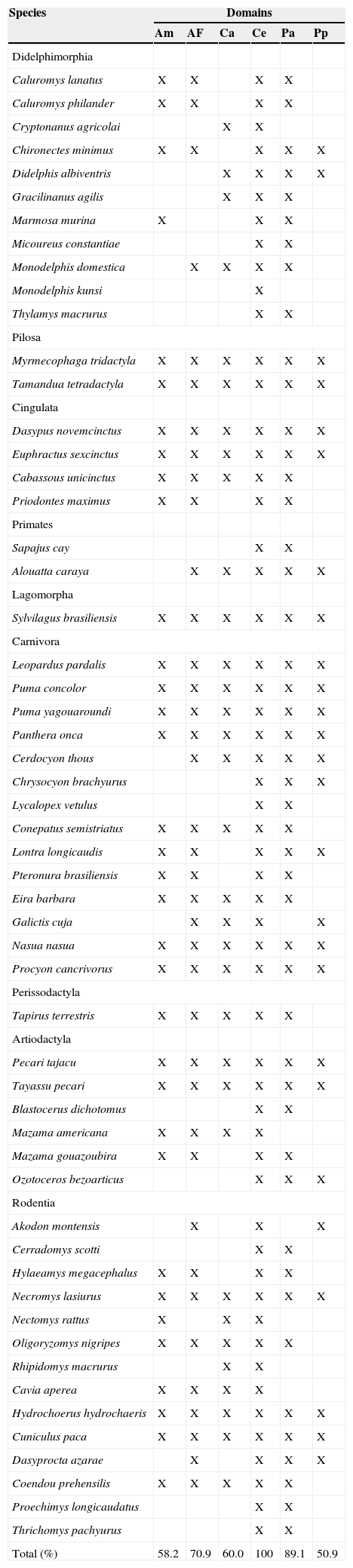
The non-volant mammal communities of the Maracaju Mountains are composed particularly by species that occur in the Cerrado domain. The Pantanal is the domain that has the highest similarity of non-volant mammal species with Maracaju Mountains, since 89.1% (49 spp.) occur in both domains. Therefore, the majority of species that occur in the region present distributions associated more with the Cerrado and Pantanal domains (Table 2). However, in Maracaju Mountains occur species that are also distributed in forest domains, like Hylaeamys megacephalus and Proechimys longicaudatus, that also occur in the Amazonian forest, and Akodon montensis, that is also found in the Atlantic Forest. The other species present broader distributions, occurring in several domains in Brazil (Table 2).
Species of non-volant mammals recorded in Maracaju Mountains and surroundings, Mato Grosso do Sul state, southwestern Brazil, during the period of 2006 to 2010, and their distributions in the Brazilian phytogeographic domains. Classification in accordance to Paglia et al. (2012)
| Species | Domains | |||||
|---|---|---|---|---|---|---|
| Am | AF | Ca | Ce | Pa | Pp | |
| Didelphimorphia | ||||||
| Caluromys lanatus | X | X | X | X | ||
| Caluromys philander | X | X | X | X | ||
| Cryptonanus agricolai | X | X | ||||
| Chironectes minimus | X | X | X | X | X | |
| Didelphis albiventris | X | X | X | X | ||
| Gracilinanus agilis | X | X | X | |||
| Marmosa murina | X | X | X | |||
| Micoureus constantiae | X | X | ||||
| Monodelphis domestica | X | X | X | X | ||
| Monodelphis kunsi | X | |||||
| Thylamys macrurus | X | X | ||||
| Pilosa | ||||||
| Myrmecophaga tridactyla | X | X | X | X | X | X |
| Tamandua tetradactyla | X | X | X | X | X | X |
| Cingulata | ||||||
| Dasypus novemcinctus | X | X | X | X | X | X |
| Euphractus sexcinctus | X | X | X | X | X | X |
| Cabassous unicinctus | X | X | X | X | X | |
| Priodontes maximus | X | X | X | X | ||
| Primates | ||||||
| Sapajus cay | X | X | ||||
| Alouatta caraya | X | X | X | X | X | |
| Lagomorpha | ||||||
| Sylvilagus brasiliensis | X | X | X | X | X | X |
| Carnivora | ||||||
| Leopardus pardalis | X | X | X | X | X | X |
| Puma concolor | X | X | X | X | X | X |
| Puma yagouaroundi | X | X | X | X | X | X |
| Panthera onca | X | X | X | X | X | X |
| Cerdocyon thous | X | X | X | X | X | |
| Chrysocyon brachyurus | X | X | X | |||
| Lycalopex vetulus | X | X | ||||
| Conepatus semistriatus | X | X | X | X | X | |
| Lontra longicaudis | X | X | X | X | X | |
| Pteronura brasiliensis | X | X | X | X | ||
| Eira barbara | X | X | X | X | X | |
| Galictis cuja | X | X | X | X | ||
| Nasua nasua | X | X | X | X | X | X |
| Procyon cancrivorus | X | X | X | X | X | X |
| Perissodactyla | ||||||
| Tapirus terrestris | X | X | X | X | X | |
| Artiodactyla | ||||||
| Pecari tajacu | X | X | X | X | X | X |
| Tayassu pecari | X | X | X | X | X | X |
| Blastocerus dichotomus | X | X | ||||
| Mazama americana | X | X | X | X | ||
| Mazama gouazoubira | X | X | X | X | ||
| Ozotoceros bezoarticus | X | X | X | |||
| Rodentia | ||||||
| Akodon montensis | X | X | X | |||
| Cerradomys scotti | X | X | ||||
| Hylaeamys megacephalus | X | X | X | X | ||
| Necromys lasiurus | X | X | X | X | X | X |
| Nectomys rattus | X | X | X | |||
| Oligoryzomys nigripes | X | X | X | X | X | |
| Rhipidomys macrurus | X | X | ||||
| Cavia aperea | X | X | X | X | ||
| Hydrochoerus hydrochaeris | X | X | X | X | X | X |
| Cuniculus paca | X | X | X | X | X | X |
| Dasyprocta azarae | X | X | X | X | ||
| Coendou prehensilis | X | X | X | X | X | |
| Proechimys longicaudatus | X | X | ||||
| Thrichomys pachyurus | X | X | ||||
| Total (%) | 58.2 | 70.9 | 60.0 | 100 | 89.1 | 50.9 |
Am: Amazon; AF: Atlantic Forest; Ca: Caatinga; Ce: Cerrado; Pa: Pantanal; Pp: Pampas.
Most species of non-volant mammals present in the Maracaju Mountains were relatively rare in this region, being observed only in few localities (Table 1). This group has many species of small-mammals, divided in rodents and marsupials. According to Marinho-Filho, Rodrigues, and Juarez (2002), 63.4% of the non-volant mammals of the Cerrado are rare, and 50.7% of these species are small rodents and marsupials. The rarity of small mammals could be explained by the high specialization of most species in the use of habitat and their strong associations with some microhabitat variables, which means that many species have local distributions in the landscape (Lacher Jr. & Alho, 2001; Mohammadi, 2010).
In relation to medium and large mammals observed in few localities, 6 species (Priodontes maximus, Panthera onca, Pteronura brasiliensis, Tayassu pecari, Ozotoceros bezoarticus and Blastocerus dichotomus) are threatened in Brazil or on global scale (Chiarello et al., 2008; IUCN, 2013). These species are becoming even more endangered mainly due to habitat loss, fragmentation and degradation of habitats, but also by hunting for meat consumtion (P. maximus, T. pecari, O. bezoarticus and B. dichotomus). The jaguar has become threatened since the local population claim they attack livestock (P. onca) or by human oppression that consider the species as a rival related to the fish resource (P. brasiliensis) (Chiarello et al., 2008).
The natural history of each species should be considered to assess its degree of rarity in a certain region (Chiarello et al., 2008). For example, grassland species, like O. bezoarticus and B. dichotomus, are rare or absent in arboreal savannas and forest landscapes or in areas where the terrain is mountainous, such as the Maracaju Mountains, where their populations could be considered endangered. However, these species are relatively common in nearby regions in the Pantanal wetlands (Rodrigues, Medri, et al., 2002), which are near the Maracaju Mountains.
The species commonly found in this study are abundant in this region (Cáceres et al., 2010), and most of them are habitat generalists (e. g. Dasypus novemcinctus, Sylvilagus brasiliensis, Leopardus pardalis, Puma concolor, Tapirus terrestris and Dasyprocta azarae) and common in the southern portion of Cerrado (Cáceres, Bornschein, & Lopes, 2008). According to Cáceres et al. (2010), the natural remnants of Cerrado present in the central-western area of Mato Grosso do Sul still support large predators like P. concolor, medium and large omnivorous and herbivores like Pecari tajacu and T. terrestris, and large insectivores like Myrmecophaga tridactyla, and are even present in small fragments or altered areas of gallery forests.
The fauna of the Maracaju Mountains is characterized, mainly by, mammals of Cerrado and Pantanal. However, Amazonian and Atlantic Forest also contributed to the mammalian fauna of the Maracaju Mountains, mainly in relation to the small non-volant mammal species. In the diagonal of open formation of Brazil, the southwestern portion tends to be more affiliated to the Amazonian, while localities in the northeastern Brazil are more related to the Atlantic Forest (Costa, 2003). Therefore, these 2 domains share small mammal faunas with open areas (Caatinga, Cerrado, and Pantanal) across gallery forests and arboreal savannas (Costa, 2003).
Some species present in Mato Grosso do Sul State (Cáceres, Carmignotto, et al., 2008) were not registered in Maracaju Mountains since they occur only in western portions of the state, where there is a strong influence of Chaco domain (e. g. Cryptonanus chacoensis, Akodon toba, Holochilus chacarius and Oligoryzomys chacoensis) and Amazonian Forest (e. g. Marmosops ocellatus, Philander opossum, Mico melanurus, Aotus azarae, Callicebus pallescens) (Bonvicino et al., 2008; Cáceres, Ferreira, & Carmignotto, 2007; Carmignotto, 2005; Goldani, Carvalho, & Bicca-Marques, 2006, 2008a; Rodrigues, Medri, et al., 2002).
There is a group of species that occur in the state composed by some species of carnivores that are naturally rare and hardly detectable (Leopardus tigrinus, L. wiedii, L. colocolo and Speothos venaticus), all endangered in Brazil (Chiarello et al., 2008) and globally (IUCN, 2013), as well as 2 species of Cingulata that are difficult to detect or obtain indirect evidence of their presence (Tolypeutes matacus and D. septemcinctus). Therefore, we recommend that additional studies in the Maracaju Mountains should be carried out, particularly with a large sampling effort using camera-traps, to confirm these cryptic species in the region, and will contribute to a better knowledge on the composition of local mammal communities.
In the last 30 years, the Cerrado domain was reduced in area by conversion of its original vegetation to exotic grassland pastures for livestock, plantations of soybean, corn, sugar-cane and Eucalyptus spp. (Klink & Machado, 2005). Thus, more than half of its original vegetation cover has been lost, and this process is widely distributed in central Brazil. In the state of Mato Grosso do Sul, for example, the Cerrado covered approximately 61% of the territory, but with the human occupation, increased from the 60's decade, this vegetation cover was reduced to approximately 32% (Sano et al., 2010). Deforestation is the major threat to the biodiversity conservation in the High Paraguay River Basin, where the Maracaju Mountains are located. In this region, the deforestation occurs due to livestock activities, Eucalyptus plantations, expansion of charcoal harvesting (Harris et al., 2005), and more recently, for the construction of Small Hydroelectric Projects. Consequently, it is possible that in the plateaus surrounding the Pantanal wetlands, such as the Maracaju Mountains, the loss of original vegetation reaches more than 60% of the area (Harris et al., 2006). However, when compared to other regions of Cerrado in the state of Mato Grosso do Sul, especially the northeastern and eastern (Mantovani & Pereira, 1998), the Maracaju Mountains still retain large extensions of natural areas that are important to the conservation of Cerrado mammals. Therefore, these mountainous regions should be considered as important areas for the establishment of Conservation Units in the Cerrado domain, representing its southwestern portion. Although the Maracaju Mountains are inserted in the Biodiversity Corridor of Maracaju-Negro (Machado et al., 2009), there are only a few Conservation Units (MMA, 2007) in this region (Serra de Sonora State Park and Particular Reserves of Gavião de Penacho, Vale do Bugio, Lajedo and Morro da Peroba), that together protect just 92.14km2 of natural areas, which is only about 0.6% of Maracaju Mountains.
Finally, the Maracaju Mountains are important on a broader spatial scale because they connect the Emas National Park, a large Conservation Unit of Cerrado with a rich mammalian fauna (Rodrigues, Silveira, et al., 2002), with the Pantanal, that has several mammal species that also occur in the Cerrado and still maintains dense populations of rare and endangered species of ungulates, like O. bezoarticus and B. dichotomus, and large carnivores, like P. onca, P. concolor and P. brasiliensis (Harris et al., 2005; Rodrigues, Medri, et al., 2002).
The connection between the Pantanal and regions of Cerrado in central Brazil could facilitate the movement of species across biodiversity corridors, such as the Maracaju Mountains, contributing to the genetic flow and conservation of many species of mammals, making this an important reason for the establishment of Conservation Units in this region of Mato Grosso do Sul State.Acknowledgements
AcknowledgementsWe thank Dimitrius A. C. Cavalcante for field assistance, Rodrigo Neves Godoi for the translation of this manuscript to English form. Nilton Cáceres that many contributed with data and revision of this manuscript and by the data available on small mammals collection in the region of Piraputanga, municipality of Aquidauana. This manuscript is the result of a long work of environmental projects. Therefore, we thank the companies: Anambi, Arater, Bio Laqua, Ecofoco and Triad.
Didelphis albiventris: BRAZIL, Mato Grosso do Sul: Aquidauana (Acambapi – Piraputanga), N. C. Cáceres et al. cols. (UFSM 46).
Marmosa murina: BRAZIL, Mato Grosso do Sul: Aquidauana (Acambapi – Piraputanga), ♀, N. C. Cáceres et al. cols. (UFSM 49).
Thylamys macrurus: BRAZIL, Mato Grosso do Sul: Aquidauana (Córrego das Antas – Piraputanga), ♀, W. Hannibal et al. cols. (UFSM 536).
Cerradomys scotti: BRAZIL, Mato Grosso do Sul: Bela Vista (Exército), ♂, N. C. Cáceres et al. cols. (UFSM 610).
Hylaeamys megacephalus: BRAZIL, Mato Grosso do Sul: Aquidauana (Acambapi – Piraputanga), ♂, N. C. Cáceres et al. cols. (UFSM 53).
Nectomys rattus: BRAZIL, Mato Grosso do Sul: Aquidauana (Acambapi – Piraputanga), ♀, N. C. Cáceres et al. cols. (UFSM 55).
Oecomys bicolor: BRAZIL, Mato Grosso do Sul: Aquidauana (Córrego das Antas – Piraputanga), ♂, W. Hannibal et al. cols. (UFSM 537).
Proechimys longicaudatus: BRAZIL, Mato Grosso do Sul: Aquidauana (Acambapi – Piraputanga), ♀, Cáceres et al. cols. (UFSM 126).
Rhipidomys macrurus: BRAZIL, Mato Grosso do Sul: Aquidauana (Acambapi – Piraputanga), ♀, Cáceres et al. cols. (UFSM 32).
Thrychomys pachyurus: BRAZIL, Mato Grosso do Sul: Aquidauana (Acambapi – Piraputanga), ♂, Cáceres et al. cols. (UFSM 137).