To assess the bacterial load in metallic and ceramic brackets and determine which favor dental plaque retention.
Material and methodsExtracted premolars divided into 2 groups and analyzed. In one group metal brackets were placed and in the other group, ceramic brackets.
ResultsStatistical analysis were performed and it was determined that there was no significant difference.
ConclusionThe type of bracket used in the orthodontic treatment, is not a determining factor in bacteria adhesion and therefore plaque accumulation as long as proper hygiene is maintained.
Evaluar la carga bacteriana en brackets metálicos y cerámicos para determinar cuáles favorecen la retención de placa dentobacteriana.
Material y métodosSe analizaron premolares extraídos, divididos en dos grupos, uno cementados con brackets metálicos y en el otro con brackets cerámicos.
ResultadosEl análisis estadístico se realizó en el software Minitab, realizando una prueba t de Student en donde se determinó que no había diferencia significativa entre grupos (0.204).
ConclusiónEl tipo de bracket utilizado en el tratamiento de ortodoncia no es un factor determinante en la adhesión de las bacterias, y por tanto la acumulación de placa dependerá de si existe o no una higiene adecuada.
Dental plaque is an heterogenous accumulation of a diverse microbial community both aerobic and anaerobic; surrounded by an extracellular matrix of polymers, microorgansims and saliva.1 After a dental cleaning, the dental enamel is covered by a variety of proteins and glyxoproteins. This lining is called acquired pellicle (biofilm) and the first colonizers are streptocci followed by lactobacillus that are commonly found over dental surfaces.
This biofilm is mainly formed by non-patogenic bacteria however due to the ingestion of sucrose and other carbohydrates, fermenting acids are produced. This leads to enamel demineralization and eventually, caries. Among the most important microorganisms are: Porphyromonas gingivalis, Bacteroides forsythus, Actynomices actinomycetemcomitans and Treponema denticola.2–5
For a long time, orthodontic patients were considered to be low-risk and the procedures involved in their treatment, non-invasive. However, the appliances used for orthodontic treatment may be associated with oral hygiene difficulty.6–8 During treatment remnant areas which stimulate biofilm production and bacterial growth are created. One of the biggest challenges in orthodontics is to maintain an adequate oral hygiene throughout treatment. The tooth area that surrounds the brackets favors bacteria adhesion and dental plaque formation. These are difficult to eliminate and regular brushing is not sufficient for removing them in retentive zones such as the one formed by the adhesive between the bracket and the gingiva.9–12 The more common complications in orthodontic treatment due to plaque accumulation are caries and periodontal disease.13–16
The fixed passive orthodontic components are brackets which serve as support for the components that produce the force. Ceramic brackets are very popular as an aesthetic alternative for orthodontic appliances in contemporary orthodontics. Ceramics are a broad type of materials that consist of metallic and non-metallic oxides which include gemstones, glasses, clays and ceramic mixtures.17,18 Metal brackets are mainly made of high-quality stainless steel; they have good bond strength and have proved to be more resilient than ceramic brackets due to their composition.6
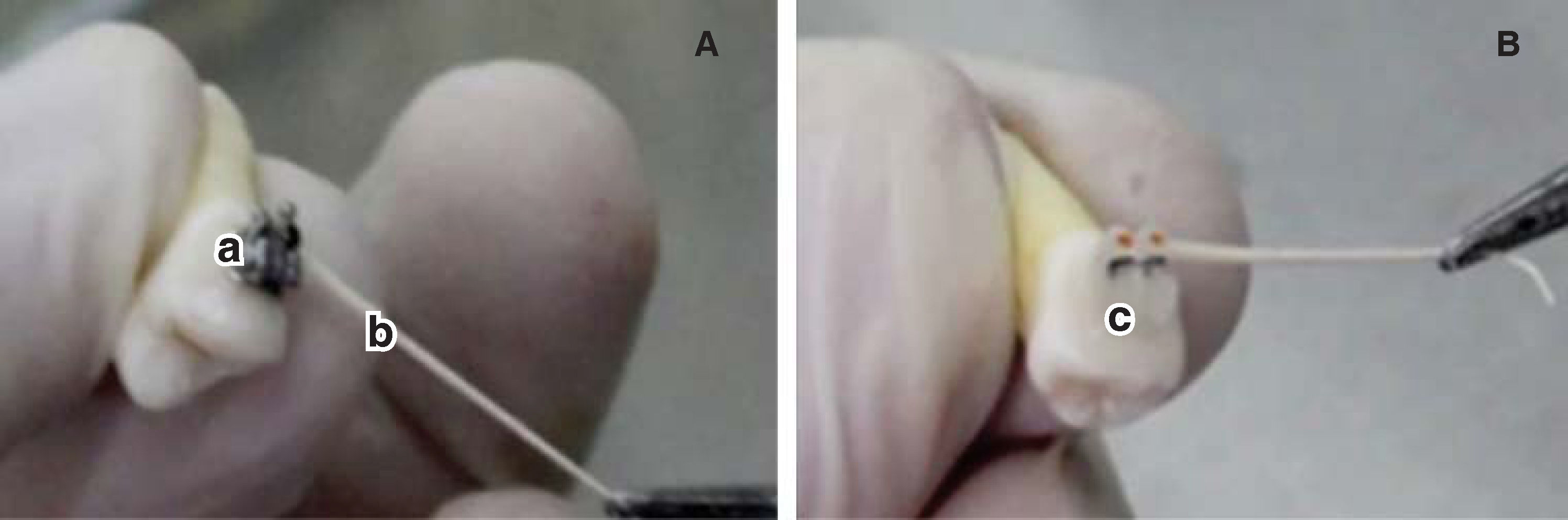
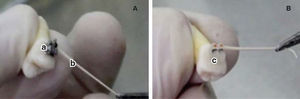
MATERIAL AND METHODSSample obtentionTwenty first premolars that had been previously removed were analyzed. They were divided into 2 groups (n = 10). Group 1 contained premolars with metal brackets bonded on them, while in group 2 ceramic brackets were bonded on the premolars (Figure 1).
Disinfection protocolThe disinfection protocol used was an ultrasonic bath. Each group was plunged into a 500mL beaker. Two solutions were used for disinfection, first 17% EDTA for 10minutes to remove the organic and inorganic matter. Subsequently, 5.25% sodium hypochlorite was used for the same amount of time in order to remove the organic matter. Between both baths, the premolars are flushed with sterile distilled water.19 Finally sample sterilization was carried with an autoclave at 121°C and 15 psi. Afterwards, the supports were cemented in the corresponding groups, Group 1: metal brackets (Ah-Kim-Pech®) and Group 2: ceramic brackets (3M Clarity®) in a laminar flow cabinet to maintain the sterility of the sample. To corroborate the sterilization process, a microbiological sample was taken from each dental component (20 in total). Using a 10μL micropipette sterile distilled water was deposited on the upper part of the bracket. On the distilled water side, three sterile # 45 paper tips (Hygienic®) were placed, each for 1minute. The first two were placed in the middle and the last was swept from one side to another. The paper points were then deposited in a test tube with 10mL of trypticase soy agar as means of transport (Figure 1). The result of the sample was negative, assessed with the McFarland scale; the sample was planted in trypticase soy agar plates where there was no microbial growth.
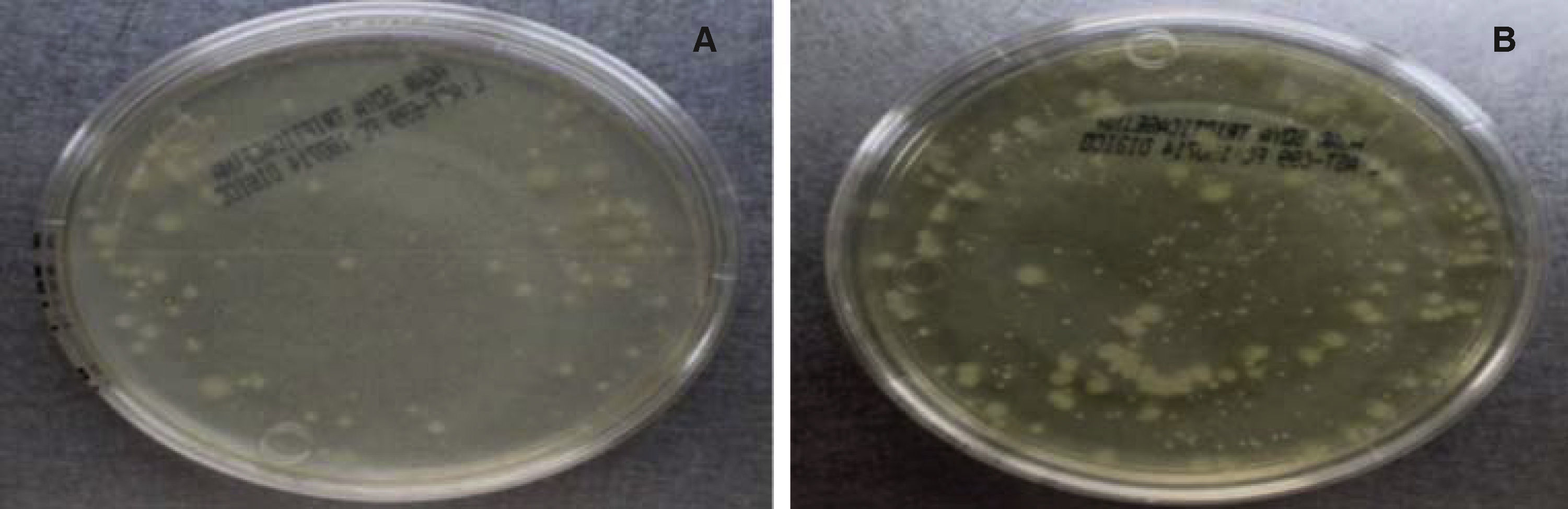
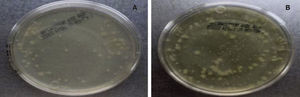
Sampling and incubationThe samples were taken from a patient who was under orthodontic treatment by using 3 sterile paper points with the method described above. They were deposited in a test tube with 10mL of trypticase soy agar and incubated for 24hours. A microbial growth with a McFarland standard of 7 was obtained; then a 0.5 McFarland standard is corresponded since this turbidity is typically found in the oral cavity. Subsequently, each tooth was placed in a test tube with 10ml of trypticase soy agar and 5 drops of the sample with bacterial growth (0.5 McFarland). Every 48hours, the Tryiticase Soy broth of each sample was changed during 10 days with the purpose of obtaining the second sample and keeping the bacteria alive. The second sample was obtained in the same manner as the first; each one was placed in 10mL of trypticase soy agar. After two hours of bacterial growth, serial dilutions were carried out between 10-1 and 10-3 in each sample. Once diluted, the sample was planted in an agar plate containing trypticase soy agar, labelled and sealed with parafilm. Afterwards, it was placed in a Felisa incubator for 24hours at a temperature of 35 ± 2°C. Then, the CFU (Colony Forming Units) count was performed. A colony counter digital pen was used. Only samples with values between 30 and 300 CFU were taken into consideration (Figure 2).20
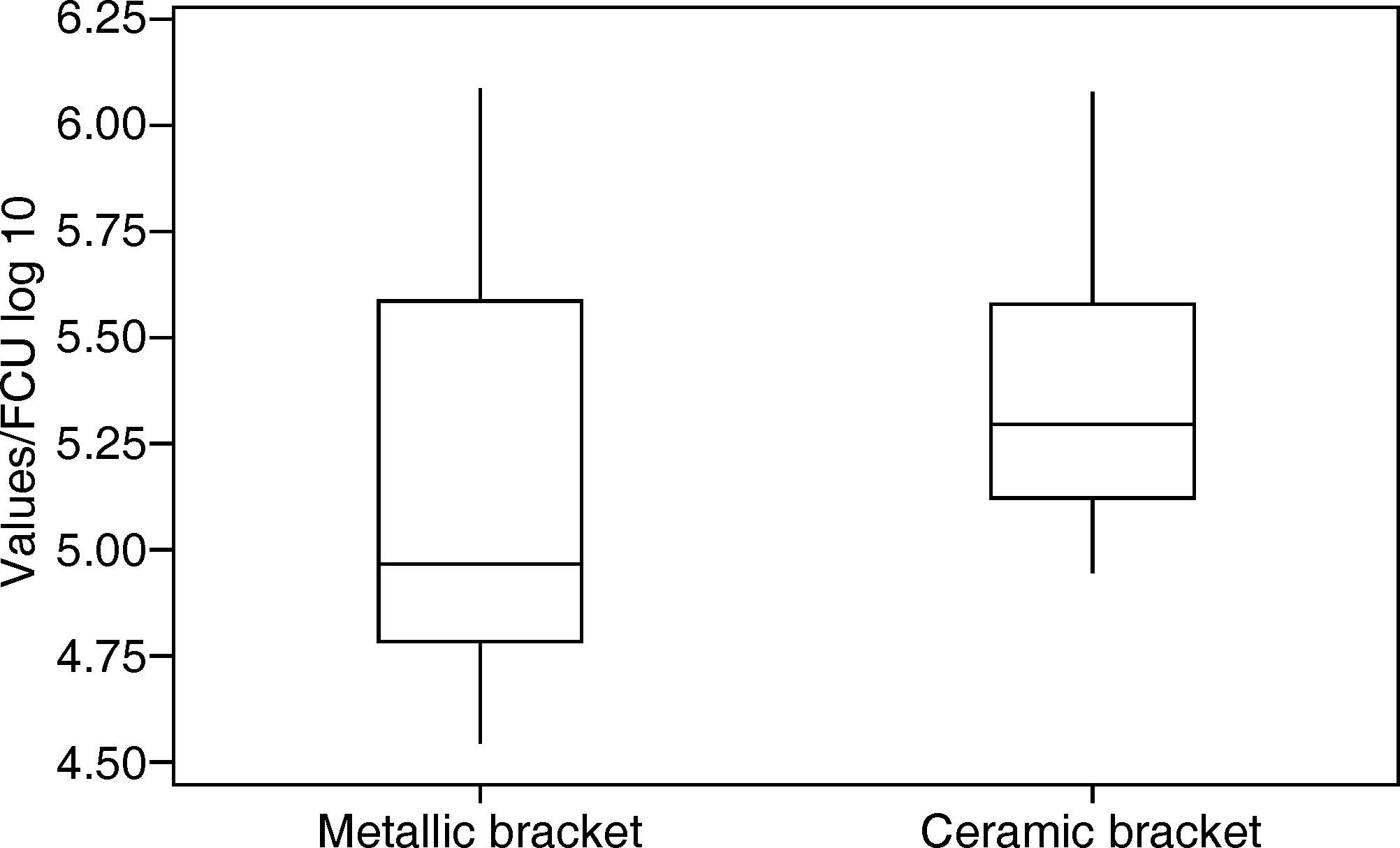
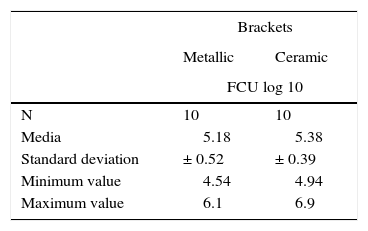
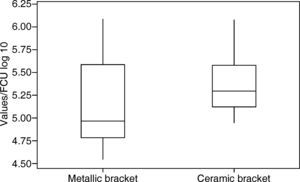
RESULTSThe values were analyzed using the Minitab software. The arithmetic mean was determined: 5.18 log 10 CFU with a standard deviation of ± 0.52 (Figure 3) for group 1 (metal brackets) and 5.38 log 10 CFU with a standard deviation of ± 0.39 (Table I) for group 2 (ceramic brackets). The difference between the two groups was compared using the Student's t-test but there were no significant differences (p = 0.204).
DISCUSSIONThe biological imbalance caused in the oral cavity by the presence orthodontic attachments has been object of several studies. Some of the factors that may facilitate bacterial adhesion to brackets are: surface roughness, saliva composition and flow, incubation time, the frequency of sucrose ingestion and oral hygiene.
Ahnin21 reported that metal brackets have less bacterial load in comparison with ceramic brackets, while Anhury22,23 Sharp and Papaioannou24 mentioned that there was no difference in the bacterial load between the two types of brackets. All these comparative studies on the microbial adhesion between different types of brackets show conflicting results. Our study agrees that there is no difference in the bacterial load between bracket types; this is probably due to the homogeneous conditions of sterility in both groups.
Eliades et al.25 assessed microorganism adhesion which indicates the influence of phenomena such as the surface and hydrophobic free energy. A significant correlation was observed between the surface and the retention capacity of the plate material. A favorable effect on bacterial adhesion was shown. According to this study, a metal has a free energy of 40.0 dynes/cm2, which is higher compared to that of ceramic brackets thus suggesting a higher bacterial adhesion in metal brackets. In this research both groups had similar conditions and a tendency for adhesion was identified in the ceramic brackets. However, there was no statistical difference with respect to the metal brackets. Bacterial adhesion to the brackets would probably be more complex if the study was performed in the oral cavity, where various interactions between salivary film, bacteria and bracket surface are present.23 In addition, the presence of other materials related to the appliances such as metal bands, archwires, elastomeric modules and resins may affect bacterial adhesion.22 The results showed bacterial adhesion in both groups perhaps due to a lack of exposure to the patient's habits such as diet and oral hygiene. As an additional factor, the use of orthodontic appliances probably favors food accumulation and makes hygiene difficult which results in the presence of biofilm.
Camargos26 mentioned that there was no significant difference between bracket types when bacterial colonization is assessed in the long term. The author suggested that the microbial adhesion of the bracket is directly related to time. The longer the appliances remain in the oral cavity, the lower the differences will be regardless of the bracket composition. In our results, no bacterial load was observed at the beginning of the study, we have hypothesized that this was due to the sterilization process performed in the brackets. Our results agree with what has been mentioned by Camargos:26 that there is no difference in the microbial load.
CONCLUSIONSBased on the results of this study, no significant differences were found in the bacterial load between both groups. Therefore, the type of brackets used in an orthodontic treatment does not influence bacterial adhesion and dental plaque accumulation.
We suggest that the presence or absence of bacteria is due to different factors, such as diet, salivary flow and oral hygiene. In vivo studies are recommended to observe the behavior of these variables.
This research was supported by the Orthodontics and Dentofacial Orthopedics Department of the University of San Luis Potosí, Mexico.
Postgraduate.
This article can be read in its full version in the following page: http://www.medigraphic.com/ortodoncia