The Lacandona rainforest represents one of the most diverse Mexican tropical wet forests. Although some studies have described the amphibians and reptiles of the region, most herpetological lists come from the northern part of the Lacandona, and there are no confirmed records for many of the expected species. We reviewed databases of scientific collections, taxonomy, and published herpetological lists to produce the most recent updated list of amphibian and reptile species in the region (35 amphibians and 90 reptiles). Furthermore, based on recent inventories (2007–2013) we establish 40 range extensions of 8 amphibians and 32 reptiles for the southeastern part of the Lacandona rainforest. Four out of these 40 records confirmed the occurrence of Dermophis mexicanus, Eleutherodactylus leprus, Pantherophis flavirufus, and Bothriechis schlegelii in the region.
La selva Lacandona representa uno de los bosques húmedos tropicales más diversos de México. Aunque algunos estudios han descrito a los anfibios y reptiles de la región, la mayoría de los listados herpetológicos proceden de la parte norte de la lacandona y no existen registros confirmados para muchas de las especies esperadas. Se han revisado bases de datos de colecciones científicas, la taxonomía y listados herpetológicos publicados para generar la lista más actualizada de especies de anfibios y reptiles de la región (35 anfibios y 90 reptiles). Además, con base en inventarios recientes (2007–2013) se estableció la extensión del área de distribución de 8 anfibios y 32 reptiles para la parte sureste de la selva Lacandona. Cuatro de estos 40 registros confirmaron la presencia de Dermophis mexicanus, Eleutherodactylus leprus, Pantherophis flavirufus y Bothriechis schlegelii en la región.
Mexico is considered the country with the second and fifth highest number of amphibian and reptile species, respectively in the world (Espinosa, Ocegueda, Flores, Llorente-Bousquets, & Vázquez, 2008; Flores-Villela & García-Vázquez, 2014; Parra-Olea, Flores-Villela, & Mendoza-Almeralla, 2014). The Lacandona rainforest contributes greatly to the herpetofaunistic diversity of Mexico and Mesoamerica, being nowadays considered a priority area for biodiversity conservation (Conabio-Conanp-TNC-Pronatura-FCF, UANL, 2007). Yet the available information on species richness and distribution of amphibians and reptiles in the region is incomplete and largely inaccurate (Hernández-Ordóñez et al., 2014).
Hepetofaunal studies in the Lacandona rainforest started in the first half of the 20th century with Smith and Taylor's expeditions (Lee, 1996), and the first published species list for the region reported 77 species, 23 amphibians, and 54 reptiles (Lazcano-Barrero, Gongora-Arones, & Vogt, 1992). After that publication, a number of inventories have reported new records for the region (Ferreira-García & Canseco-Márquez, 2006; Lee, 1996; Muñoz, 2010), including the first Mexican record of the caecilian Gymnopis syntrema (González-Hernández, Hernández-Ordoñez, Cervantes-López, & Reynoso, 2014). New range extensions also have been reported for this rainforest (Paredes-León & Reynoso, 2005a, 2005b, 2005c, 2005d; Percino-Daniel, Barcenas, & Sarabia, 2012; Percino-Daniel, Bénard-Valle, García-del Valle, & Mendelson III, 2013). Nevertheless, in the Lacandona rainforest most studies have been focused on its northern part, and many species have been hypothesized to be present in the region, without any field record that confirms their presence (Köhler, 2011; Lazcano-Barrero et al., 1992).
Here, databases of scientific collections and published herpetological lists that provide a checklist of amphibians and reptiles in the Lacandona rainforest were reviewed. Based on new inventories carried out from 2007 to 2013 in the Marqués de Comillas region, we describe 40 range extensions (8 amphibians and 32 reptiles) for the southeastern Lacandona rainforest. Four out of these 40 records confirmed the occurrence of two amphibian and two reptile species, improving thus the accuracy of the information on the species geographic distributions in the region. We also corrected our previous published species list (Hernández-Ordóñez et al., 2014), by changing Anolis sericeus to Anolis unilobatus, and Marisora unimarginata to Marisora brachypoda, and by excluding Drymarchon corais from this list, as suggested by Crother et al. (2003). Thus, this paper represents the most recent updated list of amphibian and reptile species for the region.
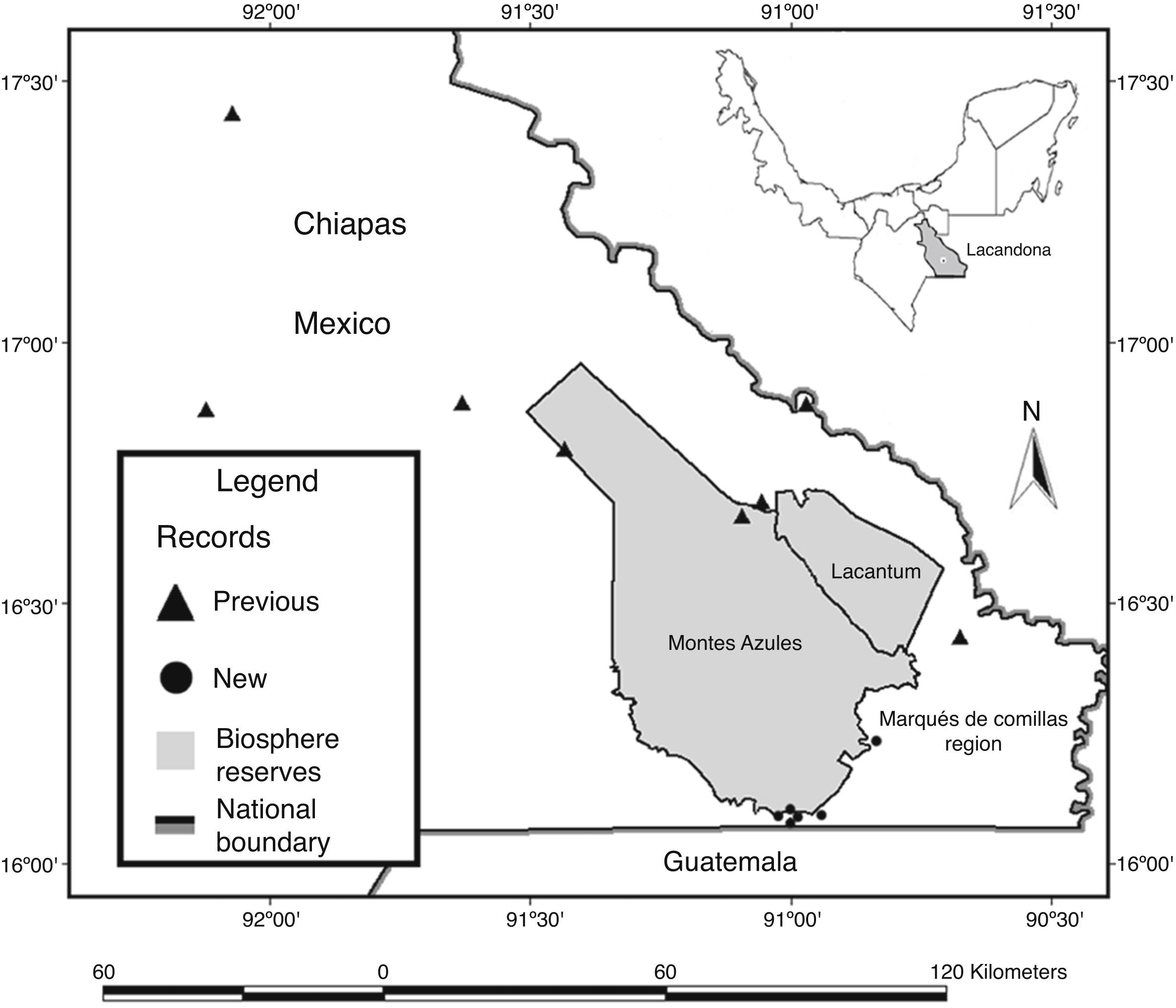
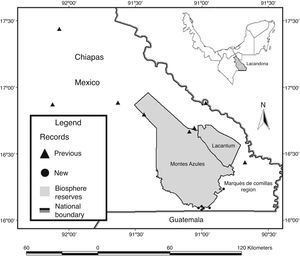
Material and methodsThe Lacandona rainforest is located in the southern part of the Mexican state of Chiapas, in southern Mexico (100–1,500m elev.; Fig. 1). The region has an extension of 13,000km2, bordered to the south and east by Guatemala, and to the east and north by the Mexican state of Tabasco and the Chiapas highlands, respectively; the Palenque National Park represents the northernmost limit of the region (INE, 2000). The Lacandona rainforest is part of the Mayan forest, which represents one of the largest forested areas in Mesoamerica (Rodstrom, Oliviery, & Tangley, 1999). Our study was focused on lowland tropical rainforests (<500m elev.), dominated by a hot and humid climate, with mean annual precipitation and temperature of 2874mm and 25°C, respectively (INE, 2000; Pennington & Sarukhán, 2005).
To update the checklist of amphibians and reptiles in the Lacandona rainforest, we reviewed all published species lists and range distribution notes from the region (Campbell & Lamar, 2004; Ferreira-García & Canseco-Márquez, 2006; González-Hernández et al., 2014; Lazcano-Barrero et al., 1992; Lee, 1996; Muñoz, 2010; Paredes-León & Reynoso, 2005a, 2005b, 2005c, 2005d; Percino-Daniel et al., 2012, 2013). We consulted databases of the Colección Nacional de Anfibios y Reptiles at the Instituto de Biología (CNAR) and Museo de Zoología at the Facultad de Ciencias (MZFC), both at the Universidad Nacional Autónoma de México (UNAM, Mexico City), the Colección Herpetológica de El Colegio de la Frontera Sur, Unidad San Cristóbal de las Casas (ECOSUR-SC) and the Colección Herpetológica (IHNHERP) of the Secretaría de Medio Ambiente e Historia Natural, both located in Chiapas. Additional data were obtained from the Global Biodiversity Information Facility database, where only records from specimens shelved in scientific collections were considered. We found a specimen of Dermophis mexicanus and a specimen of Coluber mentovarius shelved at CNAR not previously reported for the region. Thus, we confirmed the occurrence of Dermophis mexicanus in the Lacandona rainforest, and extended the distribution range of Coluber mentovarius. Both records are described in Table 1 and Appendix A.
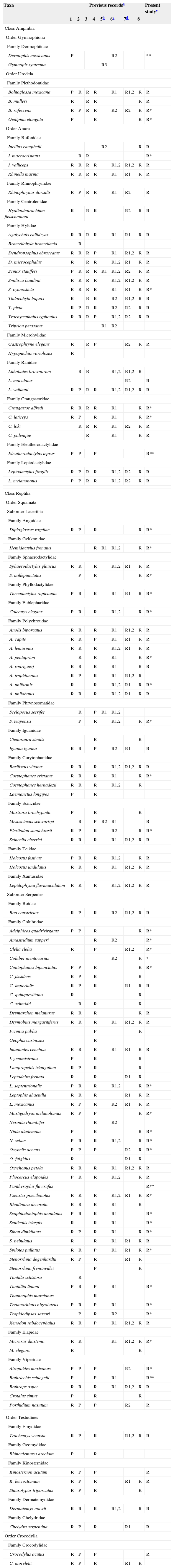
Updated list of amphibian and reptile species in the Lacandona rainforest, Mexico.
| Taxa | Previous recordsa | Present studye | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5b | 6c | 7d | 8 | ||
| Class Amphibia | |||||||||
| Order Gymnophiona | |||||||||
| Family Dermophiidae | |||||||||
| Dermophis mexicanus | P | R2 | ** | ||||||
| Gymnopis syntrema | R3 | ||||||||
| Order Urodela | |||||||||
| Family Plethodontidae | |||||||||
| Bolitoglossa mexicana | P | R | R | R | R1 | R1,2 | R | R | |
| B. mulleri | R | R | R | R | R | ||||
| B. rufescens | R | P | R | R | R2 | R2 | R | R* | |
| Oedipina elongata | P | R | R | R* | |||||
| Order Anura | |||||||||
| Family Bufonidae | |||||||||
| Incilius campbelli | R2 | R | R | ||||||
| I. macrocristatus | R | R | R* | ||||||
| I. valliceps | R | R | R | R | R1,2 | R1,2 | R | R | |
| Rhinella marina | R | R | R | R | R1 | R1 | R | R | |
| Family Rhinophrynidae | |||||||||
| Rhinophrynus dorsalis | R | P | R | R | R1 | R2 | R | ||
| Family Centrolenidae | |||||||||
| Hyalinobatrachium fleischmanni | R | R | R | R2 | R | R | |||
| Family Hylidae | |||||||||
| Agalychnis callidryas | R | R | R | R | R1 | R1 | R | R | |
| Bromeliohyla bromeliacia | R | ||||||||
| Dendropsophus ebraccatus | R | R | R | P | R1 | R1,2 | R | R | |
| D. microcephalus | R | R | R | R1,2 | R1 | R | R | ||
| Scinax staufferi | P | R | R | R | R1 | R1,2 | R2 | R | R |
| Smilisca baudinii | R | R | R | R | R1,2 | R1,2 | R | R | |
| S. cyanosticta | R | R | R | R | R1 | R1 | R | R* | |
| Tlalocohyla loquax | R | R | R | R2 | R1,2 | R | R | ||
| T. picta | R | P | R | R | R2 | R2 | R | R | |
| Trachycephalus typhonius | R | R | R | P | R1,2 | R2 | R | R | |
| Triprion petasatus | R1 | R2 | |||||||
| Family Microhylidae | |||||||||
| Gastrophryne elegans | R | R | P | R2 | R | R | |||
| Hypopachus variolosus | R | ||||||||
| Family Ranidae | |||||||||
| Lithobates brownorum | R | R | R1,2 | R1,2 | R | ||||
| L. maculatus | R2 | R | |||||||
| L. vaillanti | R | P | R | R | R1,2 | R1,2 | R | R | |
| Family Craugastoridae | |||||||||
| Craugastor alfredi | R | R | R | R | R1 | R | R* | ||
| C. laticeps | R | P | R | R1 | R | R* | |||
| C. loki | R | R | R | R1 | R2 | R | R | ||
| C. palenque | R | R1 | R | R | |||||
| Family Eleutherodactylidae | |||||||||
| Eleutherodactylus leprus | P | P | P | R** | |||||
| Family Leptodactylidae | |||||||||
| Leptodactylus fragilis | R | P | R | R | R1,2 | R2 | R | R | |
| L. melanonotus | P | P | R | R | R1,2 | R2 | R | R | |
| Class Reptilia | |||||||||
| Order Squamata | |||||||||
| Suborder Lacertilia | |||||||||
| Family Anguidae | |||||||||
| Diploglossus rozellae | R | P | R | R | R* | ||||
| Family Gekkonidae | |||||||||
| Hemidactylus frenatus | R | R1 | R1,2 | R | R* | ||||
| Family Sphaerodactylidae | |||||||||
| Sphaerodactylus glaucus | R | R | R | R1,2 | R1 | R | R | ||
| S. millepunctatus | P | R | R | R* | |||||
| Family Phyllodactylidae | |||||||||
| Thecadactylus rapicauda | P | R | R | R1 | R1 | R | R* | ||
| Family Eublepharidae | |||||||||
| Coleonyx elegans | P | R | R | R1,2 | R | R* | |||
| Family Polychrotidae | |||||||||
| Anolis biporcatus | R | R | R | R1 | R1,2 | R | R | ||
| A. capito | R | R | P | R1 | R1 | R | R | ||
| A. lemurinus | R | R | R | R1,2 | R1 | R | R | ||
| A. pentaprion | R | R | R1 | R | R* | ||||
| A. rodriguezi | R | R | R | R1 | R | R | |||
| A. tropidonotus | R | P | R | R1 | R1,2 | R | |||
| A. uniformis | R | R | R1,2 | R1 | R | R* | |||
| A. unilobatus | R | R | R | R1,2 | R1 | R | R | ||
| Family Phrynosomatidae | |||||||||
| Sceloporus serrifer | R | P | R1 | R1,2 | |||||
| S. teapensis | P | R | R1,2 | R | R* | ||||
| Family Iguanidae | |||||||||
| Ctenosaura similis | R | R | |||||||
| Iguana iguana | R | R | P | R2 | R1 | R | |||
| Family Corytophanidae | |||||||||
| Basiliscus vittatus | R | R | R | R1,2 | R1,2 | R | R | ||
| Corytophanes cristatus | R | R | R | R1 | R | R* | |||
| Corytophanes hernadezii | R | R | R | R1,2 | R | ||||
| Laemanctus longipes | P | R | |||||||
| Family Scincidae | |||||||||
| Marisora brachypoda | P | R | R | ||||||
| Mesoscincus schwartzei | R | P | R2 | R1 | R | ||||
| Plestiodon sumichrasti | R | P | R | R2 | R | R* | |||
| Scincella cherriei | R | R | R | R1 | R1,2 | R | R | ||
| Family Teiidae | |||||||||
| Holcosus festivus | P | R | R | R1,2 | R | R | |||
| Holcosus undulatus | R | R | R | R1 | R1,2 | R | R | ||
| Family Xantusidae | |||||||||
| Lepidophyma flavimaculatum | R | R | R | R1,2 | R1,2 | R | R | ||
| Suborder Serpentes | |||||||||
| Family Boidae | |||||||||
| Boa constrictor | R | P | R | R2 | R1,2 | R | R | ||
| Family Colubridae | |||||||||
| Adelphicos quadrivirgatus | P | P | R | R | R* | ||||
| Amastridium sapperi | R | R2 | R* | ||||||
| Clelia clelia | R | P | R1,2 | R* | |||||
| Coluber mentovarius | R2 | R | * | ||||||
| Coniophanes bipunctatus | P | P | R | R | R* | ||||
| C. fissidens | R | P | R | R | |||||
| C. imperialis | R | P | R | R1 | R | R | |||
| C. quinquevittatus | R | R | |||||||
| C. schmidti | R | R | R | ||||||
| Drymarchon melanurus | R | R | R | R | R | ||||
| Drymobius margaritiferus | R | R | R | R1 | R1,2 | R | R | ||
| Ficimia publia | P | R | |||||||
| Geophis carinosus | R | ||||||||
| Imantodes cenchoa | R | R | R | R1 | R1 | R | R | ||
| I. gemmistratus | P | R | R | ||||||
| Lampropeltis triangulum | R | P | R | R | |||||
| Leptodeira frenata | R | R | R1 | R | |||||
| L. septentrionalis | P | R | R | R1,2 | R | R* | |||
| Leptophis ahaetulla | R | R | R | R1 | R | R | |||
| L. mexicanus | R | P | R | R2 | R1 | R | R | ||
| Mastigodryas melanolomus | R | P | P | R | R* | ||||
| Nerodia rhombifer | R | R2 | |||||||
| Ninia diademata | P | R | R | R* | |||||
| N. sebae | P | R | R | R1,2 | R | R* | |||
| Oxybelis aeneus | P | P | P | R2 | R | R* | |||
| O. fulgidus | R | R1 | R | ||||||
| Oxyrhopus petola | R | R | R | R1 | R1,2 | R | R | ||
| Pliocercus elapoides | P | R | R | R1,2 | R | R | |||
| Pantherophis flavirufus | R** | ||||||||
| Pseustes poecilonotus | R | R | R | R1,2 | R1 | R | R* | ||
| Rhadinaea decorata | R | R | R | R1 | R | ||||
| Scaphiodontophis annulatus | P | R | R | R1 | R* | ||||
| Senticolis triaspis | R | R | R1 | R* | |||||
| Sibon dimidiatus | R | P | R | R1 | R | R* | |||
| S. nebulatus | R | R | R1 | R1 | R | R | |||
| Spilotes pullatus | R | R | P | R1 | R1 | R | R* | ||
| Stenorrhina degenhardtii | R | P | R | R1 | R | ||||
| Stenorrhina freminvillei | P | R | |||||||
| Tantilla schistosa | R | ||||||||
| Tantillita lintoni | P | R | P | R1 | R* | ||||
| Thamnophis marcianus | R | ||||||||
| Tretanorhinus nigroluteus | P | R | P | R1 | R* | ||||
| Tropidodipsas sartori | P | R | R2 | R* | |||||
| Xenodon rabdocephalus | R | R | P | R1 | R1,2 | R | R | ||
| Family Elapidae | |||||||||
| Micrurus diastema | R | R | R1 | R1,2 | R | R* | |||
| M. elegans | R | R | |||||||
| Family Viperidae | |||||||||
| Atropoides mexicanus | P | P | P | R2 | R* | ||||
| Bothriechis schlegelii | P | P | R1 | R** | |||||
| Bothrops asper | R | R | R | R1 | R1,2 | R | R | ||
| Crotalus simus | P | R | R | ||||||
| Porthidium nasutum | R | P | P | R2 | R | ||||
| Order Testudines | |||||||||
| Family Emydidae | |||||||||
| Trachemys venusta | R | P | R | R1,2 | R | R | |||
| Family Geomydidae | |||||||||
| Rhinoclemmys areolata | P | R | |||||||
| Family Kinosternidae | |||||||||
| Kinosternon acutum | R | P | P | R | |||||
| K. leucostomum | R | P | R | R1 | R | R | |||
| Staurotypus triporcatus | R | P | R | R | |||||
| Family Dermatemydidae | |||||||||
| Dermatemys mawii | R | R | R | R1,2 | R | R | |||
| Family Chelydridae | |||||||||
| Chelydra serpentina | R | P | R | R1 | R | ||||
| Order Crocodylia | |||||||||
| Family Crocodylidae | |||||||||
| Crocodylus acutus | R | P | P | R | |||||
| C. moreletii | R | P | R | R1 | R | ||||
Previous records: 1. Lazcano-Barrero et al. (1992); 2. Ferreira-García and Canseco-Márquez (2006); 3. Muñoz (2010); 4. Lee (1996); 5. New records published between 2005 and 2014; 6. Scientific collections of the Universidad Nacional Autónoma de México (UNAM); 7. Scientific collections in Chiapas state; 8. Online database (Global Biodiversity Information Facility). In all cases, we indicate the species recorded (R) or cited as probable (P) in the region.
New records: 1. Paredes-León and Reynoso (2005a, 2005b, 2005c, 2005d); 2. Percino-Daniel et al. (2012, 2013); and 3. González-Hernández et al. (2014).
Scientific collections in UNAM: 1. Museo de Zoología de la Facultad de Ciencias (MZFC); 2. Colección Nacional de Anfibios y Reptiles (CNAR).
Scientific collections in Chiapas state: 1. Colección Herpetológica Secretaría de Medio Ambiente e Historia Natural (IHNHERP); 2. El Colegio de la Frontera Sur, Unidad San Cristóbal de las Casas (ECOSUR-SC).
*Records that represented range extensions for the southeastern part of the Lacandona rainforest (i.e., ≥40km in straight line from the closest previous records); **records that confirmed the occurrence of certain species in the Lacandona rainforest. In the case of Dermophis mexicanus and Coluber mentovarius, we did not add an ‘R’ in the column for the present study because we have not recorded these species in the region, but we used unpublished records of these species deposited in the Colección Nacional de Anfibios y Reptiles (CNAR).
We also carried out herpetological surveys in the poorly known southeastern part of the Lacandona rainforest, including two adjacent areas separated by the Lacantún River: the Marqués de Comillas region (MCR) and the Montes Azules Biosphere Reserve (MABR), located approximately at 16° 04′N and 90° 45′W, and covering an altitudinal range of 100–500m elev. (Fig. 1). Inventories were conducted during 2007 (February, April, July, September, and November), 2008 (January, March, and July), 2010 (July and October), 2011 (March and August), 2012 (May and June), and 2013 (February) as part of OHO's M.Sc. and Ph.D. thesis (Hernández-Ordóñez, 2009).
Inventories in MCR included old-growth forest patches, secondary forests, crops, bean fields, cocoa plantations, pastures, streams and rivers, human settlements, and roads. We used multiple sampling techniques, including visual encounter surveys, acoustic encounter surveys, drift-fences, pitfalls traps, and boat tours along the Lacantún River (see details of these methods in Corn, 1994; Crump & Scott, 1994; Zimmerman, 1994). Because of the low vagility and the strong philopatry of amphibians and reptiles (Blaustein, Wake, & Sousa, 1994; Böhm et al., 2013; Stuart et al., 2008), the specimens collected ≥40km in a straight line from the closest previous records were considered as extensions of their geographic range (Canseco-Márquez, Gutiérrez-Mayén, & Salazar-Arenas, 2000; Chrapliwy, 1956; Kraus, 2010).
All specimens were collected under permit No. SGPA/DGVS/02132 of SEMARNAT (Mexican Government) to VHR, and shelved in the Colección Nacional de Anfibios y Reptiles, Instituto de Biología, UNAM. Luis Canseco-Márquez verified most of the specimens. Edmundo Pérez-Ramos verified Adelphicos quadrivirgatus, Joseph Mendelson III verified Incilius macrocristatus, and Alberto Cruz-Silva verified Pantherophis flavirufus.
ResultsOur herpetological inventories and detailed revisions of scientific databases revealed a total of 35 species of amphibians from 11 families, and 90 species of reptiles from 22 families for the Lacandona rainforest (Table 1). Within amphibians, anurans had the highest number of species (29 species), followed by salamanders (four species), and caecilians (two species). Regarding reptiles, squamates were the richest with 52 species of snakes and 29 species of lizards, followed by seven species of turtles and two species of crocodiles (Table 1).
We found 40 range extensions for the southeastern part of the Lacandona rainforest (8 amphibians and 32 reptiles; Table 1; Appendix A; Fig. 1). Within these new records, we confirmed the presence of four species (Dermophis mexicanus, Eleutherodactylus leprus, Pantherophis flavirufus, and Bothriechis schlegelii) that had been previously suggested to be present in the region (Table 1).
DiscussionThe very high number of species of amphibians and reptiles recorded to date highlights the importance of the Lacandona rainforest to the Mexican herpetofaunistic diversity, as this rainforest represents the second most diverse tropical region in Mexico, just behind the Los Tuxtlas region (Hernández-Ordóñez et al., 2014). Although the Lacandona rainforest covers 0.66% of the Mexican continental territory (about 2,000,000km2), according to recently published estimates for amphibians (376 species: Parra-Olea et al., 2014; 377 species: González-Hernández et al., 2014) and reptiles (864 species: Flores-Villela & García-Vázquez, 2014), our study indicates that it maintains approximately 9.3% and 10.4% of the amphibian and reptile species in Mexico, respectively. These figures may increase, as there are still some expected species that have not been confirmed for the region. Hernández-Ordóñez et al. (2014) reported 21 species (16 reptiles and 5 amphibians) that have been suggested to be present in the Lacandona rainforest, from which here we confirmed the occurrence of 4 species (2 amphibians and 2 reptiles). Additional studies are therefore needed, particularly in the central and southeastern part of the Marqués de Comillas region (Fig. 1), to have a more accurate herpetofaunal checklist. If the presence of these probable species is confirmed, the Lacandona rainforest will be recognized as the richest rainforest in the country. This information will be essential for biogeographical studies, and will provide a baseline for the ecology and conservation of biodiversity as a whole (Gotelli, 2011; Mace, 2004).
The range extensions between the northern and southern parts of the Lacandona rainforest were relatively expected, as very few surveys have been carried out in the Marqués de Comillas region. In contrast, areas such as the Palenque and Yaxchilán National Parks have been surveyed for decades (Ferreira-García & Canseco-Márquez, 2006; Lee, 1996). Interestingly, range extensions of amphibian species were mainly from Plethodontidae and Craugastoridae, which are known to have restricted distributions (e.g., Oedipina elongata; Campbell, 1998; Lee, 1996). For reptiles, most range extensions were from snakes (Colubridae), that are known to be rare (i.e., with small populations) and/or with low detection probability (Zug, Vitt, & Caldwell, 2001), preventing their registration in herpetological studies. Similarly, range extensions of lizards were mainly from species that are rare or with arboreal habits, such as Diploglossus rozellae and Thecadactylus rapicauda (Campbell, 1998; Lee, 1996). In our study, however, range extensions for common species were also recorded (e.g., Anolis uniformis, Anolis pentaprion, Sceloporus teapensis, and Corytophanes cristatus), most probably because of the scarcity of surveys in the region and the small sampling effort. We also documented the range extension of Hemidactylus frenatus, an exotic species that is usually associated with human settlements, and hence, it can be easily overlooked in surveys carried out in forested habitats (Lee, 1996).
Overall, consistent with recent studies of small rodents (San-José, Arroyo-Rodríguez, & Sánchez-Cordero, 2014), mid- and large-sized mammals (Garmendia, Arroyo-Rodríguez, Estrada, Naranjo, & Stoner, 2013), primates (Arroyo-Rodríguez, González-Perez, Garmendia, Solà, & Estrada, 2013), and plants (Hernández-Ruedas et al., 2014), our study highlights that this region should be considered a priority area for biodiversity conservation. This region maintains the largest rainforest remnant in Mexico, and one of the most important in Mesoamerica (Dirzo & Robles-Gil, 1994). Unfortunately, it is increasingly deforested and fragmented. The annual deforestation rate in the region is between 1 and 8% (Couturier, Núñez, & Kolb, 2012; Mora, 2008), mainly to create agricultural and pasture lands. Management and conservation efforts should focus on preventing additional forest loss in the region, as: (1) many amphibians and reptiles are endemic to the Mayan forest; (2) the region shares <60% of the species with neighboring Mexican tropical forests, and (3) some species are particularly vulnerable to habitat loss and degradation (reviewed by Hernández-Ordóñez et al., 2014).
This research was supported by several grants from the Consejo Nacional de Ciencia y Tecnología (MABOTRO projects Semarnat-Conacyt 2002 C01-0597 and Sep-Conacyt CB-2005-01-510), and the Dirección General de Asuntos del Personal Académico (PAPIIT-DGAPA, UNAM; grants IN-227210, IA-203111, IB-200812 and RR-280812). We thank Gilberto, Fermín, Pascual, Miguel, Emanuel, Santos Jamangape, Martín Cervantes, and Ricardo Bolaños for their invaluable assistance in the field. We also thank Joseph Mendelson III (Department of Herpetology, Zoo Atlanta), Luis Canseco and Edmundo Pérez (Museo de Zoología de la Facultad de Ciencias, UNAM), and Alberto Cruz Silva (Colección Nacional de Anfibios y Reptiles, Instituto de Biología) for their help with the identification of collected specimens. Ricardo Bolaños assisted in the elaboration of the map. We also thank Luis Antonio Muñoz Alonso from the Colección Herpetológica de El Colegio de la Frontera Sur, Unidad San Cristóbal (ECOSUR-SC) for providing us invaluable information. OHO thanks Conacyt for his MSc and PhD scholarships. Idea Wild provided field equipment.
Detailed information on range extensions of 8 amphibian and 32 reptile species for the southeastern part of the Lacandona rainforest, Mexico. Abbreviations: MCR, Marqués de Comillas region, Chiapas; MABR, Montes Azules Biosphere Reserve, Chiapas; GBIF, Global Biodiversity Information Facility database; CNAR, Colección Nacional de Anfibios y Reptiles, UNAM, Mexico City; IHNHERP, Colección Herpetológica, Secretaría de Medio Ambiente e Historia Natural (SEMAHN), Tuxtla Gutiérrez, Chiapas. All distances are air line based.
Dermophis mexicanus (Mexican Caecilian). Mexico, Chiapas. Municipality: Palenque; Locality: Ruinas de Palenque (17°29′40.00″N, −91°01′9.00″W; 56m elev.) 9 June 1986. CNAR IBH 19471. This is the first record for the Lacandona rainforest. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Lazcano-Barrero et al., 1992), extending its former known distribution 102km E from Teapa, Tabasco, Mexico (GBIF, 2014).
Bolitoglossa rufescens (Northern Banana Salamander). Mexico, Chiapas. Municipality: Ocosingo; Locality: Ejido Loma Bonita, MCR (16°06′22.91″N, −90°59′7.63″W; 172m elev.) 24 October 2010. CNAR-IBH 25335. We confirmed the presence of the species in the southeastern part of Lacandona region (Köhler, 2011), extending its former known distribution 71km E from Lagunas de Montebello, Trinitaria, Chiapas (GBIF, 2014), and 190km SE from Palenque, Chiapas (Lee, 1996). The specimen was collected at night, in a secondary growth forest on a shrub at 1m above ground level. Several specimens were also recorded in remnants and continuous old-growth tropical rainforest.
Oedipina elongata (Central American Worm Salamander). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Loma, Ejido Loma Bonita, MCR (16°05′55.6″N, −91°00′18.53″W; 167m elev.) 29 January 2008. Locality: Ruinas, MABR (16°06′37.18″N, −91°00′30.88″W; 193m elev.) 25 May 2012. Locality: Héctor, Ejido Loma Bonita, MCR (16°05′46.69″N, −90°59′13.56″W; 170m elev.) 6 June 2012. CNAR-IBH 23600, 26110, and 26115. Those specimens are the second record of the species for the Lacandona region, and southernmost record for Mexico, extending its known distribution 110km SE from Monte Líbano, Ocosingo, Chiapas, and 260km NW from Puerto Barrios, Guatemala (Lee, 1996). The specimens were found under a dead tree stump in a 20-ha remnant of tropical rainforest beside a stream and in a well-conserved continuous tropical rainforest (MARB), behind a stream over a fallen log, and in a secondary forest (MCR).
Incilius macrocristatus (Large Crested Toad). Mexico, Chiapas. Municipality: Ocosingo; Locality: Ruinas, MABR (16°06′35.21″N, −91°00′59.10″W; 300m elev.). 7 November 2007. Municipality: Marqués de Comillas; Locality: Selva Playón, Ejido Playón de la Gloria, MCR (16°05′55.64″N, −91°00′18.53″W; 186m a.s.l) 1 July 2012. CNAR-IBH 23431 and 26086. This is the first record of the species for the southeastern part of the Lacandona rainforest, extending its known distribution 71km E from Lagunas de Montebello, Trinitaria, Chiapas (GIFB 2012), and 70km S from Area Natural Protegida Lacandonia, Ocosingo, Chiapas (CNAR). Both specimens were found on leaf litter; the first in a well-conserved continuous tropical rainforest (MABR), and the second in an abandoned cocoa field (MCR).
Smilisca cyanosticta (Blue-spotted Mexican Treefrog). Mexico, Chiapas. Municipality: Ocosingo; Locality: Ruinas, MABR (16°06′37.18″N, −91°00′30.88″W; 193m elev.) 25 May 2012. CNAR-IBH 26112. This is the third record of the species for the Lacandona rainforest, extending its former known distribution 66km NE from Lacanjá-Chansayab, Ocosingo (IHNHERP), 95km SE from Lago Ocotal, Ocosingo, Chiapas, and 89km NW from Chinajá Alta Verapaz, Guatemala (Lee, 1996). The specimen was collected on a shrub, in a well-conserved continuous tropical rainforest (MABR).
Craugastor alfredi (Alfred's Rain Frog). Mexico,Chiapas. Municipality: Ocosingo; Locality: Ruinas, MABR (16°06′37.18″N, −91°00′30.88″W; 193m elev.) 18 September 2007. Municipality: Ocosingo; Locality: El Kárstico, MABR (16°06′54.68″N, −90°59′12.76″W; 449m elev.) 22 June 2012. CNAR-IBH-RF 065 and CNAR-IBH-26103. This is the first record of the species for the southeastern part of the Lacandona rainforest, extending its former known distribution 90km S from Yaxchilán, Chiapas (Ferreira-García & Canseco-Márquez, 2006), and 95km SE from Lago Ocotal, Ocosingo, Chiapas (GBIF, 2014). Both specimens were collected at night in a well-conserved continuous tropical rainforest (MABR), in shrubs at 1.5m above ground level.
Craugastor laticeps (Broadhead Rainfrog). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Loma, Ejido Loma Bonita, MCR (16°06′22.91″N, −90°59′07.63″W; 172m elev.) 7 June 2012. Municipality: Marqués de Comillas; Locality: Ejido Playón de la Gloria, MCR (16°08′42.00″N, −90°52′51.60″W; 182m elev.) 1 July 2012. CNAR-IBH 25304, 25340, 26101, 26091, 26104, 26105, 26106, 26114, 26116, 26117 and 26119. These records extend the known distribution of the species 95km SE from Lago Ocotal, Ocosingo, Chiapas (Lee, 1996), and 60km N from Finca Chilbac, Barillas Huehuetenango, Guatemala (GBIF, 2014). The specimens were collected at night on leaf litter in tropical rainforest remnants (MCR).
Eleutherodactylus leprus (Leprus Chirping Frog). Mexico, Chiapas. Municipality: Ocosingo; Locality: Ruinas, MABR (16°06′37.19″N, −91°00′30.89″W; 193m elev.) 18 March 2011. CNAR IBH 23605. We confirmed the presence of the species in the Lacandona rainforest (Lazcano-Barrero et al., 1992). This is the southernmost record for the species in Mexico, extending its known distribution 89km NW from Chinajá, Alta Verapaz, Guatemala (Lee, 1996). The specimen was collected at night on leaf litter, in a well-conserved continuous tropical rainforest (MABR).
Diploglossus rozellae (Rozella's Lesser Galliwasp). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Cuatro, MABR (16°06′49.50″N, −91°00″17.99″W; 162m elev.) 8 April 2011. CNAR-IBH 25309. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its former known distribution 55km SE from Lago Oaxaca, Marqués de Comillas, Chiapas (Lazcano-Barrero et al., 1992), and 117km S from Piedras Negras, El Petén, Guatemala (Lee, 1996). The specimen was collected in a pitfall drift-fence, near a small stream within well-conserved continuous tropical rainforest (MABR).
Thecadactylus rapicauda (Turnip-tailed Gecko). Mexico, Chiapas. Municipality: Ocosingo; Locality: Gil Selva, Ejido Loma Bonita, MCR (16°03′3.73″N, −90°34′54.83″W; 172m elev.) 14 September 2011. CNAR-IBH 26073. This record extends the known distribution of the species 80km S from Yaxchilán, Ocosingo, Chiapas (Ferreira-García & Canseco-Márquez, 2006), 85km NW from Chinajá, El Petén, Guatemala (Lee, 1996), and 190km SE from Palenque, Palenque, Chiapas (IHNHERP). The specimen was collected at night on the base of a tree in a tropical rainforest remnant (MCR).
Hemidactylus frenatus (Common House Gecko). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: Ejido Chajul, MCR (16°06′49.57″N, −90°55′27.72″W; 165m elev.) 3 June 2012. CNAR-IBH 25415 and 25431. This record extends the known distribution of the species 57km SE from Rizo de Oro, Las Margaritas, Chiapas (GBIF, 2014), and 77km S from Frontera Corozal, Ocosingo, Chiapas (Paredes-León & Reynoso-Rosales, 2005a). The individuals were collected at night in a house (MCR).
Sphaerodactylus millepunctatus (Spotted Gecko). Mexico, Chiapas. Municipality: Ocosingo; Locality: Estación Chajul, MABR (16°06′38.43″N, −90°56′23.70″W; 151m elev.) 4 July 2012. CNAR-IBH-RF 069. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its former known distribution 190km SE from Palenque, Palenque, Chiapas (Lee, 1996), and 89km NW from Chinajá, Alta Verapaz, Guatemala (Lee, 1996). The specimen was photographed at night on leaf litter in a well-conserved continuous tropical rainforest (MABR).
Colenyx elegans (Elegant Banded Gecko). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: Arca de Noé, Ejido Chajul, MCR (16°00.6′11″N, −90°55′35″W; 151m a.s.l) 10 August 2008. Locality: Selva Playón de la Gloria, Ejido Playón de la Gloria, MCR (16°09′08.69″N, −90°49′8.88W; 185m elev.) 1 July 2012. CNAR-IBH-RF 026a, 026b, and CNAR-IBH 26085. This is the third record of the species for the Lacandona rainforest, extending its former known distribution 120km S from El Real, Ocosingo (American Museum of Natural History), and 70km S from Reserva Comunal la Cruz, Ocosingo (CNAR). The specimens were collected at night, on the ground in old-growth tropical rainforest remnants (MCR).
Anolis pentaprion (Lichen Anole). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Gumer, Ejido Loma Bonita, MCR (16°05′19.94″N, −90°59′40.52″W; 188m elev.) 15 June 2012. CNAR-IBH 26089. This record extends the known distribution of the species 90km S from Yaxchilán, Ocosingo, Chiapas (Ferreira-García & Canseco-Márquez, 2006). The specimen was collected in a liana, at 1.5m from ground level, in old-growth tropical rainforest remnants (MCR).
Anolis uniformis (Lesser Scaly Anole). Mexico, Chiapas. Municipality: Ocosingo; Locality: Loma Bonita, MCR (16°06′22.91″N, −90°59′7.63″W; 172m elev.) 5 April 2007. Locality: El Siete, MABR (16°06′37.18″N, −91°00′30.88″W; 193m elev.) 29 October 2007. CNAR-IBH 23414 and 23439. Third record for the Lacandona rainforest, extending its known distribution 77km S from La Cojolita, Ocosingo, Chiapas (CNAR), and 80km SE from Lacanja-Chansayab, Ocosingo, Chiapas (IHNHERP). The specimens were collected on leaf litter, the first specimen in a well-conserved continuous tropical rainforest (MABR), and the second specimen on the leaf litter in an abandoned cocoa field (MCR).
Sceloporus teapensis (Teapen Rosebelly Lizard). Mexico, Chiapas. Municipality: Ocosingo, Locality: Desviación a Loma Bonita, Ejido Loma Bonita, MCR (16°00′00.32″N, −90°59′57.14″W; 195m elev.) 3 June 2007 and 13 June 2012. CNAR-IBH-RF 066 and 073. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its known distribution 119km SE from El Real, Ocosingo, Chiapas (GBIF, 2014), and 190km SE from Palenque, Palenque, Chiapas (Lee, 1996). The specimens were photographed at midday, roadside, on a rock (MCR).
Corytophanes cristatus (Smoothhead Helmeted Basilisk). Mexico, Chiapas. Municipality: Ocosingo; Locality: Héctor, Loma Bonita, MCR (16°05′42.49″N, −91°00′33.90″W; 177m elev.) 21 August 2012. CNAR-IBH 26885. This record extends the known distribution of the species 66km SE from Lacanjá, Chansayab, Ocosingo, Chiapas (Lee, 1996). The specimen was collected on the branch of a tree in an intermediate secondary forest (MCR).
Plestiodon sumichrasti (Sumichrat's Skink). Mexico, Chiapas. Municipality: Ocosingo; Locality: Gil Selva, Ejido Loma Bonita, MCR (16°03′3.73″N, −90°34′54.83″W; 172m elev.) 17 June 2012. Municipality: Ocosingo; Locality: Selva Cuatro, MABR (16°06′49.5″N, −91°00″17.99″W; 162m elev.) 13 May 2012. CNAR-IBH-RF 067 and 068. These represent the southernmost records from the Lacandona rainforest for the species, extending its known distribution 66km NE from Lacanjá-Chansayab, Ocosingo, Chiapas, and 190km NW from Palenque, Palenque, Chiapas (Lee, 1996). One specimen was photographed on the base of a tree in old-growth tropical rainforest remnant (MCR), and the other was captured by a pitfall trap, in a well-conserved continuous tropical rainforest (MABR).
Adelphicos quadrivirgatus (Middle American Earth Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Loma, Loma Bonita, MCR (16°05′48.62″N, −91°00′19.03″W; 167m elev.) 29 January 2008. Locality: Selva Pule, MCR (16°05′25.04″N, −90°59′18.75″W; 177m elev.) 5 September 2011. CNAR-IBH 21956 and CNAR-IBH 26075. This record extends the known distribution of the species 82km SE from La Florida, Ocosingo, Chiapas, and 40km NE from Tres Ranchos, Huehuetenango, Guatemala (GBIF, 2014). Both specimens were collected on leaf litter in old-growth tropical rainforest remnants (MCR). A Variable Coral snake (Micrurus diastema) was predating 1 specimen.
Amastridium sapperi (Sapper's Rustyhead snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: El Siete, MABR (16°06′22.91″N, −90°59′07.63″W; 175m elev.) 14 March 2007. CNAR-IBH-RF 064. This record extends the known distribution of the species190km SE from Palenque, Chiapas, 176km NW from Las Cañas, El Petén-Alta Verapaz, Guatemala (Lee, 1996), and 79km S from El Encaño, Sierra de la Cojolita, Ocosingo, Chiapas (CNAR). The snake was found under the trunk of a tree, in a well-conserved continuous tropical rainforest (MABR).
Clelia clelia (Mussurana). Mexico, Chiapas. Municipality: Marqués de Comillas, Locality: Carretera fronteriza a Chajul, MCR (16°04′58.54″N, 90°55′07.86″W; 169m elev.) 11 September 2011. CNAR-IBH 26074. This is the third record of the species for the Lacandona rainforest, extending its former known distribution 89km NW from Chinajá, Alta Verapaz, Guatemala (Lee, 1996), 190km SE from Palenque, Chiapas, and 157km SW from Chancalá, Palenque, Chiapas (IHNHERP). The specimen was collected on the road, near a secondary forest (MCR).
Coluber mentovarius (Neotropical Whipsanke). Mexico, Chiapas. Municipality: Ocosingo; Locality: Ejido La Cascada (16°52′05.39″N, −91°10′32.17″W; 370m elev.) 10 May 2006. CNAR IBH 256204. This is the third record of the species for the Lacandona rainforest, extending its former known distribution 130km S from Emiliano Zapata, Palenque, Chiapas (CNAR) and 127km SE from Yaxoquintela, Ocosingo, Chiapas (GBIF, 2014).
Coniophanes bipunctatus (Two spotted Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Loma, Loma Bonita, MCR (16°05′00.02″N; −91°57′37.11″W; 195m elev.) 23 June 2012. CNAR-IBH 26108 and 26129. This record extends the known distribution of the species 89km NW from Chinajá, Alta Verapaz, Guatemala, and 190km SE from Palenque, Chiapas, Mexico (Lee, 1996). The specimens were collected at night on leaf litter, in old-growth tropical rainforest remnants (MCR).
Leptodeira septentrionalis (Northern Cat-eyed snake). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: road to Playón de la Gloria, Playón de la Gloria (16°08′57.57″N, −90°53′55.78″W; 158m elev.) 1 September 2011. CNAR-IBH 26080. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its former known distribution 90km SW from Yaxchilán, Ocosingo, Chiapas (Ferreira-García & Canseco-Márquez, 2006), 60km S from Reserva Chan-kin, Ocosingo, Chiapas (CNAR), and 117km S from Piedras Negras, El Petén, Guatemala (Lee, 1996). The specimen was collected on the road, near an old-growth tropical rainforest remnant (MCR).
Mastigodryas melanolomus (Common Lizard Eater). Mexico, Chiapas. Municipality: Ocosingo; Locality: Sitio Rafa, Loma Bonita, MCR (16°02′52.79″N, −90°35′32.41″W; 207m elev.) 12 January 2008. CNAR-IBH 23594. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its known distribution 90km SW from Yaxchilán, Ocosingo, Chiapas (Ferreira-García & Canseco-Márquez, 2006). The specimen was collected on leaf litter in an intermediate secondary forest (MCR).
Ninia diademata (Ringneck Coffee Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Sitio Gumer, Ejido Loma Bonita, MCR (16°05′10.38″N, −90°58′18.98″W; 172m elev.) 08 August 2011. CNAR-IBH 26077. This is the first record of the species for the southeastern part of the Lacandona rainforest, extending its known distribution 80km SE from Campo Alegre, Las Margaritas, Chiapas (GBIF, 2014), and 190km SE from Palenque, Chiapas (Lee, 1996). The specimen was captured in a pitfall in an intermediate secondary forest (MCR).
Ninia sebae (Red Coffee Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Pule Selva, Ejido Loma Bonita, MCR (16°05′27.84″N, −90°58′23.56″W; 167m elev.) 24 August 2011. CNAR-IBH 26095. This is the first record of the species for the southeastern part of the Lacandona rainforest, extending its known distribution 53km E from Amparo Agua Tinta, Las Margaritas, Chiapas (GBIF, 2014), and 81km SE from La Florida, Ocosingo, Chiapas (Lee, 1996). The specimen was captured at night, on leaf litter in an old-growth tropical rainforest remnant (MCR).
Oxybelis aeneus (Gray Vine Snake). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: Fragmento de Reforma, Ejido Reforma, MCR (16°15′16.16″N, −90°49′54.34″W; 183m elev.) 23 April 2011. CNAR-IBH-RF 063. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its known distribution 107km SE from Palestina, Ocosingo, Chiapas (GBIF, 2014), and 57km NW from El Petén, Alta Verapaz, Guatemala (Lee, 1996). The snake was found on a small shrub, in a 1500ha old-growth tropical rainforest remnant (MCR).
Pantherophis flavirufus (Tropical rat snake). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: Carretera Ejido Playón de La Gloria, MCR (16°08′34.15″N, −90°54′06.12″W; 167m elev.) 25 August 2011. CNAR-IBH 26158. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its former known distribution 108km SW from Tikal, El Petén, Guatemala (Lee, 1996) and 166km NE from San Jerónimo Tacaná, Cacahoatán, Chiapas (GBIF, 2014). The specimen was found dead on the road between two old-growth tropical rainforest remnants (MCR).
Pseustes poecilonotus (Puffing Snake). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: Reserva Reforma, Ejido Reforma Agraria, MCR (16°14′59.37″N, −90°50′19.42″W; 172m elev.) 11 May 2012. Municipality: Ocosingo; Locality: Camino a Ruinas, MABR (16°06′12.94″N, −91°00′42.40″W; 179m elev.) 20 May 2012. CNAR-IBH (in process) and CNAR-IBH-RF 070. This is thefirst record of the species for the southeastern part of the Lacandona rainforest, extending its known distribution 50km SE from Sabana de San Quintin, Ocosingo, Chiapas (GBIF, 2014) and 66km S from Lacanjá-Chansayad, Ocosingo, Chiapas (Lee, 1996; IHNHERP). The first specimen was photographed on leaf litter in a well-conserved continuous tropical rainforest (MABR), and the second one was found dead on the road between two old-growth tropical rainforest remnants (MCR).
Scaphiodontophis annulatus (Guatemala Neckband Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Gil, Ejido Loma Bonita, MCR (16°05′3.95″N, −90°58′2.85″W; 165m elev.) 31 May 2012. Municipality: Ocosingo; Locality: Carretera al Trece, Ejido el Trece, MCR (16°07′59.40″N, −91°05′52.30″W; 219m elev.) 06 June 2012. CNAR-IBH (in process) and CNAR-IBH 26083. This represents the first record of the species for the southeastern part of the Lacandona rainforest, extending its known distribution 90km S from Yaxchilán, Ocosingo, Chiapas (Ferreira-García & Canseco-Márquez, 2006), 190km SE from Palenque, Palenque, Chiapas, and 84km NW from Alta Verapaz, Guatemala (Lee, 1996). Both specimens were collected at night, on leaf litter, in old-growth tropical rainforest remnants (MCR).
Senticolis triaspis (Green Rat Snake). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: Playón de la Gloria, MCR (16°08′09.49″N, −90°54′04.24″W; 173m elev.) 23 May 2012. CNAR-IBH 26126. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its known distribution 80km S from Crucero San Javier, Ocosingo, Chiapas (Lee, 1996). The specimen was found dead on the road between two old-growth tropical rainforest remnants (MCR).
Sibon dimidiata (Slender Snail Sucker). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Gil, Ejido Loma Bonita, MCR (16°06′22.91″N, −90°59′07.63″W; 172m elev.) 14 September 2011. CNAR-IBH 26072. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its known distribution 80km SE from Las Margaritas, Las Margaritas, Chiapas (GBIF, 2014), 90km S from Yaxchilán, Ocosingo, Chiapas, and 95km SE from Lago Ocotal, Ocosingo, Chiapas (Lee, 1996). The specimen was collected at night on the base of a tree, in old-growth tropical rainforest remnants (MCR).
Spilotes pullatus (Tropical Rat Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Carretera Froteriza, MCR (16°04′59.91″N, −90°59′52.51″W; 191m elev.) 10 May 2012. CNAR-IBH 26141. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2011), extending its known distribution 80km SE from Las Margaritas, Las Margaritas, Chiapas (GBIF, 2014), 90km S from Yaxchilán, Ocosingo, Chiapas, and 95km SE from Lago Ocotal, Ocosingo, Chiapas (Lee, 1996). The specimen was found dead on the road between two old-growth tropical rainforest remnants (MCR).
Tantillita lintoni (Linton's Dwarf Short-tailed Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Sitio Selva Loma, Loma Bonita, MCR (16°03′25.09″N, −91°00′17.80″W; 204m elev.) 13 June 2012. CNAR-IBH 25300, 25301 and 26088. The record extends the known distribution of the species 90km S from Yaxchilán, Ocosingo, Chiapas (Ferreira-García & Canseco-Márquez, 2006). The specimens were captured with drift-fence and pitfall traps in old-growth tropical rainforest remnants (MCR).
Tretanorhinus nigroluteus (Orangebelly Swamp Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Hector, Ejido Loma Bonita, MCR (16°05′46.69″N, −90°59′13.56″W; 170m elev.) 21 June 2008. CNAR-IBH 23416. This represents the second record of the species for the Lacandona rainforest, and first record for the southeastern part of this rainforest (MCR), extending itsknown distribution 90km S from Yaxchilán, Ocosingo, Chiapas (Ferreira-García & Canseco-Márquez, 2006), and 89km NW from Chinajá, Alta Verapaz, Guatemala (Lee, 1996). The specimen was collected at night in a stream in a secondary forest (MCR).
Tropidodipsas sartorii (Terrestrial Snail Sucker). Mexico, Chiapas: Municipality: Ocosingo; Locality: Selva Gil, Ejido Loma Bonita, MCR (16°05′10.38″N, −90°58′18.98″W; 172m elev.) 14 September 2012. Locality: Arroyo Seco, MABR (16°07′14.17″N, −90°55′54.00″W; 165m elev.) 23 February 2013. CNAR-IBH-RF 074 and CNAR-IBH 26888. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its known distribution 190km SE from Palenque, Chiapas, and 70km SW from Rio La Pasión, El Petén, Guatemala (Lee, 1996). The first specimen was photographed in a 40ha old-growth tropical rainforest remnants (MCR) and the second was collected on leaf litter in a well-conserved continuous tropical rainforest (MABR).
Micrurus diastema (Variable Coral Snake). Mexico, Chiapas. Municipality: Ocosingo; Locality: Sitio Héctor, Ejido Loma Bonita, MCR (16°05′69.91″N, −90°00′49.47″W; 204m elev.) 16 September 2007. CNAR-IBH 23449. The record extends the known distribution of the species 53km E from Amparo Agua Tinta, Las Margaritas, Chiapas (GBIF, 2014), and 66km SE from Lacanjá-Chansayad, Ocosingo, Chiapas (Lee, 1996; IHNHERP). The specimen was found on the leaf litter in a secondary forest (MCR).
Atropoides mexicanus (Central American Jumping Pitviper). Mexico, Chiapas. Municipality: Marqués de Comillas; Locality: Ejido Playón de la Gloria, MCR (16°08′42.00″N, −90°52′51.6″W; 182m elev.) 12 August 2011. Municipality: Ocosingo; Locality: El Kárstico, MABR (16°11′31.10″N, −91°08′41.30″W; 44m elev.) 22 June 2012. CNAR-IBH-RF 062 and 071. We confirmed the presence of the species in the southeastern part of the Lacandona rainforest (Köhler, 2008), extending its known distribution 66km SE from Lacanjá-Chansayad, Ocosingo, Chiapas (ECO-SH) and 190km SE from Palenque, Chiapas (Campbell & Lamar, 2004). Specimens were photographed on a dead tree in a cocoa plantation (MCR), and active at night on the leaf litter in a well-conserved continuous tropical rainforest (MABR), respectively.
Bothriechis schlegelii (Eyelash viper). Mexico, Chiapas. Municipality: Ocosingo; Locality: Selva Rafa, Ejido Loma Bonita, MCR (16°04′67.03″N, −90°59′28.51″W; 160m elev.) 25 May 2011. Locality: Ruinas, MARB (16°11′02.00″N, −91°00′51.50″W; 193m elev.) 28 May 2011. CNAR-IBH 26082 and 26093. We confirmed the presence of the species for the southeastern part of the Lacandona rainforest (MCR) (Köhler, 2008); the record extends its known distribution 100km from Paso Subín, El Petén, Guatemala (Lee, 1996) and 249km SE from Rayón, Rayón, Chiapas (ECO-SCH). Both specimens were surveyed on vines (2m at soil level) beside a stream; one specimen was collected in a 30ha old-growth tropical rainforest remnant (MCR) and another was collected in a well-conserved continuous tropical rainforest (MABR).
Peer Review under the responsibility of Universidad Nacional Autónoma de México.