The first record about the pathogenic fungus Batrachochytrium dendrobatidis in Mexican plethodontid salamanders dates back to the 1970s. However, little is known of the patterns of infection in wild populations and the effects of changes in temperature on the degree of infection. This work quantified Bd infection in a population of Pseudoeurycea leprosa in La Malinche National Park, Puebla, Mexico from June 2011 to September 2012. A total of 160 adult salamanders were experimentally exposed to temperatures of 10, 15, 20, 23, 25, or 28°C for 10 weeks. The results of this study revealed that: (1) the population of P. leprosa in La Malinche National Park is infected with Bd throughout the year at a low prevalence of between 0 and 17%; (2) 20.6% of the salamanders that were Bd negative at the time of collection expressed chytridiomycosis after exposure to the experimental temperature treatments; (3) temperature was the cause of death in each treatment, with temperatures of 25°C and 28°C affecting the survival of P. leprosa; (4) the infection load in certain P. leprosa individuals exhibited cycles of increasing and decreasing zoospore genomic equivalents over time.
El primer registro del hongo patógeno Batrachochytrium dendrobatidis (Bd) en salamandras pletodóntidas de México data de la década de 1970. Sin embargo, se conoce muy poco sobre los patrones de infección por Bd en poblaciones silvestres y el efecto de los cambios de temperatura sobre el grado de infección. Este trabajo cuantificó la infección por Bd en la población de Pseudoeurycea leprosa en el Parque Nacional La Malinche, Puebla, México, de junio de 2011 a septiembre de 2012. Se expusieron 160 individuos adultos a tratamientos de 10, 15, 20, 23, 25 o 28°C durante 10 semanas. Los resultados de este estudio revelaron que: (1) la población de P. leprosa del Parque Nacional La Malinche presenta infección por quitridiomicosis a lo largo del año con prevalencias bajas, entre el 0 y el 17%; (2) el 20.6% de los organismos que resultaron negativos (sin infección) al momento de la recolecta resultaron positivos después de los experimentos de temperatura; (3) la temperatura fue la causa de la muerte en los experimentos realizados a 25°C y 28°C, afectando directamente la supervivencia de P. leprosa; (4) la carga de infección en algunos individuos de P. leprosa presentó ciclos de aumento y disminución de la infección durante los tratamientos.
The wild populations of many species are decreasing drastically around the world, which has led scientists to propose that we are in the midst of a sixth mass species extinction event (Medina-Vogel, 2010; Wake & Vredenburg, 2008). This phenomenon has been attributed to a variety of factors, including habitat destruction, pollution, overexploitation, climate change and emerging infectious diseases (Dobson & Foufopoulos, 2001; Medina-Vogel, 2010). Emerging diseases become increasingly important as new cases appear around the world (Medina-Vogel, 2010). To address this threat, scientists have suggested that research on emerging diseases should focus on the causes of animal diseases, transmission of diseases via hosts, physiology and ecology of the pathogens, and impacts of global climate change (Harvell et al., 2002; Medina-Vogel, 2010).
Many pathogens and their hosts are limited by the temperature of the environment (Harvell et al., 2002). Thus, when climate conditions change, the parasite–host relationship can be disrupted, which may increase the virulence of the pathogens and/or the susceptibility of the hosts (Harvell et al., 2002). Examples of diseases that have caused population declines and are dependent on specific temperatures include distemper in seals, parvovirus in lions, white-nose syndrome in bats, and chytridiomycosis in amphibians (Daszak, Cunningham, & Hyatt, 2003; Harvell et al., 2002).
Chytridiomycosis occurs worldwide and has caused the decline of more than 200 amphibian species (Skerratt et al., 2007). The pathogenic fungi that cause the disease are Batrachochytrium dendrobatidis, which was discovered in the 1990s and occurs in all of the amphibian orders (Olson et al., 2013), and Batrachochytrium salamandrivorans, which was discovered in 2013 in European salamanders (Martel et al., 2014).
Chytridiomycosis is characterized by the colonization of the keratinized layers of amphibian epidermis or larval mouthparts (Berger et al., 1998; Martel et al., 2014; Pessier, Nichols, Longcore, & Fuller, 1999). Motile zoospores develop into sporangia in the keratinized parts of the amphibian skin and release zoospores through a discharge tube. This can result in epidermal hyperplasia and hyperkeratosis. In addition, these changes disrupt the osmoregulatory function of the skin, leading to dehydration, electrolyte imbalances, and death caused by cardiac arrest (Berger et al., 1998; Brutyn et al., 2012; Carver, Bell, & Waldman, 2010; Marcum, St-Hilaire, Murphy, & Rodnick, 2010; Voyles et al., 2007; Voyles, Rosenblum, & Berger, 2011).
To date, more than 500 amphibian species have been infected with Bd (Olson et al., 2013). However, the most dramatic examples of the appearance of Bd with a consequent population decline occurred in the anurans Bufo periglenes and Atelopus varius in Costa Rica in the 1980s (Pounds & Crump, 1994). Because of the seasonality of infectious Bd outbreaks, Lips, Diffendorfer, Mendelson, and Sears (2008) and Cheng, Rovito, Wake, and Vredenburg (2011) suggested that the pathogen arrived in North America in the early 1970s and moved southward into Central America and South America through introduced species or other vectors as well as by water currents (Cheng et al., 2011; Lips et al., 2008). In Mexico and Central and South America, the epidemiological outbreaks and the prevalence of Bd may also have been caused by more suitable climate conditions for the pathogen (Lips et al., 2008; Ron, 2005).
The earliest record of Bd infection in Mexico is from plethodontid salamanders collected in 1972 (Cheng et al., 2011). According to the cited study, chytrid infections were present in voucher specimens of several salamander genera collected in the 1970s and 1980s from the states of Veracruz, Oaxaca, and Hidalgo, where severe salamander population declines were reported by Parra-Olea, Garci¿a-París, and Wake (1999) and Rovito, Parra-Olea, Vásquez-Almazán, Papenfuss, and Wake (2009). These results led Cheng et al. (2011) to propose that an outbreak of chytridiomycosis might have been related to these declines. Thus far, Bd has been found in 50 species of Mexican amphibians, including Pseudoeurycea leprosa (Mendoza-Almeralla, Burrowes, & Parra-Olea, 2015), and the affected amphibians are mainly distributed in mountainous regions, extending from the north to the south of the country.
Despite the progress made in gathering information about the presence of Bd in Mexico, little is known about its infection dynamics in amphibian species distributed in the country. Therefore, this study had the following objectives: (1) determine whether the population of P. leprosa in La Malinche National Park is infected with Bd year-round; (2) determine if stress caused by temperature treatments affects the Bd infection load in P. leprosa, and (3) determine the effect of temperature and Bd infection on the survival of P. leprosa.
Materials and methodsThe Malinche National Park is in the Trans-Mexican Volcanic Belt, where 2 of the continent's biogeographic regions converge. The park is located in the Mexican states of Puebla and Tlaxcala, and this region is characterized by high species diversity and endemism (López-Domínguez & Acosta, 2005). The park has a total area of 45,711ha and a maximum elevation of 4,461 m.a.s.l. (Melo, 1977). Below 2,800 m.a.s.l., the climate is temperate with temperatures ranging between 12 and 18°C, and above 2800 m.a.s.l., the climate is cold with temperatures from 5 to 12°C (García, 2004).
We visited La Malinche National Park 15 times from June 2011 to September 2012; we collected specimens according to standard protocols and wore surgical gloves when handling and swabbing the salamanders (Van Rooij et al., 2011). The swab was then placed in a sterile 2ml vial, and each organism was placed in a plastic bag to prevent cross-contamination. A number of the adult individuals were transported to the laboratory inside a plastic cooler in their individual plastic bags. In the laboratory, the salamanders were placed into a 250ml plastic container (7.3cm high, 8cm upper diameter and 6.4cm lower diameter), which contained a damp paper substrate. The salamanders were fed 2 flies or 2 crickets every 4 days during the experiments, and they were maintained at room temperature for 5 days before being used in the temperature experiments. After the experiments, 160 salamanders were deposited in the Colección Nacional de Anfibios y Reptiles (CNAR), Universidad Nacional Autónoma de México.
Each container was placed in a cooling system (wine cellar) fixed at a constant temperature of 10, 15, 20, 23, 25, or 28°C. Humidity was maintained constant between 50 and 60% by spraying bottled water into the containers and changing the substrates every 3 days. The temperature and humidity were checked every 3 days with a thermohydrometer.
The experiment lasted for 10 weeks, and each salamander was swabbed once every week. Thirty salamanders were used for each of the temperature treatments (10, 15, 20, 23, and 25°C); the first 10 salamanders subjected to 28°C died within the first few days, so this temperature treatment was discontinued.
Detection and quantification of BdDNA was extracted from each swab using the PrepMan protocol (Applied Biosystems, Carlsbad, CA, USA) and analyzed following standard protocols with a real-time PCR assay to quantify Bd infection (Boyle, Boyle, Olsen, Morgan, & Hyatt, 2004). The assay uses genetic markers specific to Bd and compares each sample to a set of standards to calculate a genomic equivalent. DNA was extracted from each swab and analyzed according to standard protocols with a real-time PCR assay to quantify Bd infection (Boyle et al., 2004). The qPCR was performed on a StepOne Real-Time PCR System (Applied Biosystems). The PCR conditions consisted of an initial denaturation at 95°C for 10min, followed by 50 cycles of 10s at 95°C and 1min at 60°C. Each sample was assayed in duplicate together with standards of known Bd quantity (0.1, 1, 10, 100, and 1,000 zoospores) and with negative controls (5μl double distilled water). Following the quantification of the Bd zoospore genomic equivalents (ZGEs), each value was multiplied by the dilution factor to obtain the total equivalent zoospores of each sample.
We calculated the Bd prevalence by dividing the number of infected salamanders by the total number of specimens swabbed per visit. The differences in the number of salamanders infected before and after the temperature treatments were evaluated using a McNecmar test for each treatment, and the infection load was expressed as the ZGE. To test for differences in the infection load caused by temperature stress, we ran a nonparametric analysis of variance (ANOVA) in SPSS version 21.0.0.0 (IBM Corporation Armonk, NY, USA) using the treatment data, the number of salamanders, and their infection loads before and after treatment.
To determine the effects of temperature on mortality in P. leprosa, we compared survivorship curves for each treatment using the survival package in R (Therneau, 2014). We ran an initial analysis that included all of the salamanders regardless of their infection load, and then divided them into 2 groups: salamanders that were Bd negative during the entire experiment and salamanders that became Bd positive during the experiment. In the survival analysis, greater importance was given to deaths that occurred at the beginning of the experiment (option rho=1, implemented in the survdiff function) because temperature and humidity are known to affect Bd-amphibian dynamics by physiologically limiting vital processes for both the pathogen and the host. In this analysis, the temperature treatment was considered a level, and the collection month was considered a stratum. We tested for significant differences between all survivorship curves using a Kaplan–Meier analysis, and the probability of death associated with each temperature treatment was determined with Cox models of proportional risk.
To understand the relationship between infection load and survival in P. leprosa, the salamanders were classified into 3 groups: (1) the No-Cycle group, which included animals in which the infection load was either maintained or increased over time; (2) the 1–2 Cycles group, which included animals in which the infection load decreased/increased once or twice, and (3) the >3 Cycles group, which included animals in which the infection load decreased/increased 3 or more times. Using these data, we tested the hypothesis that survival depends on the ability to clear or reduce Bd infection. For these analyses, we also applied the Kaplan Meier analysis and Cox's proportional risk model. We included the cumulative number of zoospores over the course of the experiment (i.e., the sum of the ZGEs for 10 weeks) as an additional variable to explain the risk of mortality in Cox's proportional risk model.
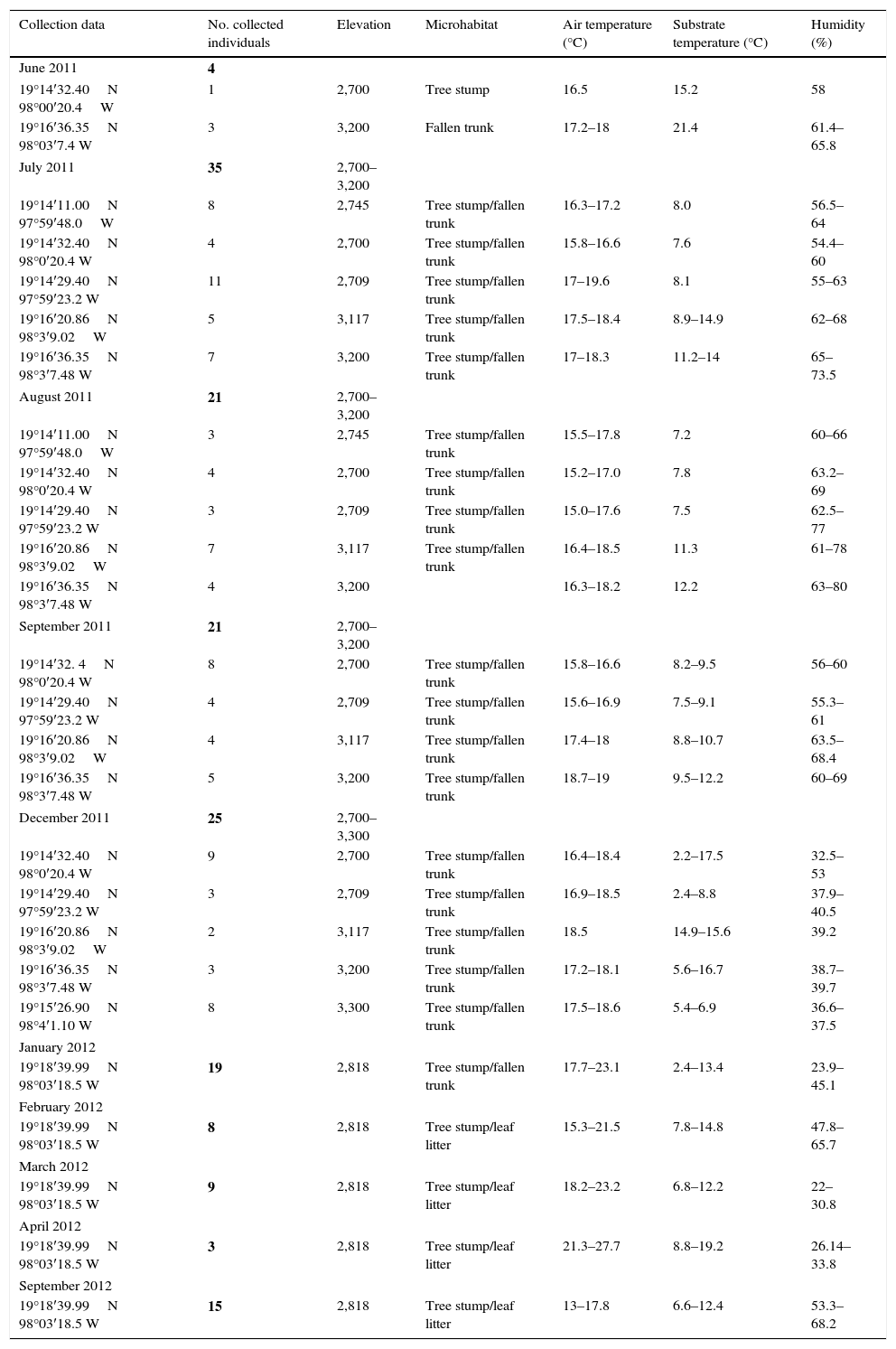
ResultsOver the 15 visits to La Malinche National Park from June 2011 to September 2012, we collected 160 adult P. leprosa (Table 1). Information on the microhabitat, specific locality, and ambient temperatures are provided in Appendix.
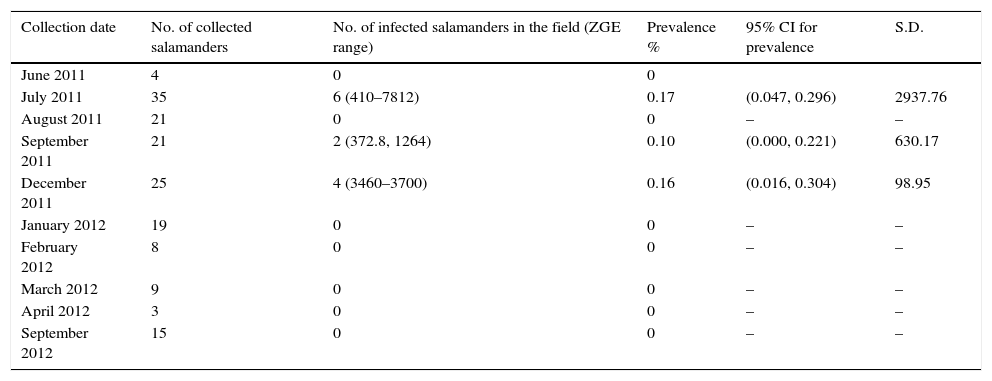
Batrachochytrium dendrobatidis (Bd) prevalence in the field and after temperature treatments. The ZGE (zoospore genomic equivalent) range represents the infection load range. Prevalence is measured as the number of infected salamanders divided by the total number of organisms collected.
| Collection date | No. of collected salamanders | No. of infected salamanders in the field (ZGE range) | Prevalence % | 95% CI for prevalence | S.D. |
|---|---|---|---|---|---|
| June 2011 | 4 | 0 | 0 | ||
| July 2011 | 35 | 6 (410–7812) | 0.17 | (0.047, 0.296) | 2937.76 |
| August 2011 | 21 | 0 | 0 | – | – |
| September 2011 | 21 | 2 (372.8, 1264) | 0.10 | (0.000, 0.221) | 630.17 |
| December 2011 | 25 | 4 (3460–3700) | 0.16 | (0.016, 0.304) | 98.95 |
| January 2012 | 19 | 0 | 0 | – | – |
| February 2012 | 8 | 0 | 0 | – | – |
| March 2012 | 9 | 0 | 0 | – | – |
| April 2012 | 3 | 0 | 0 | – | – |
| September 2012 | 15 | 0 | 0 | – | – |
Our results show that only 12 (7.5%) of the salamanders were infected with Bd in the months of July, September, and December, and the prevalence was low in all 3 months, with values of 0.17, 0.09, and 0.16, respectively. The highest infection load in the field was 7812 ZGEs, which was observed in 1 individual in July (Table 1).
In all of the temperature treatments, 45 of the exposed salamanders (24.82%) were positive for Bd, although only 12 animals were Bd positive in the field, whereas the other 33 were recorded as Bd negative at the time of collection and became Bd positive at some point during the temperature treatments. These data indicate that in their natural habitat, the salamanders had subclinical levels of infection, and temperature stress caused an increase in Bd load. For each temperature treatment, the McNecmar analysis was performed to determine the number of salamanders infected before and after the experiment, and it returned a significant difference (p≤0.05) between the 10 and 20°C treatments, indicating that exposing P. leprosa to certain temperatures affects pathogen expression.
The number of infected salamanders and the infection loads appeared to differ across treatments (Table 2), although the nonparametric ANOVA did not reveal significant differences (p≥0.05) in the infection load between the 10, 15, 20, 23 and 25°C temperature treatments. This result led us to reject the hypothesis that the degree of infection is temperature dependent.
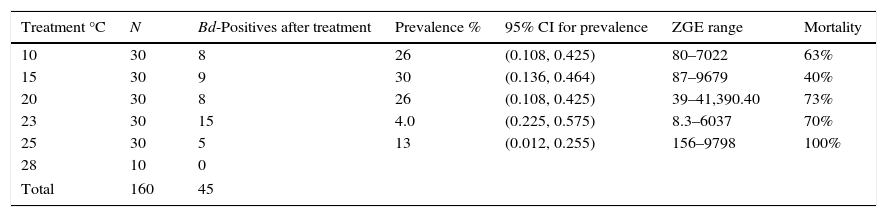
Results of the temperature treatments. N=number of organisms in the experiment. Prevalence is measured as the number of infected salamanders divided by the total number of organisms in the experiment. The ZGE range is the zoospore genomic equivalent loads or the infection load. Mortality is the percentage of dead salamanders divided by the total number of organisms exposed to each treatment.
| Treatment °C | N | Bd-Positives after treatment | Prevalence % | 95% CI for prevalence | ZGE range | Mortality |
|---|---|---|---|---|---|---|
| 10 | 30 | 8 | 26 | (0.108, 0.425) | 80–7022 | 63% |
| 15 | 30 | 9 | 30 | (0.136, 0.464) | 87–9679 | 40% |
| 20 | 30 | 8 | 26 | (0.108, 0.425) | 39–41,390.40 | 73% |
| 23 | 30 | 15 | 4.0 | (0.225, 0.575) | 8.3–6037 | 70% |
| 25 | 30 | 5 | 13 | (0.012, 0.255) | 156–9798 | 100% |
| 28 | 10 | 0 | ||||
| Total | 160 | 45 | ||||
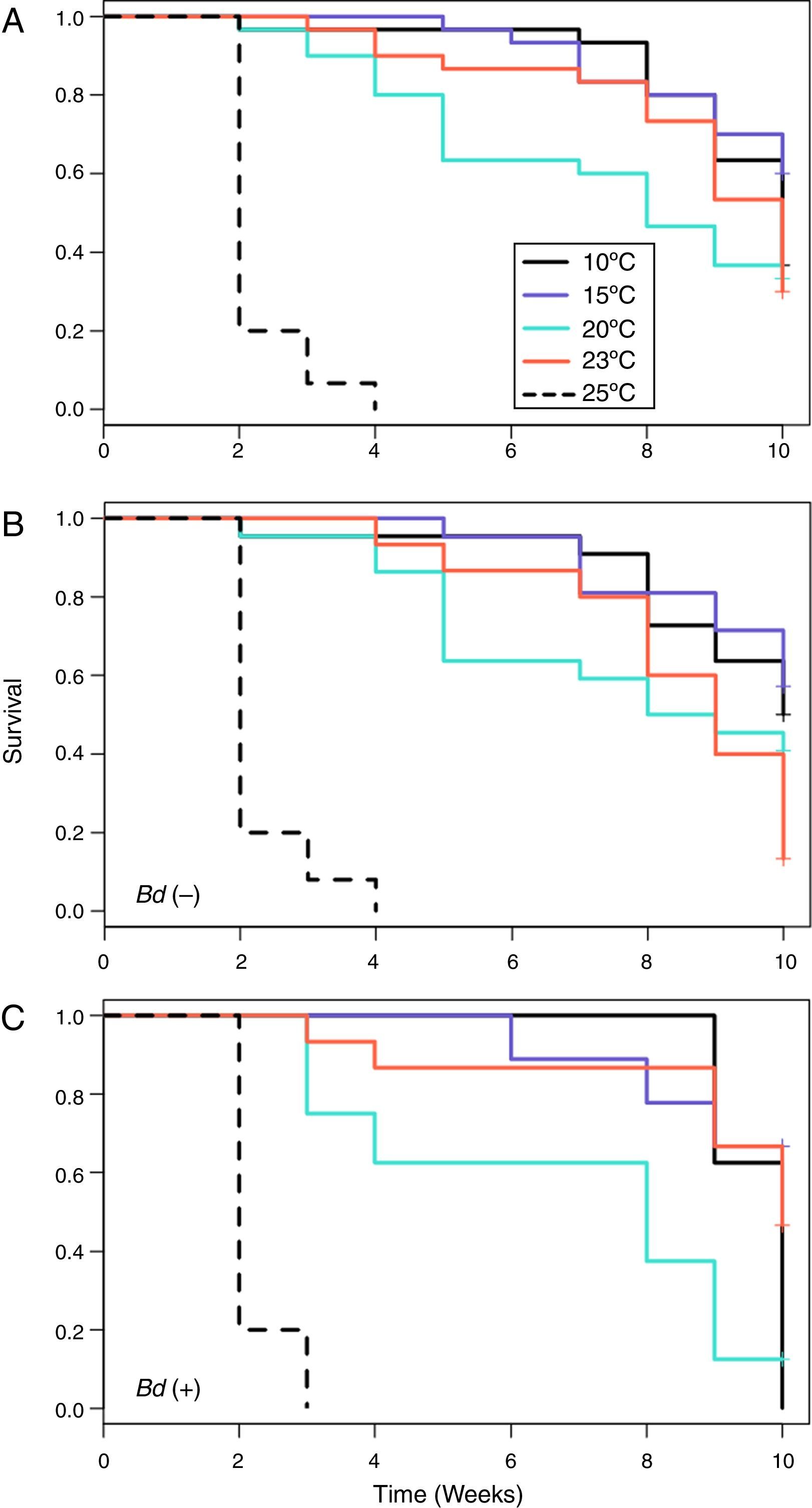
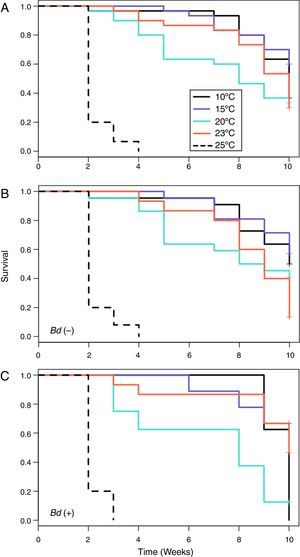
When all of the individuals were included in the analyses, the Kaplan–Meier survival analysis showed significant differences between temperatures (=59.4, DF=4, p<0.001) (Table 3) and Cox's proportional risk models using the 10°C treatment as a reference revealed significant differences in survival with the treatment at 25°C (LRT=68.8, DF=4, p<0.001), which had a risk rate of 63.5 (Table 3, Fig. 1A)
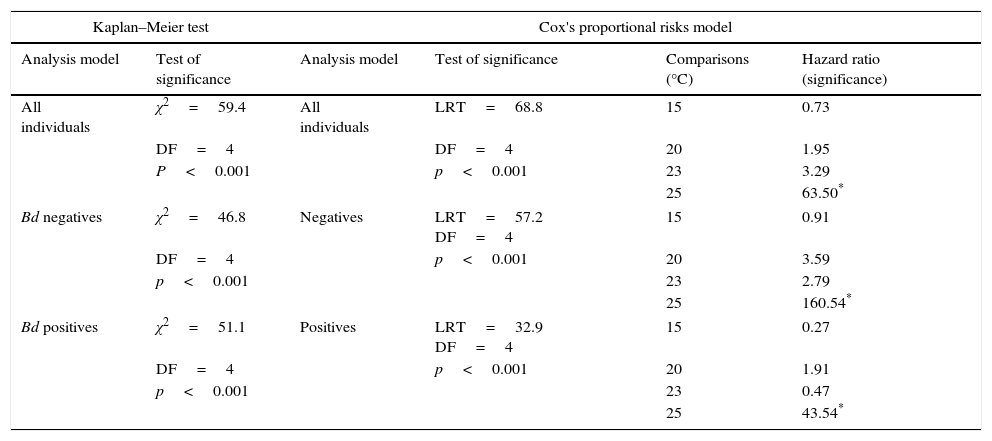
Summary of the survivorship analyses that measured the probability of death associated with each temperature treatment. Cox's proportional risk models used the 10°C treatment as a reference. For the analyses, each temperature treatment was considered a level, and the collection month was considered a stratum.
| Kaplan–Meier test | Cox's proportional risks model | ||||
|---|---|---|---|---|---|
| Analysis model | Test of significance | Analysis model | Test of significance | Comparisons (°C) | Hazard ratio (significance) |
| All individuals | χ2=59.4 | All individuals | LRT=68.8 | 15 | 0.73 |
| DF=4 | DF=4 | 20 | 1.95 | ||
| P<0.001 | p<0.001 | 23 | 3.29 | ||
| 25 | 63.50* | ||||
| Bd negatives | χ2=46.8 | Negatives | LRT=57.2 DF=4 | 15 | 0.91 |
| DF=4 | p<0.001 | 20 | 3.59 | ||
| p<0.001 | 23 | 2.79 | |||
| 25 | 160.54* | ||||
| Bd positives | χ2=51.1 | Positives | LRT=32.9 DF=4 | 15 | 0.27 |
| DF=4 | p<0.001 | 20 | 1.91 | ||
| p<0.001 | 23 | 0.47 | |||
| 25 | 43.54* | ||||
For the Bd-negative group, the Kaplan–Meier analysis also showed a difference in the probability of survival (χ2=46.8, DF=4, p<0.001), Cox's proportional risk models showed significant differences with the treatment at 25°C (LRT=57.2, DF=4, p<0.001), and the hazard ratio for this treatment was 160.54 (Table 3, Fig. 1B). For the Bd-positive group, the Kaplan–Meier analysis indicated significant differences (χ2 test=51.1, DF=4, p<0.001), whereas Cox's proportional risk models showed significant differences with the treatment at 25°C (LRT=32.9, DF=4, p<0.001), with a hazard ratio of 43.54 (Table 3, Fig. 1C).
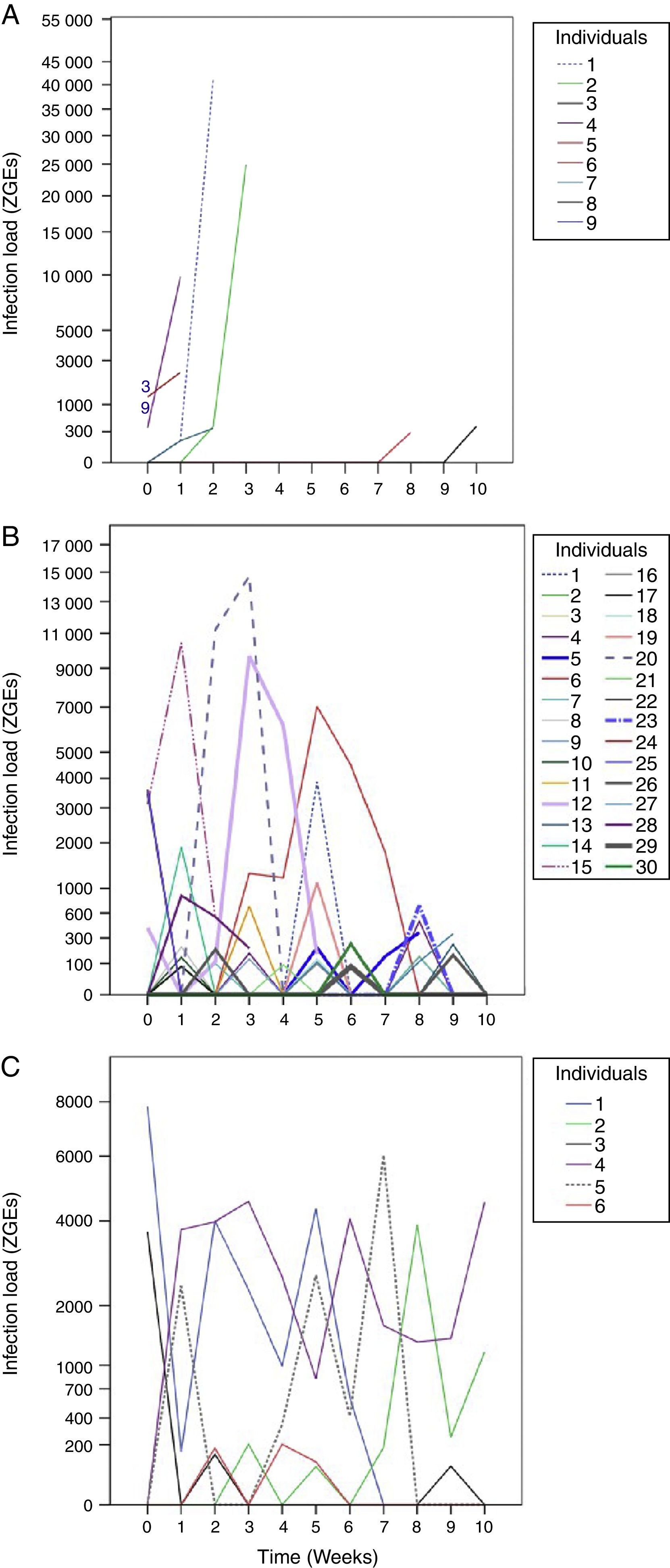
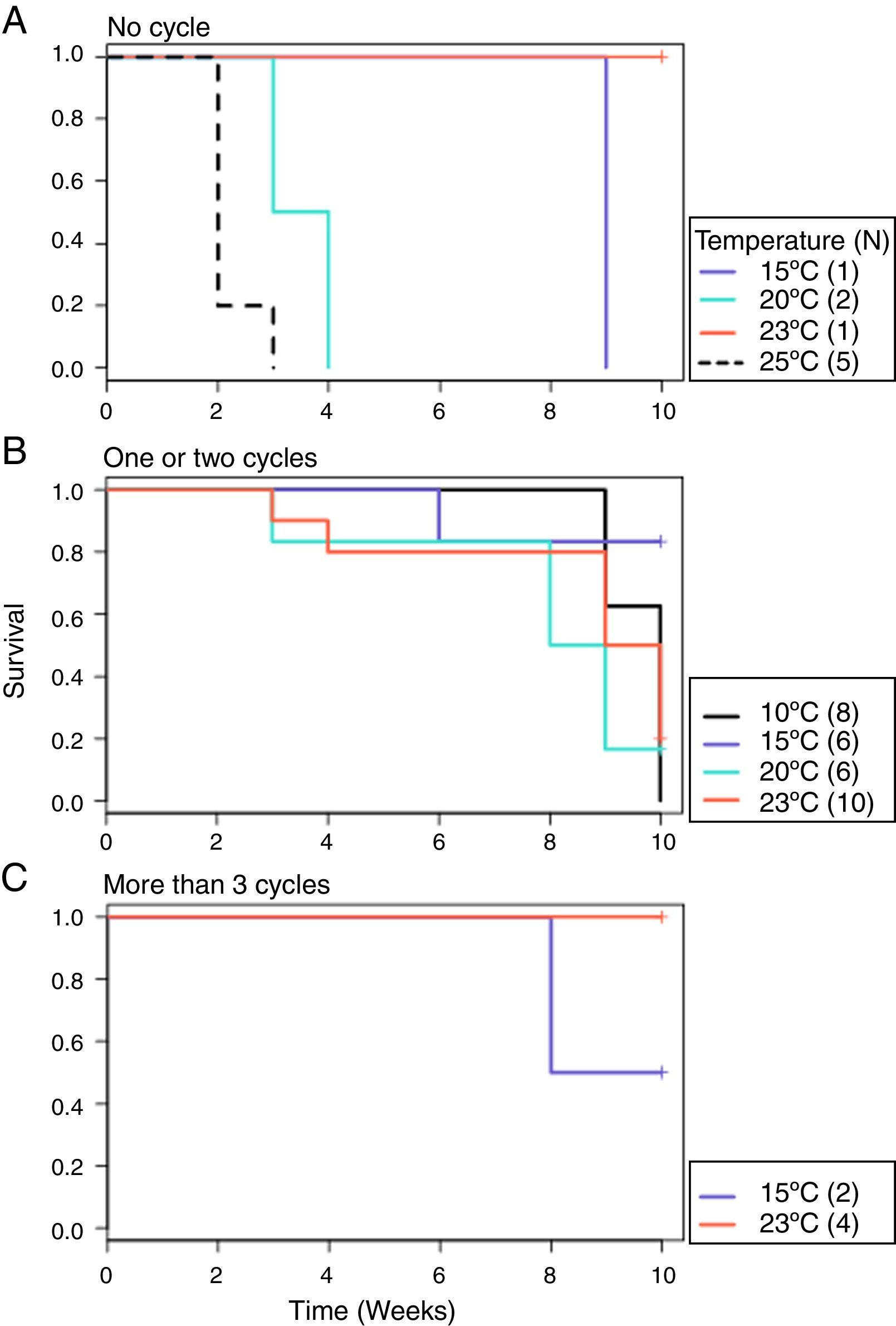
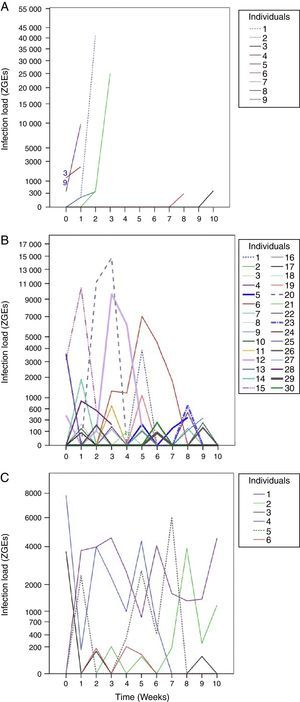
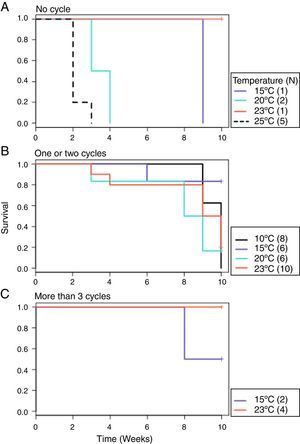
From the first week of temperature treatments to the end of the experiment, 115 salamanders were healthy and 45 were infected (Table 2). Of the 45 infected salamanders, 8 died immediately after manifesting the infection (Fig. 2A); 9 maintained the infection at similar infection loads; 30 exhibited cycles of increasing–decreasing ZGEs once or twice and then presented as Bd negative for the remainder of the experiment (Fig. 2B), and 6 showed more than 3 cycles of increasing–decreasing ZGEs during the experiment (Fig. 2C). The Kaplan–Meier analysis of infection loads revealed a significant difference between the No-Cycle group and the animals that fell into 1 of the 2 cycle categories. The Cox analysis also detected significant differences between these groups (Table 4, Fig. 3A–C).
Batrachochytrium dendrobatidis (Bd) infection loads. (A) No cycle: animals in which the infection load was either maintained or increased over time. Individuals 3 and 9 died before 1 week of treatment. (B) 1–2 cycles: animals in which the infection load decreased/increased once or twice. (C) >3 cycles: animals in which the infection load decreased/increased 3 or more times.
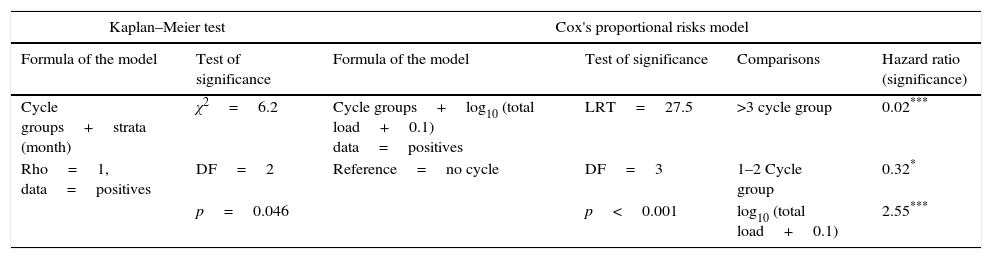
Summary of the survivorship analyses that measured the relationship between the infection load and survival in Pseudoeurycea leprosa.
| Kaplan–Meier test | Cox's proportional risks model | ||||
|---|---|---|---|---|---|
| Formula of the model | Test of significance | Formula of the model | Test of significance | Comparisons | Hazard ratio (significance) |
| Cycle groups+strata (month) | χ2=6.2 | Cycle groups+log10 (total load+0.1) data=positives | LRT=27.5 | >3 cycle group | 0.02*** |
| Rho=1, data=positives | DF=2 | Reference=no cycle | DF=3 | 1–2 Cycle group | 0.32* |
| p=0.046 | p<0.001 | log10 (total load+0.1) | 2.55*** | ||
Significance level
Probability of survival. (A) No cycle: animals in which the infection load was either maintained or increased over time. Individuals 3 and 9 died before 1 week of treatment. (B) 1–2 cycles: animals in which the infection load decreased/increased once or twice. (C) >3 cycles: animals in which the infection load decreased/increased 3 or more times.
Reductions in amphibian populations have frequently been associated with chytridiomycosis, a disease caused by the aquatic fungus Bd. Globally, forest-associated amphibians that live in or along streams are more likely to present declines compared with species in other assemblages (Ryan, Lips, & Eichholz, 2008; Stuart et al., 2008; Woodhams & Alford, 2005). Differences in habitat characteristics and life history traits as well as taxonomic variations in susceptibility to Bd could affect the prevalence of Bd in these communities.
Although Bd has been detected in numerous salamander species (Byrne, Davie, & Gibbons, 2008; Chatfield, Moler, & Richards-Zawacki, 2012), fully terrestrial plethodontid salamanders appear to be less susceptible to chytridiomycosis compared to anurans (Rothermel et al., 2008; Timpe, Graham, Gagliardo, Hill, & Levy, 2008). We detected Bd in P. leprosa from La Malinche, although the overall prevalence of the pathogen was relatively low when compared with other reports for Mexican amphibians. Field studies in Mexico have reported higher prevalence rates: for example, Frías-Álvarez et al. (2008) reported Bd in 50–100% of the ambystomatid salamanders and anurans analyzed from different parts of the country, Van Rooij et al. (2011) reported a prevalence between 25 and 100% in plethodontid salamanders in central and southern Mexico, and Luja, Rodríguez-Estrella, Ratzlaff, Parra-Olea, and Ramírez-Bautista (2012) also reported a high prevalence of Bd in the Baja California tree frog. However, all of these previous studies had small sample sizes, which substantially reduced the probability of an accurate measure for prevalence. Our study provides additional data to the limited number of previous studies that have systematically sampled terrestrial salamander assemblages (Caruso & Lips, 2013; Keitzer, Goforth, Pessier, & Johnson, 2011; Moffitt et al., 2015; Muletz, Caruso, Fleischer, McDiarmid, & Lips, 2014; Rothermel et al., 2008), and the results are consistent with the proposal that salamanders are less affected by chytridiomycosis and may act as an important vector of the disease (Chatfield et al., 2012; Garner et al., 2005).
Ambient environmental conditions, such as temperature and precipitation, are known to affect the vital physiological processes of amphibians, and these conditions have a strong correlation with the outcome of Bd infections (population-level infection prevalence and host mortality rates) (James et al., 2015; Rowley & Alford, 2013). In culture, Bd grows best between 17 and 25°C, with low temperatures retarding the pathogen's growth. The air and substrate temperatures observed at the time of collection (Appendix) and those reported for wild populations of P. leprosa (Guisado-Rodríguez & García-Vázquez, 2010) are generally lower than optimal for Bd to thrive (Piotrowski, Annis, & Longcore, 2004); therefore, these less than optimal temperatures may be the most simple explanation for the observed low prevalence of Bd infection and an important factor in the local abundance of this salamander species despite the epidemic wave of chytridiomycosis that has affected several salamander populations (Rovito et al., 2009).
Although environmental conditions in La Malinche are favorable for the survival of P. leprosa with a low prevalence of Bd, when the salamanders were exposed to temperature experiments and conditions ideal for Bd growth, 33 new cases of infection were detected, indicating that these salamanders had subclinical levels of infection in the field. Over the course of the experiments, we observed variations in the infection loads, and the salamanders exhibited cycles of increasing and decreasing ZGEs over time. A similar pattern was described by Shin, Bataille, Kosch, and Waldman (2014), who found that the ZGEs of individual frogs significantly varied over a 5-day period because they intermittently released zoospores. Our results might also reflect cycles in the release of Bd zoospores; however, we cannot rule out the effects of the defense mechanisms of the 19 individuals that expressed Bd once and were then Bd negative for the remainder of the experiment.
Elevated host body temperatures have been shown to clear frogs of Bd infection. Woodhams, Alford, and Marantelli (2003) and Rowley and Alford (2013) showed that individual probabilities of infection by Bd decreased strongly with an increasing percentage of body temperatures above 25°C in tropical species. For P. leprosa, the 3 survival analyses revealed that 25°C is a critical temperature for the survival of these organisms and identified this temperature as the most important factor influencing the survival of the salamanders rather than infection by Bd; therefore, both the pathogen and the host are negatively affected by temperatures of 25°C or higher.
P. leprosa is the most abundant species of plethodontid salamander in Mexico, and its populations currently appear to be stable (García-Vázquez, Gutiérrez-Mayén, Hernández-Jiménez, and Aurioles-López, 2006). However, studies of climate change in mountainous ecosystems have predicted an increase of 2°C to 3°C and a decrease in annual precipitation of between 3 and 20% by the year 2050 in the Trans-Mexican Volcanic Belt, where La Malinche National Park is located (Villers-Ruiz & Castañeda-Aguado, 2013). Furthermore, a loss of 75% of the distributional area of P. leprosa has also been predicted by the year 2050 because of climate change (Parra-Olea, Martínez-Meyer, & Pérez-Ponce de León, 2005). This study provides evidence that a synergistic effect between temperature stress and infection with Bd increases mortality in this species, and the results may provide an example of how temperate species of anurans might be affected by climate change.
We thank the Secretaría del Medio Ambiente y Recursos Naturales, Mexico for providing scientific collecting permits (FAUT 0106 and special permit 08657/14 to GP-O). Laura Márquez and Lidia Cabrera helped with the laboratory work. Funding was provided by PAPIIT-UNAMIN209914 and UC-MEXUS CONACyT (CN-13-614) to GPO. Mirna García-Castillo, Rafael Barzalobre and Mariana Meneses helped with the field work. This article fulfills part of the requirements of the first author to obtain the degree of Doctor of Sciences from the Graduate Studies in Biological Sciences Program of the UNAM. The first author expresses her gratitude to the Graduate Studies in Biological Sciences Program, UNAM for support and Conacyt for awarding a graduate studies scholarship (CVU/Becario, No. 2 05284/202580).
| Collection data | No. collected individuals | Elevation | Microhabitat | Air temperature (°C) | Substrate temperature (°C) | Humidity (%) |
|---|---|---|---|---|---|---|
| June 2011 | 4 | |||||
| 19°14′32.40N 98°00′20.4W | 1 | 2,700 | Tree stump | 16.5 | 15.2 | 58 |
| 19°16′36.35N 98°03′7.4 W | 3 | 3,200 | Fallen trunk | 17.2–18 | 21.4 | 61.4–65.8 |
| July 2011 | 35 | 2,700–3,200 | ||||
| 19°14′11.00N 97°59′48.0W | 8 | 2,745 | Tree stump/fallen trunk | 16.3–17.2 | 8.0 | 56.5–64 |
| 19°14′32.40N 98°0′20.4 W | 4 | 2,700 | Tree stump/fallen trunk | 15.8–16.6 | 7.6 | 54.4–60 |
| 19°14′29.40N 97°59′23.2 W | 11 | 2,709 | Tree stump/fallen trunk | 17–19.6 | 8.1 | 55–63 |
| 19°16′20.86N 98°3′9.02W | 5 | 3,117 | Tree stump/fallen trunk | 17.5–18.4 | 8.9–14.9 | 62–68 |
| 19°16′36.35N 98°3′7.48 W | 7 | 3,200 | Tree stump/fallen trunk | 17–18.3 | 11.2–14 | 65–73.5 |
| August 2011 | 21 | 2,700–3,200 | ||||
| 19°14′11.00N 97°59′48.0W | 3 | 2,745 | Tree stump/fallen trunk | 15.5–17.8 | 7.2 | 60–66 |
| 19°14′32.40N 98°0′20.4 W | 4 | 2,700 | Tree stump/fallen trunk | 15.2–17.0 | 7.8 | 63.2–69 |
| 19°14′29.40N 97°59′23.2 W | 3 | 2,709 | Tree stump/fallen trunk | 15.0–17.6 | 7.5 | 62.5–77 |
| 19°16′20.86N 98°3′9.02W | 7 | 3,117 | Tree stump/fallen trunk | 16.4–18.5 | 11.3 | 61–78 |
| 19°16′36.35N 98°3′7.48 W | 4 | 3,200 | 16.3–18.2 | 12.2 | 63–80 | |
| September 2011 | 21 | 2,700–3,200 | ||||
| 19°14′32. 4N 98°0′20.4 W | 8 | 2,700 | Tree stump/fallen trunk | 15.8–16.6 | 8.2–9.5 | 56–60 |
| 19°14′29.40N 97°59′23.2 W | 4 | 2,709 | Tree stump/fallen trunk | 15.6–16.9 | 7.5–9.1 | 55.3–61 |
| 19°16′20.86N 98°3′9.02W | 4 | 3,117 | Tree stump/fallen trunk | 17.4–18 | 8.8–10.7 | 63.5–68.4 |
| 19°16′36.35N 98°3′7.48 W | 5 | 3,200 | Tree stump/fallen trunk | 18.7–19 | 9.5–12.2 | 60–69 |
| December 2011 | 25 | 2,700–3,300 | ||||
| 19°14′32.40N 98°0′20.4 W | 9 | 2,700 | Tree stump/fallen trunk | 16.4–18.4 | 2.2–17.5 | 32.5–53 |
| 19°14′29.40N 97°59′23.2 W | 3 | 2,709 | Tree stump/fallen trunk | 16.9–18.5 | 2.4–8.8 | 37.9–40.5 |
| 19°16′20.86N 98°3′9.02W | 2 | 3,117 | Tree stump/fallen trunk | 18.5 | 14.9–15.6 | 39.2 |
| 19°16′36.35N 98°3′7.48 W | 3 | 3,200 | Tree stump/fallen trunk | 17.2–18.1 | 5.6–16.7 | 38.7–39.7 |
| 19°15′26.90N 98°4′1.10 W | 8 | 3,300 | Tree stump/fallen trunk | 17.5–18.6 | 5.4–6.9 | 36.6–37.5 |
| January 2012 | ||||||
| 19°18′39.99N 98°03′18.5 W | 19 | 2,818 | Tree stump/fallen trunk | 17.7–23.1 | 2.4–13.4 | 23.9–45.1 |
| February 2012 | ||||||
| 19°18′39.99N 98°03′18.5 W | 8 | 2,818 | Tree stump/leaf litter | 15.3–21.5 | 7.8–14.8 | 47.8–65.7 |
| March 2012 | ||||||
| 19°18′39.99N 98°03′18.5 W | 9 | 2,818 | Tree stump/leaf litter | 18.2–23.2 | 6.8–12.2 | 22–30.8 |
| April 2012 | ||||||
| 19°18′39.99N 98°03′18.5 W | 3 | 2,818 | Tree stump/leaf litter | 21.3–27.7 | 8.8–19.2 | 26.14–33.8 |
| September 2012 | ||||||
| 19°18′39.99N 98°03′18.5 W | 15 | 2,818 | Tree stump/leaf litter | 13–17.8 | 6.6–12.4 | 53.3–68.2 |
Peer Review under the responsibility of Universidad Nacional Autónoma de México.