The aims of this research are to identify and describe a periphyton community of thermophilic microalgae in order to expand our knowledge on biodiversity of a particular environment. Conspicuous biomass of thermophilic microalgae (48°C) inhabits the cooling towers of the thermoelectric power plant of Villa de Reyes (Central Mexico). Aggregate samples or microalgal mats were taken in three different areas of the top of a cooling tower, for identification. According to the sequencing analysis of 16S and 18S rDNA genes, the community is dominated by 3 species of Cyanoprokaryota: Chlorogloeopsis fritschii, Arthronema africanum and Chroococcidiopsis sp., previously reported as thermophiles. Also, 2 species of the Chlorophyte or green algae Scenedesmus. Finally, 12 species of diatoms comprise the microalgal community; diatoms were only microscopically identified within the mats, suggesting that the mats constitute a suitable microenvironment in thermal ambiences. The identified species are of particular interest because their habitat represents an extreme and an artificial biotope. To the best of our knowledge, this is the first report of thermophilic communities of microalgae in Mexico from a power plant; also, this is the first report of A. africanum for the country.
Esta investigación tiene por objetivo identificar y describir la comunidad perifítica de microalgas termófilas, para expandir nuestro conocimiento de la biodiversidad en ambientes particulares, como las microalgas termófilas (48°C) que crecen de manera conspicua en la zona superior de la torre de enfriamiento de la central termoeléctrica de Villa de Reyes (centro de México). Se tomaron muestras de agregados o tapetes microalgales en 3 zonas distintas de la parte superior de una torre de enfriamiento, para su identificación. Una vez realizada la amplificación, la clonación y el análisis de los genes que codifican para las subunidades 16S y 18S del rDNA, se observó el predominio de 3 especies de Cyanoprokaryota: Chlorogloeopsis fritschii, Arthronema africanum y Chroococcidiopsis sp., especies descritas como termófilas en trabajos previos. Además, se identificaron 2 especies de Chlorophyta (algas verdes) del género Scenedesmus y 12 especies de diatomeas; la identificación de diatomeas se realizó a partir de observaciones por microscopia electrónica de barrido. Característicamente, las diatomeas solo se observaron dentro los densos tapetes algales que se conforman, sugiriendo que estos tapetes constituyen un microambiente conveniente en ambientes térmicos. Las especies identificadas son de particular interés, ya que su hábitat representa un biotopo extremo y artificial. Por lo que sabemos, este trabajo constituye el primer registro de microalgas termófilas que habitan en torres de enfriamiento y Arthronema africanum se documenta por primera vez para México.
The importance of expanding our knowledge on biodiversity and identifying and characterizing microorganisms, from extreme environments, responds to the social, scientific and technological requirements worldwide (Connolly et al., 2011). This knowledge could serve to develop new and sustainable technologies to obtain food, nutritional supplements, medicines, biofuels, fibers, biopolymers, colorants or other biomaterial, and for bioremediation and biorestoration tasks.
Temperature is one of the main factors determining the distribution and abundance of species due to its effects on enzymatic activities (Aguilera, Souza-Egipsy, & Amils, 2012). Therefore, thermophilic algae have thermal tolerant molecules that constitute their cells, while their metabolism is based on thermostable enzymes (Singleton & Amelunxen, 1973). A good example of a biotechnological use of thermophilic algae is the bioethanol production (Li, Du, & Liu, 2008) and the obtaining of poly-β-hydroxybutyrate from Synechococcus spp., a compound used to degrade plastics (Nishioka et al., 2002), among other biorefinery products. Another interesting suggestion comes from Ramachandra, Mahapatra, and Gordon (2009): where thermophilic diatoms that harbor symbiotic nitrogen-fixing Cyanoprokaryota for use in solar panels subject to solar heating.
It is well known that thermal ecosystems support microalgal communities dominating total ecosystem biomass and productivity (Aguilera et al., 2012; Hindák, Kvíderová, & Lukavský, 2013; Jonker, van Ginkel, & Olivier, 2013; Nikulina & Kociolek, 2011; Reisser, 2013; Stockner, 1967). Since 1969, Castenholz comments that thermal pollution from the water-coolant of power plants (nuclear and conventional) promoted microalgae growth. Previous reports indicated the presence of thermophilic microalgae in thermoelectric power plants, as those overgrowing on the concrete walls of a cooling tower at 4 different plants in the Czech Republic (Hauer, 2010) or in Belchatów, central Poland (Hindák, Wo¿owski, & Hindáková, 2011), as well as in the woody structure of cooling towers of the thermoelectric power plant of Villa de Reyes in San Luis Potosí (Central Mexico), at 48⿿50°C and pH 7.5 (Covarrubias, 2011).
The cooling towers are used to remove the heat from water via their partial vaporization through a heat-exchanger, via convective heat transfer with dry and cold air. Because the towers are exposed to solar radiation, their woody structures are colonized by a vast biomass of thermophilic microalgae. The presence of microalgae is also explained because the cooling systems are supplied with wastewater from the city of San Luis Potosí. The thermoelectric power plant aims to design a cooling pond for heat rejection as an algae bioreactor pond with the algae that inhabit the top of the cooling towers (sensuLeffler, Bradshaw, Groll, & Garimella, 2012), because (1) these algae are removed periodically in order to prevent clogging of filter systems of the water recycler; (2) the algae may fix CO2 from flue gases, and (3) they seek to use the biomass generated, as biofuel or biofertilizer. Therefore, the objective of this work was to identify the thermophilic microalgae colonizing the top of a cooling tower, a thermal and human-made ambience. We are interested in these conspicuous microorganisms and are removed periodically from the cooling towers, as they may be used in an algae bioreactor pond.
Materials and methodsMicroalgae and water samples were taken in the middle of the Summer (August 2009), in one of the cooling towers (Fig. 1a) of the thermoelectric power plant of Villa de Reyes in Central Mexico (21°48⿲19⿳ N, 100°56⿲00⿳ W). The thermoelectric plant is located in a high-altitude zone of the semiarid Mexican Plateau (1,820m.a.s.l.).
The cooling tower in the thermoelectric power plant of Villa de Reyes, San Luis Potosí, Central Mexico (a). The samples of biofilms composed by microalgae were taken at the top of the tower (b). Floor of the cancels in the top of the tower with microalgal mats, showing the turbulence of the water (c).
The sampled microalgae were growing as abundant and dense brown-green mats, loosely attached to the wooden floor (Fig. 1b) on the top of a cooling tower, with quite constant temperature and considerably water turbulence (Fig. 1c). Three samples of microalgae mats were collected with a spatula; the mats were submerged and attached to the wooden structure of the cooling tower. The samples were mixed to obtain one mixed sample, which was finally transferred to 2 glass flasks of 250mL, previously acid rinsed (24h in 15% HNO3) and sterilized. In the laboratory, a flask with the mixed sample of microalgae was used for microscopic observation, and the second flask was stored at ⿿70°C until use for total DNA extraction. A sample of the water (500mL) was also collected per triplicate, in the same site where the microalgae were taken. The water samples were transported to the laboratory in a cooler and kept refrigerated until the chemical analysis. In the sampling site, pH (pHmeter Orion 3STAR BenchTop), temperature and light intensity (Photometer EXTECH, 401027) were recorded.
The water samples were prepared for their chemical analysis, which includes the concentration of soluble sulfates (SO42⿿) by turbidimetry, carbonates (CO32⿿) and bicarbonates (HCO3⿿) by titration, nitrites (NO2⿿) and nitrates (NO3⿿) by spectrophotometry (Shimadzu, model UV-2501 PC); biochemical and chemical oxygen demand (BOD and DOQ) were obtained using the Winkler and digestion in close flux methods, respectively. The analyses were done according the Mexican Norm NOM-001-Semarnat (1996). Sodium (Na), calcium (Ca), and potassium (K) were also quantified in the water samples using an atomic absorption spectroscopy (Perkin Elmer 2380). Previously, the water samples were filtered with a 0.45-μm pore size membrane (cellulose). All the analyses were done in the Laboratory of Environmental Engineering (Faculty of Chemistry, UASLP), per triplicate.
DNA extraction, PCR amplification and phylogenetic analysisThe harvested samples were vacuum dried, frozen in liquid nitrogen, and ground to a fine powder using a mortar and pestle under liquid nitrogen. Total DNA extraction from microalgae was performed using harsh lyses method described by Gabor, de Fries, and Janssen (2003) and Casas-Flores, Gómez-Rodríguez, and García-Meza (2015) with minor modification. Samples were centrifuged at 3,000rpm during 15min, in order to concentrate the biomass. 150μL of mat sample was suspended in 750μL of lysis buffer (100mM Tris pH 8.0, 100mM EDTA pH 8.0, 1.5M NaCl, 1% CTAB) containing 2.5mg/mL of lysozyme, 1mg/mL of proteinase K and 0.7g of 0.1-mm-diameter zirconium beads; afterward, the suspension was incubated at 37°C for 8min. Samples were mixed in a mini-bead beater at top speed for 8min in the presence of 200μL SDS at 20% (w/v), incubated at 65°C for 2h mixing every 30min. The obtained unique sample was centrifuged for 10min, and the buttons were washed with 500μL of lysis buffer, mixed, incubated at 65°C for 10min and centrifuged; afterward, 24:1 chloroform: isoamyl alcohol was added, and the mixture was centrifuged for 10min. Nucleic acids were precipitated from aqueous phase with 0.6 volume of isopropanol and incubated at 4°C overnight. The DNA pellet was then washed with 70% ethanol and suspended in TE buffer 1ÿ (pH 8).
Total DNA was preserved at ⿿20°C, and this was used as template for PCR amplification of the 16S and 18S rDNA, using 1μL of total DNA for a final volume of 25μL. For 16S, oligonucleotides 533F (5⿲-GAGTTTGATC/TA/CTGGCTCAG-3⿲) and 1492R (5⿲-CGGC/TTACCTTGTTACGAC-3⿲) were used to amplify a 950-bp region of the 16S rDNA gene (Bond, Smriga, & Banfield, 2000). The reaction mixture containing, 1ÿ PCR buffer, 200μM of dNTPs, 1μM of each forward and reverse primers, and 1.25U of Taq polymerase (Promega). Negative controls with water were included. Touch gene gradient or TC-2720 thermocycler (Applied Biosystems) was used to incubate reactions with an initial denaturizing step at 94°C during 2min, followed by 30 cycles at 94°C for 1min, 60°C for 1min and 72°C for 1:30min and completed with an extension at 72°C per 7min. PCR products were resolved on a 1.5% agarose gel. Amplified PCR product was directly cloned into pGEM-T Easy (Promega) according to the manufacturer's instructions, Escherichia coli Top-10F⿿cells prepared for transformation and recombinants were selected with Luria-Bertani agar plates, containing 0.1mg/mL of carbenicillin, and incubated at 37°C for 18h. Recombinant plasmids were extracted as described by Birnboim and Doly (1979), and recombinant plasmids were purified with Kit Wizard Sv Gel and PCR clean-up system. Finally, amplicons were sequenced according to Sanger, Nicklen, and Coulson (1977).
For the rDNA 18S, we used the universal eukaryote primers 82F (5⿲-GAAACTGCGAATGGCTC-3⿲) Proto/5R (5⿲-GACGGGCGGTGTGTAC-3⿲) (Auinger, Pfandl, & Boenigk, 2008) amplified in a PCR as shown in fingerprints. Touchgene gradient or TC-2720 thermocycler (Applied Biosystems) was used to incubate reactions with an initial denaturizing step at 94°C for 5min; 35 cycles at 94°C for 30s (lowering 0.2°C each cycle) for 30s and 72°C for 1min, and completed with an extension at 72°C per 7min. From here on, the samples were treated as described in 16S rDNA genes.
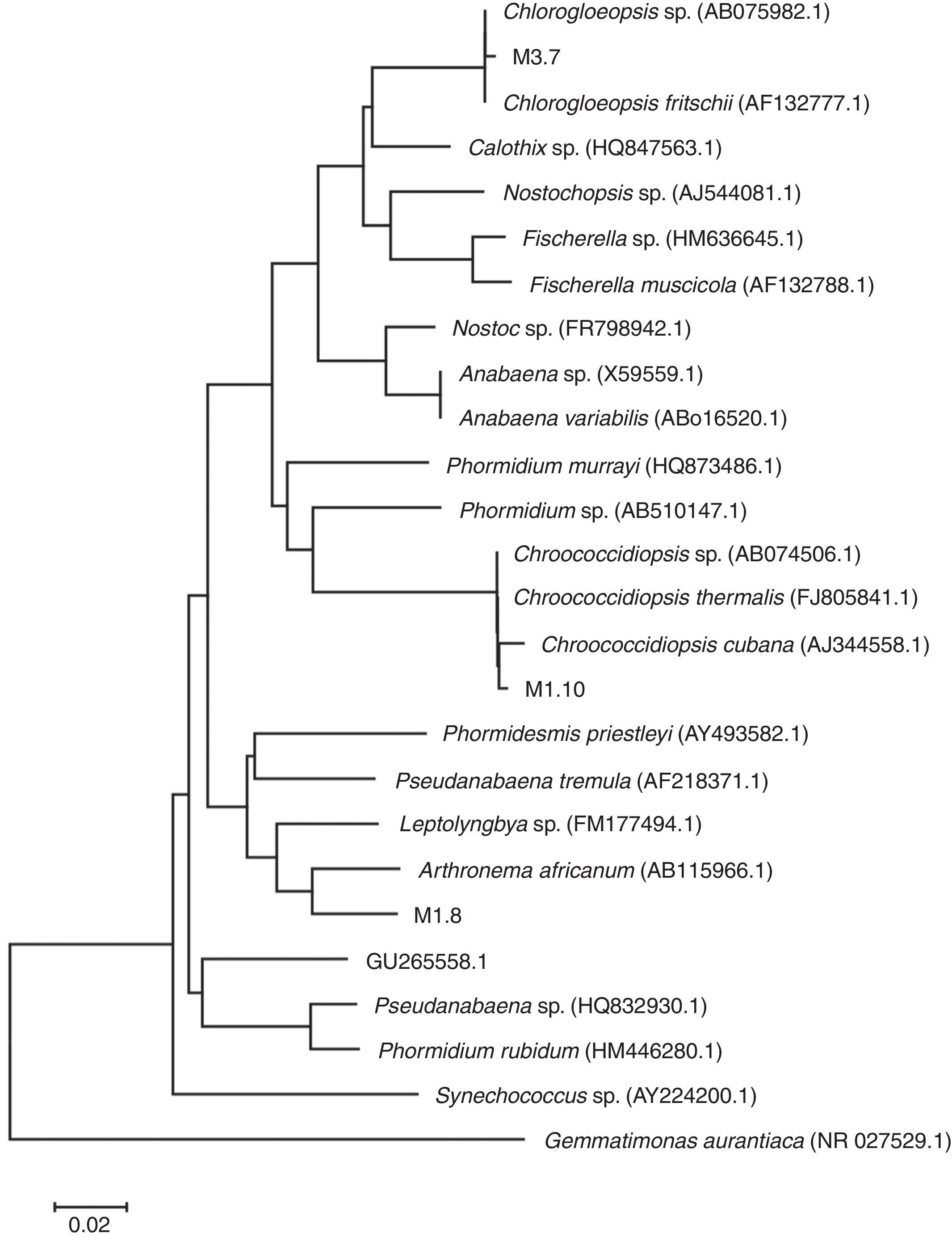
The phylogenetic relationships were determined by comparing their 16S or 18S rDNA sequences against those of NCBI (http://blast.ncbi.nlm.nih.gov/) database, using the BLAST program. The 16S rDNA sequences obtained from NCBI were aligned with those obtained in this work using the MEGA5 software (version 5.1) (Tamura, Stecher, Peterson, Filipsk, & Kumar, 2013) by the neighbor-joining method. Thus, the evolutionary history was inferred by using the maximum likelihood method based on the Saitou-Nei model (Saitou & Nei, 1987). The tree with the highest log likelihood (0.92306470) is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura, Nei, & Kumar, 2004) and are in the units of the number of base substitutions per site. The analysis involved 26 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 786 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
The obtained sequences of this study were deposited in GenBank and their accession numbers are listed in Table 1.
Some characteristics of the identified microalgae sampled in the cooling tower in the thermoelectric power plant of Villa de Reyes.
| No. sequence designated | Taxonomic assignment (GenBank access number) | No. of clones | Percent of identity | Features from literature |
|---|---|---|---|---|
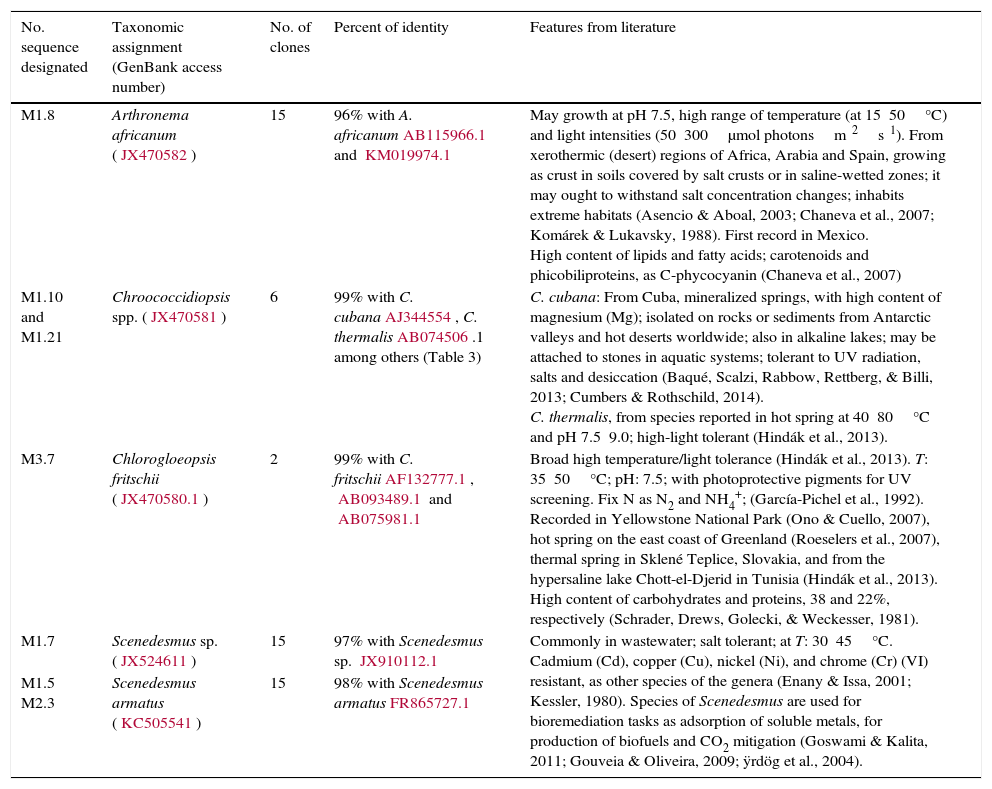
| M1.8 | Arthronema africanum (JX470582) | 15 | 96% with A. africanumAB115966.1 and KM019974.1 | May growth at pH 7.5, high range of temperature (at 15⿿50°C) and light intensities (50⿿300μmol photonsm⿿2s⿿1). From xerothermic (desert) regions of Africa, Arabia and Spain, growing as crust in soils covered by salt crusts or in saline-wetted zones; it may ought to withstand salt concentration changes; inhabits extreme habitats (Asencio & Aboal, 2003; Chaneva et al., 2007; Komárek & Lukavsky, 1988). First record in Mexico. High content of lipids and fatty acids; carotenoids and phicobiliproteins, as C-phycocyanin (Chaneva et al., 2007) |
| M1.10 and M1.21 | Chroococcidiopsis spp. (JX470581) | 6 | 99% with C. cubanaAJ344554, C. thermalisAB074506.1 among others (Table 3) | C. cubana: From Cuba, mineralized springs, with high content of magnesium (Mg); isolated on rocks or sediments from Antarctic valleys and hot deserts worldwide; also in alkaline lakes; may be attached to stones in aquatic systems; tolerant to UV radiation, salts and desiccation (Baqué, Scalzi, Rabbow, Rettberg, & Billi, 2013; Cumbers & Rothschild, 2014). C. thermalis, from species reported in hot spring at 40⿿80°C and pH 7.5⿿9.0; high-light tolerant (Hindák et al., 2013). |
| M3.7 | Chlorogloeopsis fritschii (JX470580.1) | 2 | 99% with C. fritschiiAF132777.1, AB093489.1 and AB075981.1 | Broad high temperature/light tolerance (Hindák et al., 2013). T: 35⿿50°C; pH: 7.5; with photoprotective pigments for UV screening. Fix N as N2 and NH4+; (García-Pichel et al., 1992). Recorded in Yellowstone National Park (Ono & Cuello, 2007), hot spring on the east coast of Greenland (Roeselers et al., 2007), thermal spring in Sklené Teplice, Slovakia, and from the hypersaline lake Chott-el-Djerid in Tunisia (Hindák et al., 2013). High content of carbohydrates and proteins, 38 and 22%, respectively (Schrader, Drews, Golecki, & Weckesser, 1981). |
| M1.7 | Scenedesmus sp. (JX524611) | 15 | 97% with Scenedesmus sp. JX910112.1 | Commonly in wastewater; salt tolerant; at T: 30⿿45°C. Cadmium (Cd), copper (Cu), nickel (Ni), and chrome (Cr) (VI) resistant, as other species of the genera (Enany & Issa, 2001; Kessler, 1980). Species of Scenedesmus are used for bioremediation tasks as adsorption of soluble metals, for production of biofuels and CO2 mitigation (Goswami & Kalita, 2011; Gouveia & Oliveira, 2009; ÿrdög et al., 2004). |
| M1.5 M2.3 | Scenedesmus armatus (KC505541) | 15 | 98% with Scenedesmus armatusFR865727.1 |
The sampled microalgae were observed in an optical microscopy Leica (DME) and Olympus (BX51). Observations of the sampled microalgae by light microscopy were done with a microscope Olympus, in order to describe them.
Diatoms, were also analyzed by scanning electron microscopy (SEM, Philips XL30), and specialized literature (Krammer & Lange-Bertalot, 1986, 1988, 1991), in order to identify them since the described method for DNA extraction was not useful for the cell disruption diatoms due to the silicic nature of the frustule. Diatoms were previously cleansed via thoroughly oxidizing organic material from the wall and cytoplasm using a chromic mixture of nitric acid (HNO3, 20%) and potassium dichromate (K2CrO7), according to Johansen (in García-Meza, 2009). Cleansed frustules of diatoms were placed in the sample holder and coated with gold (Au) for their observation in the SEM.
ResultsThe registered water temperature was 48±1°C at the sampling time (10⿿12h), the photic zone attains 11.5cm and the light intensity varies with depth between 32.0 and 102.9μmol photons m⿿2s⿿1 (at 11.5 and 0cm depth, respectively).
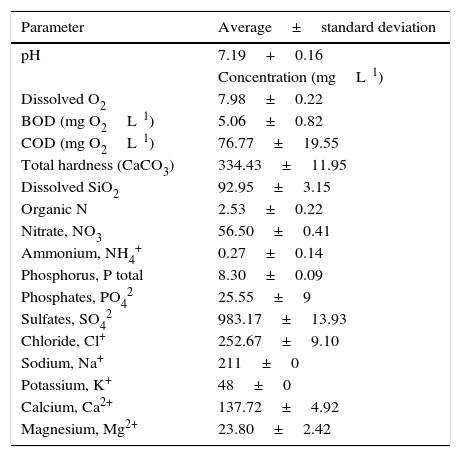
According to the chemical characteristics of the sampled water (Table 2), the ambience may be suitable to neutrophile (pH 6.9⿿7.3) and oligohalobien (salts<260mg/L) microalgae, which may use bicarbonates (HCO3⿿) and nitrates (NO32⿿) as carbon (C) and nitrogen (N) sources, respectively. The ionic dominance was SO42⿿>CO32⿿>Cl+>Ca2+>Na+>HCO3⿿>NO3⿿K+PO42⿿>Mg2+.
Some characteristics of the sampled water collected (per triplicate) in the same site where the microalgae were taken. Data: n=3.
| Parameter | Average±standard deviation |
|---|---|
| pH | 7.19+0.16 |
| Concentration (mgL⿿1) | |
| Dissolved O2 | 7.98±0.22 |
| BOD (mg O2L⿿1) | 5.06±0.82 |
| COD (mg O2L⿿1) | 76.77±19.55 |
| Total hardness (CaCO3) | 334.43±11.95 |
| Dissolved SiO2 | 92.95±3.15 |
| Organic N | 2.53±0.22 |
| Nitrate, NO3⿿ | 56.50±0.41 |
| Ammonium, NH4+ | 0.27±0.14 |
| Phosphorus, P total | 8.30±0.09 |
| Phosphates, PO42⿿ | 25.55±9 |
| Sulfates, SO42⿿ | 983.17±13.93 |
| Chloride, Cl+ | 252.67±9.10 |
| Sodium, Na+ | 211±0 |
| Potassium, K+ | 48±0 |
| Calcium, Ca2+ | 137.72±4.92 |
| Magnesium, Mg2+ | 23.80±2.42 |
BOD: biochemical oxygen demand; DOQ: chemical oxygen demand.
The highly available carbonate reacts with the CO2 from the power plant, resulting in a high bicarbonate concentration (Table 2); this buffering reaction maintains the pH of the water.
It is important to say that the water is reused 3 times in the cooling-cycle; afterwards, the water is thrown out because it increases the concentration of contained salts; the water taken during the sampling days corresponds to the first cycle, with the lowest salt and organic matter concentration, but with high concentrations of CaCO3 (>438mg/L) (Table 2).
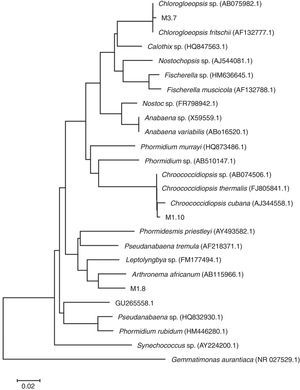
Based on their morphological description (Table 4) and their 16S rRNA sequences, the microalgae include 3 genera of Cyanoprokaryota (Fig. 2; Table 1): non-heterocytes N2-fixing Chroococcidiopsis (Pleurocapsal), a non-heterocytous filamentous Arthronema (Oscillatorial), 96% identical to A. africanum (Schwabe & Simonsen) Komárek & Lukavsky (NCBI AB115966.1 and KM019974.1), and a heterocytous Chlorogloeopsis (Nostocal), 99% identity with C. fritschii (A.K. Mitra) A.K. Mitra & D.C. Pandey (NCBI AF132777.1, AB093489.1, and AB075981.1). However, 6 cloned sequences of Chroococcidiopsis sp. JX470581 produced significant alignments and showed 99% identity with the 16S RNA genes of clones designated M1.10 and M1.21 in this work (Table 3).
Phylogenetic tree showing relationship of the sequences found in our samples to representative Cyanoprokaryota16S rDNA genes. Sequences of the cyanobacteria were retrieved from GenBank. The evolutionary history was inferred using the neighbor-joining method. The optimal tree with the sum of branch length=0.92306470 is shown. Out group: Gemmatimonas aurantiaca.
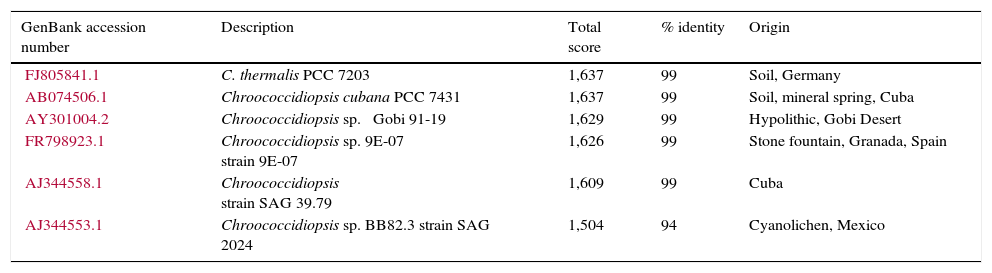
Sequences of Chroococcidiopsis sp. JX470581 producing significant alignments with gene for 16S ribosomal RNA designated M1.10 and M1.21 (from www.ncbi.nlm.nih.gov/genbank).
| GenBank accession number | Description | Total score | % identity | Origin |
|---|---|---|---|---|
| FJ805841.1 | C. thermalis PCC 7203 | 1,637 | 99 | Soil, Germany |
| AB074506.1 | Chroococcidiopsis cubana PCC 7431 | 1,637 | 99 | Soil, mineral spring, Cuba |
| AY301004.2 | Chroococcidiopsis sp. ⿿Gobi 91-19⿿ | 1,629 | 99 | Hypolithic, Gobi Desert |
| FR798923.1 | Chroococcidiopsis sp. 9E-07 strain 9E-07 | 1,626 | 99 | Stone fountain, Granada, Spain |
| AJ344558.1 | Chroococcidiopsis strain SAG 39.79 | 1,609 | 99 | Cuba |
| AJ344553.1 | Chroococcidiopsis sp. BB82.3 strain SAG 2024 | 1,504 | 94 | Cyanolichen, Mexico |
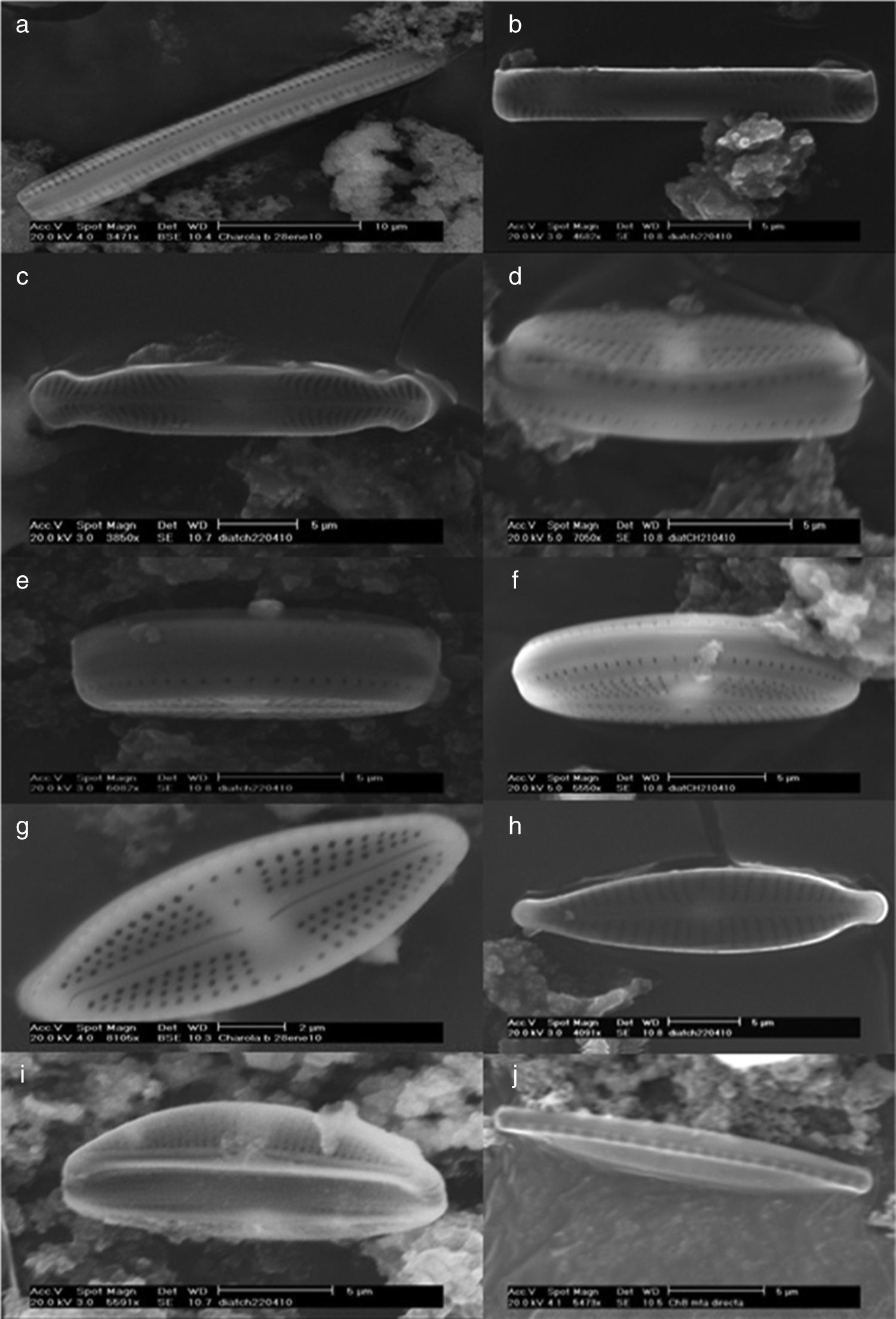
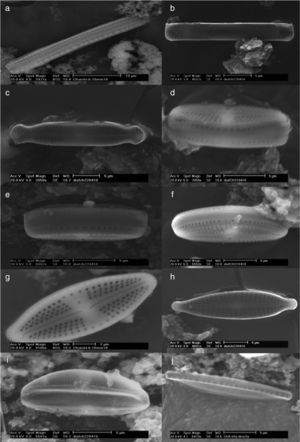
Eukaryotic microalgae are represented by 2 chlorophytes Scenedesmus spp. JX524611, and Scenedesmus armatusKC505541 (R. Chodat) R. Chodat (Tables 1 and 4) and 12 species of diatoms (Fig. 3, Table 4).
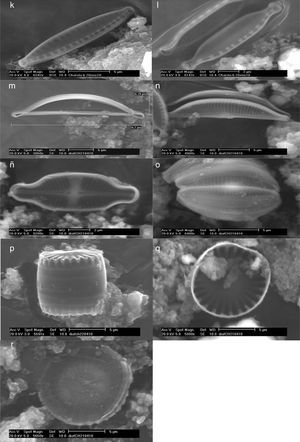
Thermophilic diatoms observed at SEM. Nitzschia sp., cingulum (a); Pinnularia latarea var. thermophila (b and c); Luticula goeppertiana, cingulum (d, e, and f) and valvar view (g); Gomphonema parvulum (h); Amphora ovalis (i); Nitzschia hantzchiana, cingulum (j) and valvar view (k); Nitzschia microcephala (l); Amphora veneta, internal (m) and valvar (n) views; Achnanthidium exiguum (ñ); Amphora pediculus (o), Stephanocyclus menenghiniana, cingular (p) and internal (q) views; Stephanodiscus sp. (r).
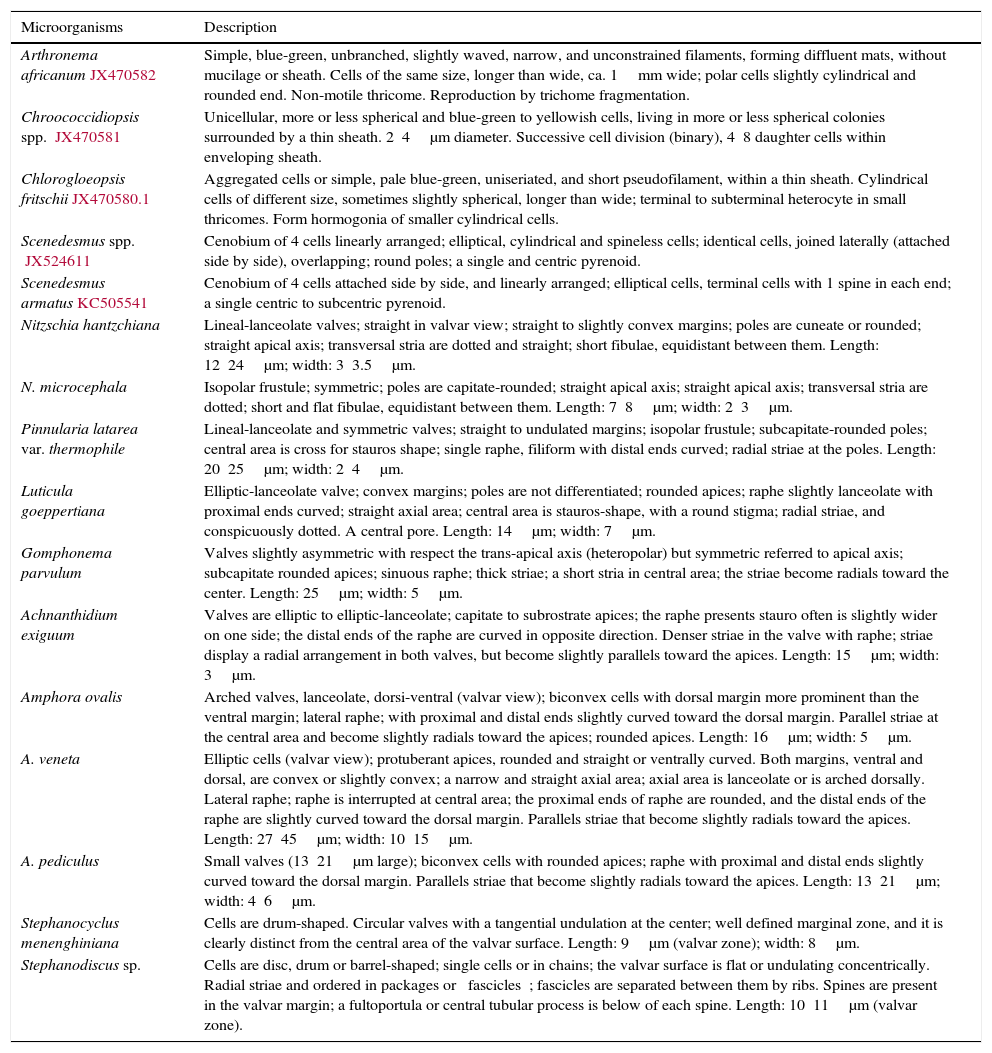
Morphological descriptions of the identified microalgae from the cooling tower in the thermoelectric power plant of Villa de Reyes.
| Microorganisms | Description |
|---|---|
| Arthronema africanumJX470582 | Simple, blue-green, unbranched, slightly waved, narrow, and unconstrained filaments, forming diffluent mats, without mucilage or sheath. Cells of the same size, longer than wide, ca. 1mm wide; polar cells slightly cylindrical and rounded end. Non-motile thricome. Reproduction by trichome fragmentation. |
| Chroococcidiopsis spp. JX470581 | Unicellular, more or less spherical and blue-green to yellowish cells, living in more or less spherical colonies surrounded by a thin sheath. 2⿿4μm diameter. Successive cell division (binary), 4⿿8 daughter cells within enveloping sheath. |
| Chlorogloeopsis fritschiiJX470580.1 | Aggregated cells or simple, pale blue-green, uniseriated, and short pseudofilament, within a thin sheath. Cylindrical cells of different size, sometimes slightly spherical, longer than wide; terminal to subterminal heterocyte in small thricomes. Form hormogonia of smaller cylindrical cells. |
| Scenedesmus spp. JX524611 | Cenobium of 4 cells linearly arranged; elliptical, cylindrical and spineless cells; identical cells, joined laterally (attached side by side), overlapping; round poles; a single and centric pyrenoid. |
| Scenedesmus armatusKC505541 | Cenobium of 4 cells attached side by side, and linearly arranged; elliptical cells, terminal cells with 1 spine in each end; a single centric to subcentric pyrenoid. |
| Nitzschia hantzchiana | Lineal-lanceolate valves; straight in valvar view; straight to slightly convex margins; poles are cuneate or rounded; straight apical axis; transversal stria are dotted and straight; short fibulae, equidistant between them. Length: 12⿿24μm; width: 3⿿3.5μm. |
| N. microcephala | Isopolar frustule; symmetric; poles are capitate-rounded; straight apical axis; straight apical axis; transversal stria are dotted; short and flat fibulae, equidistant between them. Length: 7⿿8μm; width: 2⿿3μm. |
| Pinnularia latarea var. thermophile | Lineal-lanceolate and symmetric valves; straight to undulated margins; isopolar frustule; subcapitate-rounded poles; central area is cross for stauros shape; single raphe, filiform with distal ends curved; radial striae at the poles. Length: 20⿿25μm; width: 2⿿4μm. |
| Luticula goeppertiana | Elliptic-lanceolate valve; convex margins; poles are not differentiated; rounded apices; raphe slightly lanceolate with proximal ends curved; straight axial area; central area is stauros-shape, with a round stigma; radial striae, and conspicuously dotted. A central pore. Length: 14μm; width: 7μm. |
| Gomphonema parvulum | Valves slightly asymmetric with respect the trans-apical axis (heteropolar) but symmetric referred to apical axis; subcapitate rounded apices; sinuous raphe; thick striae; a short stria in central area; the striae become radials toward the center. Length: 25μm; width: 5μm. |
| Achnanthidium exiguum | Valves are elliptic to elliptic-lanceolate; capitate to subrostrate apices; the raphe presents stauro often is slightly wider on one side; the distal ends of the raphe are curved in opposite direction. Denser striae in the valve with raphe; striae display a radial arrangement in both valves, but become slightly parallels toward the apices. Length: 15μm; width: 3μm. |
| Amphora ovalis | Arched valves, lanceolate, dorsi-ventral (valvar view); biconvex cells with dorsal margin more prominent than the ventral margin; lateral raphe; with proximal and distal ends slightly curved toward the dorsal margin. Parallel striae at the central area and become slightly radials toward the apices; rounded apices. Length: 16μm; width: 5μm. |
| A. veneta | Elliptic cells (valvar view); protuberant apices, rounded and straight or ventrally curved. Both margins, ventral and dorsal, are convex or slightly convex; a narrow and straight axial area; axial area is lanceolate or is arched dorsally. Lateral raphe; raphe is interrupted at central area; the proximal ends of raphe are rounded, and the distal ends of the raphe are slightly curved toward the dorsal margin. Parallels striae that become slightly radials toward the apices. Length: 27⿿45μm; width: 10⿿15μm. |
| A. pediculus | Small valves (13⿿21μm large); biconvex cells with rounded apices; raphe with proximal and distal ends slightly curved toward the dorsal margin. Parallels striae that become slightly radials toward the apices. Length: 13⿿21μm; width: 4⿿6μm. |
| Stephanocyclus menenghiniana | Cells are drum-shaped. Circular valves with a tangential undulation at the center; well defined marginal zone, and it is clearly distinct from the central area of the valvar surface. Length: 9μm (valvar zone); width: 8μm. |
| Stephanodiscus sp. | Cells are disc, drum or barrel-shaped; single cells or in chains; the valvar surface is flat or undulating concentrically. Radial striae and ordered in packages or ⿿fascicles⿿; fascicles are separated between them by ribs. Spines are present in the valvar margin; a fultoportula or central tubular process is below of each spine. Length: 10⿿11μm (valvar zone). |
The identified diatoms are Nitzschia hantzchiana Rabenhorst, N. microcephala Grunow, N. sp., Pinnularia latarea var. thermophile K. Krammer, Luticula goeppertiana (Bleisch) Mann, Gomphonema parvulum (Kützing) Kützing, Achnanthidium exiguum (Grunow) D. B. Czarnecki, Amphora ovalis (Kützing) Kützing, A. veneta Kützing, and A. pediculus (Kützing) Grunow ex A. Schmidt (Order Pennales), as well as Stephanocyclus menenghiniana (Kützing) Skabitschevsky and Stephanodiscus sp. (Order Centrales).
DiscussionThis work represents the first report of a thermophilic community of microalgae from a power plant in Mexico. It is an extreme human-made ambience, whose principal characteristic is the high temperature of the water used in the cooling tower (48⿿50°C). In extreme environments, phototrophic microorganisms are frequently found forming thick microbial mats (Aguilera et al., 2012; Tuchman & Blinn, 1979). Also, at the top of the studied cooling tower, the microalgae were growing as conspicuous and dense brown-green mats (Fig. 1b), mainly composed by filamentous microalgae of about 1.5⿿2cm width; such mats covered ca. 70% of the wooden floor, to which these were strongly adhered due to the continued water flux, (Fig. 1c). However, the sampling was done during the first cycle when the extent and thickness of mats was the lowest: the extent of microalgal mats changes according to water management changes, since this management alters the amount and proportion of nutrients; e.g. the water comprises 50 and 300mgL⿿1 of SiO2 and 11 and 35mgL⿿1 of phosphates in the first and third cycle, respectively.
Despite the conspicuousness of microalgal mats, the microscopically observed mats showed low species richness compared with other reports of thermal regions, as well as previous research on cooling towers in the Czech Republic, and in central Poland, wherein the algae grow on the concrete walls, as a result of sprayed water (Hauer, 2010; Hindák et al., 2011); although the algal growth in the cooling tower may be primarily due to the CO2 emanated by the power plant, as was suggested by Edwards (2008) and Douskova et al. (2009). The low species richness may be explained as consequence of the changeability of this human-made ambience; e.g. water turbulence amends constantly the quality and quantity of light; in contrast, most thermal pools are clear and usually shallow, and show low radiation extinction and have remarkably constant chemical composition and temperature (Castenholz, 1969).
The identified microalgae include 3 species of Cyanoprokaryota, previously reported as thermophilic (Chaneva, Furnadzhieva, Minkova, & Lukavsky, 2007; Hindák et al., 2013). Arthronema africanumJX470582 was the most common Cyanoprokaryota in the sampled mats (microscopic observation). A. africanum may grow under a wide range of temperatures and light intensities (Table 1) due to its protective mechanism against high irradiation intensity, e.g. contents of phycobiliproteins and carotenoids increase with temperature and light intensity (Asencio & Aboal, 2003). Furthermore, A. africanum has a pantropical distribution (Hoffmann, 1996).
Species of the genus Chlorogloeopsis have been identified as thermophiles, with broad tolerance to light intensity (Hindák et al., 2013), also due to photoprotective pigments (García-Pichel, Sherry, & Castenholz, 1992; Ono & Cuello, 2007). For this genus, our phylogenetic analysis of 16S rRNA gene sequences affiliated with Chlorogloeopsis fritschiiAF132777.1 and AB093489.1 (95% identity); the latter has already been described in thermal environments such as in hot springs of Greenland, at 49°C, for the first time by Roeselers et al. (2007).
The third Cyanoprokaryota identified was Chroococcidiopsis spp. JX470581. Some Chroococcidiopsis can survive under extreme temperatures (as C. thermalis in thermal springs), high levels of radiation, and high concentrations of salt, (like C. cubana, from mineralized springs). The clones designated M1.10 and M1.21 in this work produced significant alignments and showed 99% identity with the 16S RNA genes of 5 sequences of Chroococcidiopsis from different geographical origin and life-strategy: C. thermalis PCC 7203 (soil, Germany). C. cubana PCC 7431 (soil, Cuba), C. sp. ⿿Gobi 91-19⿿ (hypolithic, Gobi Desert), C. sp. 9E-07 (on stone fountain, Spain) (Table 3). Moreover, it showed 95% identity with a Chroococcidiopsis, C. sp. BB82.3 strain SAG 2024 (cyanolichen) from Mexico. The latter suggests that strains from very different geographical regions and habitat types are closely related, especially those from soil and aquatic-living strains from lichenized and rock associations (Donner, 2013). Furthermore, C. cubana and C. thermalis are highly similar based on highly conserved genes as 16S rDNA; however, both species showed differences in their ecology (Table 1; Donner, 2013).
A few and early reports of thermophilic strains of Scenedesmus were presented by Trukhin (1963) and Kessler (1980), while few diatoms are considered thermophilic, tolerating temperatures above 40°C (Stockner, 1967), and most of them are benthic, as the 232 species, varieties, and forms of diatoms from 10 hot springs and reservoirs of Sakhalin and Kuril Islands, where temperatures may reach ca. 70°C (Nikulina and Kociolek, 2011). In the cited work, the main represented genera are Pinnularia and Nitzschia, as in the thermo-mineral waters of Katlanovska Banja (Stavreva-Veselinovska & Todorovska, 2010) and Sao Miguel Island, Azores (Quintela et al., 2013). Meanwhile in our work, Nitzschia is the best represented while Pinnularia latarea var. thermophila and Nitzschia hantzschiana are the most common. Among the identified diatoms in this work, Achnanthidium exiguum, Gomphonema parvulum, Amphora ovalis, A. veneta and A. pediculus, have been previously reported as thermophiles, at 40⿿58°C (Mannino, 2007; Nikulina & Kociolek, 2011; Quintela et al., 2013; Stavreva-Veselinovska & Todorovska, 2010). Unlike the aforementioned studies, in the current study the diatoms were only microscopically observed within the algal mats. It is well known that diatoms acclimate better to low temperature and lower light intensity than Cyanoprokaryotas and Chlorophytes. Thus, the presence of diatoms in our sample may be also due to mucilage-producing Cyanoprokaryota, e.g. Chroococcidiopsis spp.; i.e., the surrounding mucilaginous matrix represents a suitable microenvironment for diatoms. Some species avoid high light intensity and UV light by living within soil or caves, as photobionts in lich symbiosis or within biological soil crust or dense mats or biofilms (Donner, 2013).
The scientific interest in thermophilic microalgae responds to the following facts: (1) there are considerable amount of taxa not yet described that may thrive up to 50°C; (2) they could very well represent ancient forms related to the origin and evolution of the oxygenic photosynthesis and nitrogen (N2) fixing capabilities of Cyanoprokaryota, and (3) they have potential uses as biomaterials or in diverse biotechnologies at high temperatures (Castenholz, 1969). A myriad of applications of the identified algae has been launched (Table 1), such as the power plant that aimed to use the biomass generated as biofuel or biofertilizer. Specifically, Scenedesmus quadricauda and the thermophile Chlorogloeopsis spp. have been suggested by Ono and Cuello (2007) and Goswami and Kalita (2011), respectively, for use in a CO2-mitigating photobioreactor, as is desired by the thermoelectric power plant of Villa de Reyes.
This work expands our knowledge about the environments that may be inhabited or colonized by the microalgae, as thermal (48°C) and human-made ambience. The achievement of significant biomass of microalgae deeply depends on the availability and intensity of sunlight, water, CO2, and nutrients. All these elements occur in great extent in the cooling towers of the thermoelectric power plant. The screening for species of microalgae must continue to prospect the uses to regional needs, especially now, in a time when we face a crisis of energetic, food and environmental issues. Specifically, we suggest the use of the microalgae that grow naturally in the cooling towers, as inoculum for the algae pond that the thermoelectric power plant devised in order to obtain biofuels or biofertilizer.
To the best of our knowledge, this is the first report about thermophilic communities of microalgae from a Mexican power plant. In addition, this is the first report of A. africanum in Mexico. This study opens new lines of research such as potential application; e.g. A. africanum cultivation to produce phycobiliproteins as colorants.
This work was supported by a Conacyt scholarship number 217539. Financial support for this work comes from the Laboratory of Geomicrobiology, UASLP, and from the División de Biología Molecular, IPICyT. The authors also thank to QFB Alma de Lira Santillán and Dr. Roberto Leyva Ramos of the Laboratory of Environmental Engineering (Faculty of Chemistry, UASLP), for the chemical analysis of the sampled water. This work was possible thanks to the thermoelectric power plant of Villa de Reyes; special thanks to Eng. Sergio Castillo-Zaragoza. Finally, the authors also thank the anonymous reviewers that improved the manuscript.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.