The “aje” (Llaveia axin axin) is a parasitic hemipteran of various tree species in tropical dry forests of Mexico and Guatemala. Females produce fatty extracts used since pre-hispanic times (i.e., traditional medicine and as raw material to produce handcrafts). However, very little is known about its biology and conservation status. In Michoacán and Guerrero States (Mexico), fifteen localities with historical reports of “aje” were visited 4 times (2006-2007). The incidence, seasonal abundance and distribution, host species, tree size and section preference, were determined. Only 3 populations in 2 localities were found at low altitudes (< 300m asl), close to water sources, with only 1 host tree, Acacia cochliacantha. Females were mostly found at the base of the trunk, but showed no preference for any tree size. July (2007) was the month with greater abundance. The “aje” seems highly threatened with local extinction, due to their fragmented relict populations and rapid destruction of its habitat and host species, which is considered as a weed. More basic information on its biology and population dynamics is urgently needed to implement any conservation strategy. In addition, the management of the “aje” should take into consideration biological, pharmacological and cultural connotations.
El “aje” (Llaveia axin axin) es un hemíptero parásito de especies de árboles del bosque tropical seco de México y Guatemala. Las hembras producen extractos grasos utilizados por el hombre desde tiempos prehispánicos (i.e., medicina tradicional y como materia prima para producir la técnica artesanal de maque). Sin embargo, se conoce muy poco sobre su biología y estado de conservación. En los estados de Michoacán y Guerrero, se visitaron, en cuatro ocasiones (2006-2007), 15 localidades con reportes históricos del “aje”. Se determinó la incidencia, abundancia y distribución estacional del “aje”, así como las especies hospederas y preferencia por sección y tamaño de árbol. Se localizaron tres poblaciones en dos localidades (< 300m snm), cercanas a cuerpos de agua, y se encontró presente en un sólo hospedero, Acacia cochliacantha. El “aje” fue comúnmente encontrado en la base del tronco principal, sin mostrar preferencia por el tamaño de árbol. Julio (2007) fue el mes con mayor abundancia. El “ajé” está amenazado por la extinción local de sus poblaciones, debido a su distribución agregada y a la destrucción de su hábitat y por la eliminación de su especie hospedera, considerada como maleza. Urge obtener información básica sobre su biología y dinámica poblacional, así como la implementación de alguna estrategia de conservación. El manejo del “aje” debe considerar connotaciones biológicas, farmacológicas y culturales.
Many insect species are considered a nuisance as they represent agricultural pests and disease vectors affecting several species of plants and animals (including humans); while many other species are considered beneficial because they provide food, raw material for several purposes, act as biological controls, and provide ecosystem services such as pollination and decomposition (Donkin, 1977; Kent, 1984; Miller, 1993; Losey and Vaughan, 2006; Schabel, 2008). Among these beneficial insects are the giant margarodids in the genus Llaveia (Signoret) (Coccoidea: Margarodidae). Representatives of the genus have been recorded from Mexico through Central America, Peru and Ecuador. The fatty extracts from some Llaveia species have been used by the indigenous people of Mexico and Guatemala to the present day for centuries (McBryde, 1943; Heil, 1995; Williams and Mac Vean, 1995; Martínez, 2006). The Llaveia fat product is only extracted from adult females and is used as a waterproofing and polishing agent for articles of wood and pottery, as a base for face and body paints, and artistically for binding pigments used in decorating wooden artifacts. The giant scale, Llaveia axin axin, is processed for its fat content that is used as a lacquer coating on wood products, especially art and sculpture creations; this handcraft technique is known as “maque”. The fat resembles a soft wax that is a combination of free fatty acids and triacylglycerols; when rubbed onto the substrate, with various pigments it polymerizes into a durable and decorative film (sensuMac Vean, 2008). In Mexico several towns have historically specialized in this form of artwork. Among these places are Chiapa de Corzo (Chiapas State), Olinalá (Guerrero State), Uruapán and Pátzcuaro (Michoacán State) (Jenkins, 1964, 1970; Barrera and Prado, 2003; Martínez, 2006). In folk medicine the fat is used as unguent for external wounds, swelling and skin problems (McBryde, 1943; Jenkins, 1964, 1970).
The production of L. axin axin extracts once flourished in regions of Mexico and Guatemala but recent information suggests that this prehispanic practice is vanishing because of a lack of knowledge about its production and use, and its replacement in artwork by synthetic materials (Williams and MacVean, 1995). In Michoacán, the preservation of this ancient technique has become very difficult mainly because L. axin axin populations have been overexploited for decades; approximately 17 000 adult Llaveia females yield only 2.2kg of raw material (Williams and MacVean, 1995). Additionally, habitat destruction for agriculture and cattle grazing and the lack of basic information on its natural history threatens the persistence of this valuable biotic resource. This study presents basic and novel information on the ecology of L. axin axin including its regional distribution, seasonal abundance, tree hosts and habitat preference in the southwestern region of Michoacán. To our knowledge, this is the first study on the ecology of L. axin axin as most of the published information on this species is based on its taxonomy and cultural value. Furthermore, the management practices suggested here considers a multidisciplinary approach (e.g., anthropological and biological sciences) that may help to preserve this threatened insect species.
Materials and methodsStudy site. The study took place in the Balsas River Basin of the states of Guerrero and Michoacán, Mexico. The original vegetation of the area was tropical dry forest, 4-15m in height (Rzedowski, 1978; Atlas Geográfico del estado de Michoacán, 2003); however, the natural vegetation has been drastically altered as a consequence of agricultural activities since the early 1900s (Rzedowski and Calderón de Rzedowski, 1987; Trejo-Vázquez, 1999). Some tree species characteristic of the study area are Acacia cochliacantha, A. cymbispina, Amphipterigygium glaucum, Caesalpina coriaria, Cordia eleagnoides, Guaiacum coulteri, Haematoxylon brasiletto, Pithecellobium dulce, Prosopis laevigata, and Ziziphus amole. The study region presents an annual precipitation of 975.5mm and temperatures fluctuate between 20.8 to 37.1° C. The wet season lasts from June to October; whereas the dry season from November to May (Enciclopedia de los Municipios de México, 2005). Tropical dry forest is the most abundant tropical forest type in Mexico (Masera et al., 1997). However, this tropical vegetation type is undergoing rapid rates of deforestation and/or land cover change. Annually 1.4-1.9% of all tropical dry forests in Mexico are converted to pastures and other uses (Trejo and Dirzo, 2000).
Study species. The “aje” or “axe” Llaveia axin axin (Llave, 1832) (Hemiptera: Coccoidea: Margarodidae), is a giant scale insect (females up to 2.5 cm long; Mac Vean, 2008) that parasites on several species of plants, feeding on sap drawn directly from the plant’s vascular system (Kosztarab, 1987; Gullan and Kosztarab, 1997; Ben-Dov, 2005). The species appears to be polyphagous as it has been reported to feed on different plant species (i.e., Acacia cochliacantha, A. farmesiana, Jatropha curcas, Spondias sp., Bursera sp., Pithecellobium dulce and herbs from the Labiatae family (Barrera and Pardo, 2003; Mac Vean, 2008). Adult “aje” females are wingless and secrete a powdery waxy coating that forms long wax strands protecting their body; they are almost immobile and are permanently attached to the plant they have parasitized. Their mouthparts arise between the front coxae. Adult males have wings and are believed to die within a day or 2. The ephemeral adult males are poor fliers and more often rely on walking over the host plant for a successful encounter with a female. Their range is thought to be restricted to that of the population; however, it is likely that some males in flight can travel to other parts of the host plant or to other plants (Lambdim, 2008).
Information on L. axin axin as well as on other scale insects of Mexico is incomplete, scattered throughout the literature and is often found in non scientific publications (e.g., Barrera and Prado, 2003; Martínez, 2006). Consequently, there is little detailed information on their biology and ecology. However, the available information suggests that as some other species in the Margarodidae, the “aje” has a single generation per year, that overwinters as eggs (ovisac) in the soil and that developmental time for the various stages is usually influenced by temperature and humidity (Gullan and Kosztarab, 1997; Lambdin, 2008).
Sampling design. In September 2006, based on topographic and vegetation maps of the Balsas River Basin in the South-West region of the state of Michoacán and the North of the state of Guerrero the sites were “aje” populations have been reported were located (Fig. 1). Fifteen localities were found and visited; 13 in the state of Michoacán (Rancho Tomatlán, Tziritzícuaro, San Lucas, El Coco, Rancho el Tecolote, Arroyo Hondo, San Jerónimo, Cuahutémoc, Pinzán Colorado, El Limón, La Cuchilla, and Monte Grande), and 2 in the State of Guerrero (Cutzamala and Zirándaro). In each locality, 3km trails were walked looking for the incidence of the “aje”. Once found, the geographic position of the populations was obtained with a GPS (Garmin 12 XL, Olathe, KS, USA). To verify the presence of “aje” populations, each of the 15 sites was visited 4 times throughout 2006 and 2007. In October 2006, only 2 populations in Arroyo Hondo (Municipality of Huetamo) were found. These populations are thereafter referred to as Arroyo Hondo 1 and Arroyo Hondo 2 (Fig. 1). In November 2006, a third population in San Pedrito (Municipality of San Lucas) was found. All populations were located in Michoacán (Fig. 1). No other populations were found in the remaining localities. Between the years of 1976 and 2000, the Municipalities of Huetamo and San Lucas lost ca. 30 140.4 ha of tropical dry forest at a rate of 1 255.8 ha/yr (C. Pacheco, pers. inf.).
In each of the 3 sites 3; 50m long x 6m width (300m2) permanent transects were positioned, covering a total area of 2 700m2. Because during the sampling period “ajes” were only found in isolated-relict populations it was not possible to position transects systematically (i.e., at the same distance from each other). Therefore, in Arroyo Hondo 1, distances between transects were 8 and 20m; Arroyo Hondo 2, 20 and 100m; and in San Pedrito 100 and 200m. To register the host tree species of the “aje”, as well as its seasonal incidence and abundance the study sites were visited 4 times in the case of Arroyo Hondo 1 and 2; and 3 times in the case of San Pedrito. In 2006 visits took place during October and November; whereas in 2007 in July and October. However, 2 of the 3 transects in Arroyo Hondo 2 were destroyed by the land owners and therefore for the months of July and November of 2007 data correspond to a single transect for this site. The timing and number of visits were arranged according to the security circumstances present in the study region (i.e., violent episodes due to drug trafficking).
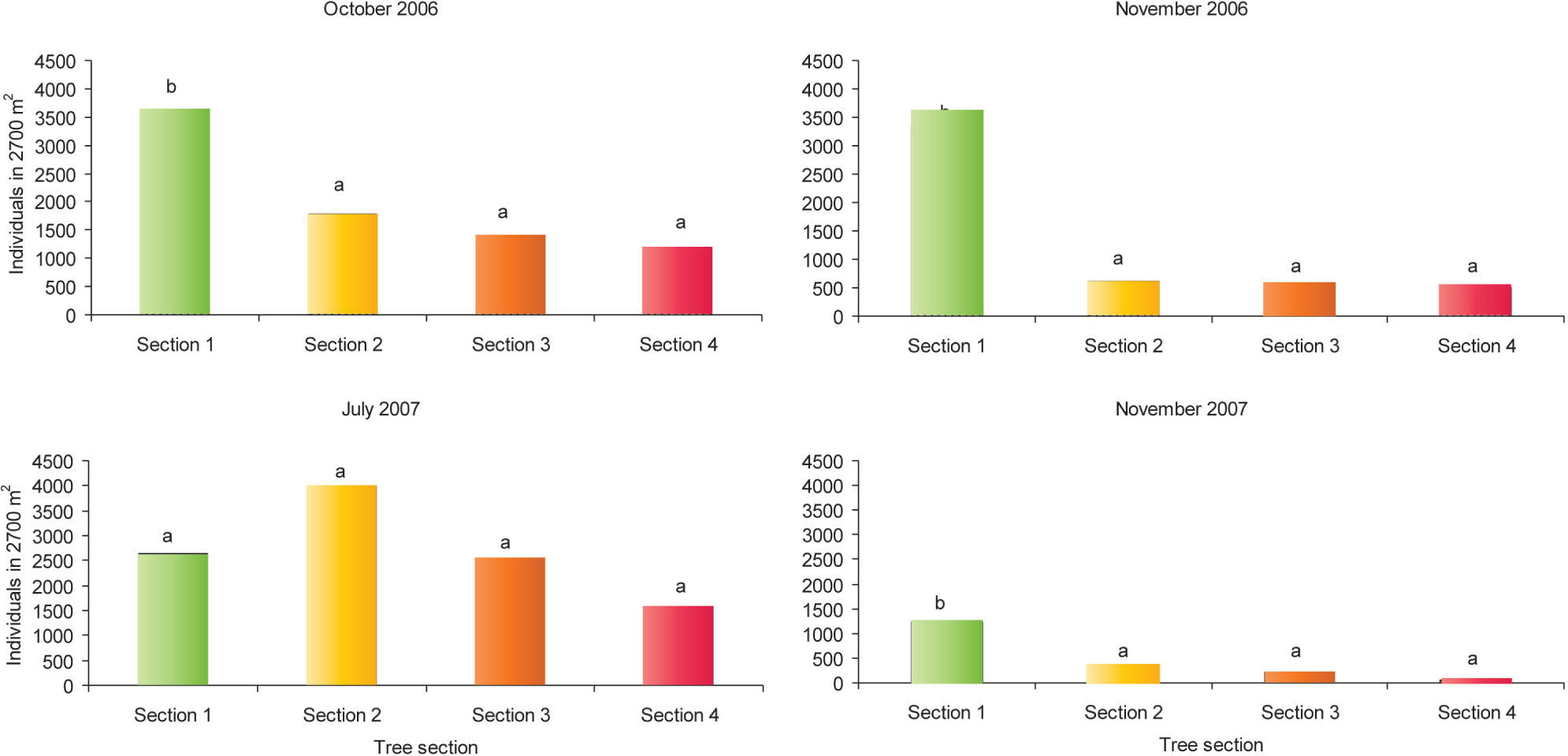
All individual trees with “aje” individuals were registered and identified to species. To determine if the “aje” showed preference for a particular tree section and plant structure (stem base, stem and/or crown, and foliage) each host tree was divided every 50 cm into 4 sections from the base of the main trunk up to 200 cm height: section 1, 0-50 cm; section 2, 51-100 cm; section 3, 101-150 cm and section 4, 151-200 cm. In each tree section the number “ajes” attached to the trunk and branches (no individual was found in the foliage and flowers) were counted. Additionally, “ajes” on the ground within a 1m radius from the trunk were also counted. Height and diameter at breast height (dbh) were measured for each host tree. These same trees were monitored during the study period to look for seasonal variations in the populations of “ajes”. Statistical analysis. Due to logistical constraints (i.e., destruction of study areas) the study ended with an unbalanced sampling design. Therefore, it was more appropriate to analyze the average number of “aje” (after data transformation) across sites rather than the total number of insects, to perform the analyses. Differences in the number of “ajes” among sites and among tree sections (section 1, 0-50 cm; section 2, 51-100 cm; section 3, 101-150 cm and section 4, 151-200 cm) within sites were analyzed using the GLIM statistical package (Crawley, 1993; Green and Payne, 1994). Because the number of “ajes” was obtained from the same trees at 4 different times, data correspond to a repeated measures analysis of variance in which the effect of time is nested within treatments (site and tree section; Crawley 1993; Mead 1994). The significance of each term was tested through the deviance that the term removed from the total. In those cases in which a term was found to be significant, the individual levels of the term were compared with a posteriori t- tests (Crawley, 1993). To determine if “ajes” had preference for certain tree size a Pearson productmoment correlation coefficient (Sokal and Rohlf, 1995) was performed. Data were log (x+1) transformed prior to analysis to normalize the data. However, figures are shown with the untransformed data. Significant differences were set at ≤0.05.
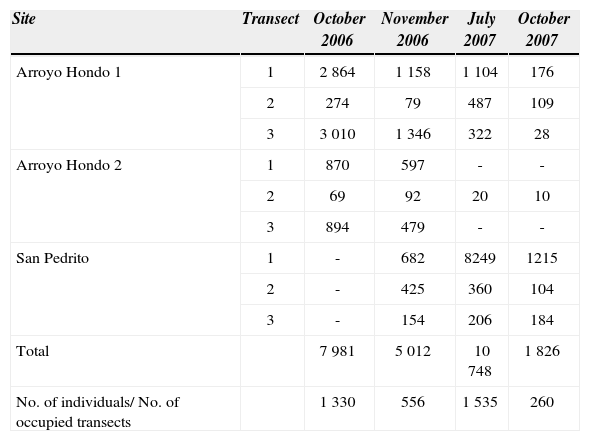
ResultsIn the 9 transects (3 per site; 2 700m2 sampled) a total of 8 tree species were recorded (i.e., Acacia farnesiana, Acacia cochliacantha, Caesalpinaceae sp., Pisonia aculeate, Salpianthus purpurascens, Cordia spinosa, Haematoxilum sp., and Pithecellobium dulce). However, A. cochliacantha was the only species hosting the “aje”. All registered individuals (N= 253) of A. cochliacantha in the 3 sites were infested with “ajes” (San Pedrito, 93 trees; Arroyo Hondo 1, 73 trees; and Arroyo Hondo 2, 87 trees). The populations of the “aje” were highly variable in time and among and within sites (Table 1). During the study period some trees presented severe “aje” infestation of the trunk and branches while others had very few individuals infesting them (range, 0-1 199 individuals). Two of the populations were found in living fences (Arroyo Hondo 1 and 2) and 1 in an old-field invaded by A. colchliacantha (San Pedrito) (Fig. 2). These sites were on an elevation gradient from 229 to 251m asl and about 0.48 to 5.22km from the River Balsas river (Table 2).
Number of Llaveia axin axin females infesting Acacia cochliacantha trees (North-West Michoacán, Mexico). Please note that the “San Pedrito” site was located until November 2006, and that 2 of the 3 transects in “Arroyo Hondo 2” were destroyed by the land owners. The total numbers of L. axin axin found and the average value considering the number of transects sampled per month are shown. The total sampling area was 2 700m2. Distances between transects within sites varied between 20 and 200m
| Site | Transect | October 2006 | November 2006 | July 2007 | October 2007 |
|---|---|---|---|---|---|
| Arroyo Hondo 1 | 1 | 2 864 | 1 158 | 1 104 | 176 |
| 2 | 274 | 79 | 487 | 109 | |
| 3 | 3 010 | 1 346 | 322 | 28 | |
| Arroyo Hondo 2 | 1 | 870 | 597 | - | - |
| 2 | 69 | 92 | 20 | 10 | |
| 3 | 894 | 479 | - | - | |
| San Pedrito | 1 | - | 682 | 8249 | 1215 |
| 2 | - | 425 | 360 | 104 | |
| 3 | - | 154 | 206 | 184 | |
| Total | 7 981 | 5 012 | 10 748 | 1 826 | |
| No. of individuals/ No. of occupied transects | 1 330 | 556 | 1 535 | 260 |
Satellite images of localities with relict populations of Llaveia axin axin. The populations were found in highly disturbed habitats corresponding to living fences and in an abandoned pasture dominated by Acacia colchiacantha trees; where: a), San Nicolás Arroyo Hondo 1; b), San Nicolás Arroyo Hondo 2, and c), San Pedrito (from Google Earth September 2011). Distance between Arroyo Hondo 1 and 2, was 1.7km; between Arroyo Hondo 1 and San Pedrito, 6.3km, and between Arroyo Hondo 2 and San Pedrito, 7.4.
Geographic location of sites with Llaveia axin axin relict populations. All sites are in the northwestern part of the Michoacán State, Mexico
| Site | Geographic coordinates | Elevation (m asl) | Distance to River Balsas (km) |
|---|---|---|---|
| Arroyo Hondo 1 | 18°32’14.7” N, 100°51’26.6” W | 251 | 5.2 |
| Arroyo Hondo 2 | 18°31’39.6” N, 100°51’11.8” W | 245 | 4.5 |
| San Pedrito | 18°28’49.2” N, 100°49’11.9” W | 229 | 0.45 |
The nested analysis of variance showed no significant differences on “aje” abundance among sites and therefore this term was removed from the model. However, there was a significant effect of the tree section on the abundance of “ajes” (F312= 9.7, p= 0.025), showing a significant greater number of scale insects at the base of the tree trunk; section 1(up to 50 cm height) (Fig. 3). The effect of time nestedwithin-section was significant (F12,100= 1.8, p = 0.025), with greater numbers of “ajes” in July 2007 than at any other time. October 2007, was the month with the lowest number of “ajes” infesting A. cochliacantha trees. Trees of A. cochliacantha had a diameter at breast height (dbh) between 1 cm to 58 cm (mean 7.6 cm ± SD 2.9), while tree height ranged between 30 cm - 610 cm (mean 3.2 ± SD 0.71). There was no significant correlation between tree size (i.e., dbh and height) and “aje” abundance. We counted a total of 786 “aje” individuals on the ground at the base of the trees (San Pedrito, 114 insects; Arroyo Hondo 1, 581 insects; and Arroyo Hondo 2, 91 insects). According to the 4 visits, adult females of “aje” start colonizing A. cochliacantha trees in July (rainy season); feed on the sap until the end of November, disappearing thereafter, probably to overwinter and for ovoposition on the ground.
Total numbers of Llaveia axin axin females at different sections from the base of the main tree trunk (Acacia colchiacantha). Individuals were registered in 4 sampling dates. Different letters above bars represent significant differences (t ≥2, p < 0.05) among tree sections: 1, up to 50 cm height; 2, 50 - 100 cm height; 3, 100 - 150 cm height, and 4, 150 -200 cm height.
Results showed that L. axin axin populations had a restricted and aggregated distribution. The fact that this insect species was found in only 2 out of 15 localities with previous reports of its presence; that these localities are strongly modified and degraded; and that its only host tree A. cochliacantha is considered as a weed; suggests that these populations are relict and highly threatened locally (Fig. 2). Two of the populations were found in living fences and 1 in an old-field dominated by A. cochliacantha; suggesting that L. axin axin persists in highly altered habitats. Rapid habitat destruction and transformation still persisting in the region may increase demographic and environmental stochasticity making these “aje” relict populations more vulnerable to local extinction (Leigh, 1981; Shaffer, 1981; Lande, 1988; Foley, 1994). Furthermore, small populations are more likely to present reduced genetic variation due to inbreeding (Willi et al., 2006).
Regarding the host species, previous studies in Michoacán have reported the presence of the “aje” not only in A. cochliacantha, but also in A. farnesiana, Spondias sp., Bursera sp. and in P. dulce (Barrera and Pardo, 2003). During the search of “ajes” special attention was given to the presence of plant species that were previously reported as hosts. Actually, a coleopteran larva in Bursera sp. and P. dulce that could be easily mistaken as L. axin axin at a glance, due to their very similar morphology and coloration, was registered.
Population dynamics. The abundance of the “ajes” changed with time, being July the month with greater abundance. Females start to disappear in December (winter) and do not appear until the beginning of the rainy season of the following year (June-July). Information on the life cycle for more studied species in the Margarodidae describe that after mating, females produce masses of pink eggs covered with a white cotton-like material which are later deposited on the ground (e.g., Ben Dov, 2005; Foldi, 2005; Lambdin, 2008). No eggs, but many females on the ground at the base of the acacias trunks were found. Most female individuals were found in the first 50 cm from the base of the tree trunks (tree section 1). Females and males must overlap at some instance for sexual reproduction to take place. Only a single winged male was observed in visiting periods to the region (September 2009) and therefore it was not possible to register the mating behavior of the “aje”. Long-term observations are urgently needed on “aje” population dynamics.
Several species on the Margarodidae are known to select multiple locations on the host plant (Ben Dov, 2005). The location on the host plant depends upon their stage of development, time of the year and free space. Furthermore, the population density of members of the Margarodidae is affected by several types of biotic and abiotic factors (Kosztarab, 1987; Gullan and Kosztarab, 1997). Mortality is attributed to adverse weather conditions, such as heavy rains and high relative humidity, or hot and dry conditions, fungal infection and predators. In this study, L. axin axin was always more abundant at the base of the main trunk but there is no information concerning factors of mortality apart from that caused by humans. On the other hand, distributional patterns of species in several families of scale insects are related to environmental preferences such as elevation, vegetation type, and climatic conditions. It has been found that the number of species on the Margarodidae tend to decrease as elevation increases (Gullan and Kosztarab, 1997; Lambdin, 2008). According to these findings, L. axin axin is restricted to disturbed tropical dry forest vegetation on low elevations (< 300m asl) along the River Balsas Basin. Therefore the species is adapted to areas with moderate humidity provided by this important water source.
Management considerations. Based on its intrinsic values, not just on anthropocentric criteria, this fascinating insect should be conserved following a multidisciplinary approach; including biological, social and medical (pharmacology) sciences. Presently, in Mexico there are no efficient efforts to conserve L. axin axin and other related species, nor their habitats and usages. This insect species could be considered as an umbrella species for other native plant and animal species in the River Balsas Basin of Michoacán. A multidisciplinary approach for the preservation of the “aje” should be implemented.
Conservation biology. There are many aspects on the biology of the “aje” that are still unknown such as its reproductive biology and behavior, population genetics, its interactions with other plants and animals (i.e., competition, predation, etc.), which may be helpful for its conservation. Information on the effects (e.g., physiological) of L. axin axin infestation on its host species is lacking, at least for Mexico. There is evidence that scale insects injure the plant surface by injecting their stylets into the tissue, producing open wounds, which may allow the entrance of plant pathogens that may lead to the death of the plant; whereas other species are known vectors of pathogens (García-Guzmán and Dirzo, 2001; Ben Dov, 2005; Lambdim, 2008). It is believed that “aje” infestations kill the edible Spondias spp. trees in Chiapas, and therefore it is considered a nuisance (J. Benítez-Malvido, pers. inf.).
Tropical dry forests in Michoacán, and elsewhere, are being rapidly degraded, yet very little is known about the potential consequences of the disappearance of specialized phytophagous insects that may drive many evolutionary and ecological processes (Crawley, 1989; Miller, 1993). The extinction of a plant species (e.g., A. colchiacantha) from a particular area may have cascading effects on other organisms, such as specialized phytophagous insects and nesting animals that rely on the host plant for survival (Didham et al., 1996; Harvell et al., 2002). Conversely, the loss of specialized phytophagous insects can affect biodiversity by releasing the host from an important source of population regulation, such as often occurs when species are introduced to new geographic areas where their natural enemies are absent (Harvell et al., 2002). Therefore, it makes good sense to conserve this scale insect species.
There are many actions that could be taken into consideration to promote the conservation of L. axín axin. Our results showed that the range of the “aje” is highly fragmented leaving small populations isolated from each other and therefore ecological connectivity should be implemented. However, given the degradation of the study region (Fig. 2), the successful connection of the “aje” habitat or the restoration of the vegetation to its historical distribution (in Michoacán) seems unlikely. Therefore, one of the major goals is to maintain these relict populations by protecting the host trees and by allowing A. colchiacantha to regenerate in old-fields. These restored and/or recovered areas could serve as refuges for the “aje” and could be favorable to the survival of other native species.
The social component. The “aje” in southwestern Michoacán is declining because of habitat destruction for agricultural purposes (e.g., pasture grasses for cattle). Additionally, there is a limited knowledge about its production and use by the local human population and because the insect fat product is being replaced by synthetic materials (Williams and Mac Vean, 1995). Local people need to be informed about this biotic resource and of its economic potential for human welfare in the region. The involvement of local people is fundamental to generate information that could be used for the preservation of the remaining “aje” populations. There is an urgent need for the long-term monitoring of this native insect species that could be carried out by the local people.
In pre-hispanic Mexico, people semi-domesticated several social insects (i.e., cochineal Cocccus spp.; stingless bees Melipona spp. and Trigona spp.) including the “aje” (Jenkins, 1970; Donkin, 1977; Kent, 1984; Schabel, 2008). These insects were maintained for different specific purposes. For example, cochineal was used for the production of a crimson dye; the “aje” was used in making lacquer based wax and bees provided for honey and wax. Semi-domesticated animals are those kept or maintained outside their natural habitat, but their breeding is not controlled by man (e.g., no genetic control) and the animals may be easily returned to the wild. Management strategies generally mimic the insects’ native habitat however, ways to breed the “aje” have to be re-evolved.
Many peasant farmers in Mexico manage complex and diverse agro-ecosystems, and constantly adapt management strategies with multiple aims. The most frequently used strategy is crop and product diversification (Astier et al., 2011). The need for more sustainable agro-ecosystems is an urgent one for farmers in the Balsas River Basin of Michoacán, and they could be interested in adopting new management strategies which include the cultivation and production of “aje”. Alternative management strategies could be attained by preserving old-fields invaded by A. colchiacantha trees and by continuing the use of this legume as living fences in cattle pastures. Actions such as this could stop further habitat degradation for the “aje” and may improve the environmental conditions to sustain human populations in the region.
The widespread usage of L. axin axin provides substantial evidence that there are no toxic or adverse effects associated with the fat of the insect (Williams and MacVean, 1995). Indigenous medicinal uses of the “aje” have been documented as early as the XVI century (del Val-de Gortari et. al., 2008). However, much information is needed on the chemical composition of its fatty products and on its potential usages in contemporary pharmacology. Furthermore, the cultural impact of insects must not be neglected. We need to record the many uses of insects soon, before it is too late, as habits change and traditions are lost, information on the role(s) of insects in human cultures and societies will ultimately become irrevocable (sensuSchabel, 2008).
This study was supported by the Project “Manejo sustentable de Hatos Ganaderos” UNAM-2007; the Casa de Artesanías of the Michoacán State and by the Centro de Investigaciones en Ecosistemas, UNAM. We are very grateful to Dr. Yair Ben-Dov (Department of Entomology Agricultural Research Organization, The Volcani Center Israel) for identifying Llaveia axin axin; and to the people from the Universidad Michoacana herbarium for the identification of the plant species. Hernesto A. Sánchez Rosas helped in the field-work and Carlos Pacheco calculated the deforestation rates.