The aim of this study was to determine the effect of an alcoholic drink (rum) in the adhesion of Transbond (3M) and Enlight (ORMCO) resins. Eighty brackets (0°) were bonded with Transbond and Enlight to healthy human teeth previously etched with phosphoric acid (37%). Each resin used its own adhesive. After bonding all teeth were immersed in physiologic serum at 7 °C for 24 hours and then thermocycled for 500 cycles; (5-55 °C). Forty samples were immersed in physiologic solution and the rest in the alcoholic drink for 12 days. At the end of 12 days, the force was measured in order to remove the bracket of each resin using a universal mechanical testing machine and the bond strength was calculated. The adhesion areas were observed in order to determine the failure site. The values were analyzed with ANOVA. The Enlight resin proved to have better bond strength than Transbond when both were immersed in serum but rum increased Transbond bond strength significantly. It was concluded that the medium changes the bond strength significantly. It was concluded that environment has an influence over bracket bond strength which could be an advantage for treatment since brackets have better adhesion but a disadvantage for the patients overall health.
El propósito de este estudio fue determinar si las resinas Transbond (3M) y Enlight (ORMCO) sufren cambios en su adhesión al diente cuando están en contacto con una bebida alcohólica (ron). Ochenta brackets (0°) fueron adheridos a dientes premolares humanos sin caries usando Transbond y Enlight, previamente grabados con ácido fosfórico al 37%. Cada resina uso su propio adhesivo. Después de estar sumergidos en suero fisiológico a 7 °C durante 24 horas, se sometieron a un termociclado de 500 ciclos (5-55 °C). Se sumergieron cuarenta muestras en solución fisiológica y el resto en la bebida alcohólica durante 12 días; transcurrido este tiempo se midió la fuerza para desprender el bracket de cada resina usando una maquina universal de pruebas mecánicas y se calculó la resistencia al desprendimiento en MPa. Se observaron las zonas de desprendimiento para determinar el sitio de falla. Los valores fueron analizados con ANOVA. Se encontró que Enlight presentó mayor resistencia al desprendimiento que Transbond cuando ambos estuvieron sumergidos en el suero, pero el ron incrementó considerablemente la resistencia al desalojo de Transbond. Se concluyó que el medio influye en la resistencia al desprendimiento de los brackets, siendo una ventaja para la estabilidad de la aparatología, ya que se adhiere más el bracket, pero una desventaja para la salud.
Adhesive resins for brackets play an important role in orthodontic treatment since brackets are bonded to teeth through mechanisms that involve adhesion.1Adhesion is defined as the force that holds together two similar materials when in close contact.2 It is due to chemical or physical bonds that can be affected when exposed to different environment.3
There are numerous problems for obtaining and preserving adhesion to dental structure. These include the aqueous nature of the oral environment, heterogeneity of tissues, the feasibility of certain dental tissues and biophysics and other restrictions imposed by the biological environment.3 On the other hand, many factors affect bracket retention during orthodontic treatment such as the enamel surface.4
A conditioned enamel surface is highly reactive, specially the organic surface. However, if saliva is allowed to get in contact with the conditioned and dry enamel, proteins adhere to the surface and affect the adhesion characteristics and resin penetration.
Candy, fruit juices, carbonated soft drinks and diet juices lower oral pH below 5.5 affecting and damaging the adhesive materials present in the oral cavity.5
It is important to determine if the patient’s eating habits influence or modify adhesion systems thus altering treatment. Studies have been done on how diet affects orthodontic treatment but they have focused on enamel demineralization and not on bracket bond strength. Gedalia,6 in 1991, conducted a study on the softening human enamel subjected to an acid drink (coca-cola) using hard cheese for enamel remineralization; the intraoral test showed that exposure of the enamel for one hour in cocacola diminished its hardness and hard cheese had a remineralization effect after 5 minutes by increasing the hardness of the enamel.
Abu Bakr7 analyzed the effect of alcoholic beverages (whiskey), low- pH beverages (coca-cola and orange juice) and deionized water over adhesion as well as color stability. Whiskey was the beverage that affected adhesion and color the most followed by coca-cola and orange juice.
Sean Beattie8 performed an in vivo study about the effect of several foods (cereal, meat, tomato sauce, chicken, rice, chocolate, orange juice, coca and milk) over dental polymers (elastic bands and latex elastics); he did not find significant differences on the patient’s daily dietary change; however there were significant differences between the used elastic bands brand (Rocky Mountain Orthodontics, 3M Unitek and American Orthodontics).
The effects of food over dental restorative polymers have been studied and several forms of degradation were found when the restorative materials were subjected to ethanol/water,9,10 ethanol/artificial saliva,11–13lactic acid, citric acid, heptane and beverages such as coffee, vinegar, whiskey, coca-cola and orange juice.7
West14 performed a study where he showed that a carbonated orange beverage with calcium caused less enamel loss when compared to a conventional carbonated beverage.
The aim of this study was to determine if an alcoholic beverage such as rum affects the bond strength of brackets bonded with Transbond (3M) and Enlight (ORMCO) resins.
Materials and methodsEighty human premolars extracted for orthodontic reasons without restorations, fractures or caries stored in physiological serum with 0.9% sodium chloride in pyrogen-free sterile injectable Baxter water. They were kept under refrigeration (5° C) for a period no longer than two months until the total sample size was obtained.
Eighty stainless steel premolar brackets with 0° prescription (ORMCO) with a mesh base and an area of 11.5 mm2 approximately, were adhered to the tooth crowns using Transbond XT resin (6KT batch) and Enlight (441020 batch). A LED lamp was used to cure the resins (Ultra Lume 5) which was tested using a thermal radiometer (model 200 from Demetron Research Comp.) to determine the generated heat (< 50 mW/cm2) and a curing radiometer (model 100 from Demetron Research Corp.) to determine light intensity (400 mW/cm2).
The teeth were divided into four groups of 20 each, they were polished with prophylactic paste free of fluoride during 10 seconds and then washed and dried with oil-free air and water for 10 seconds.
The enamel was etched with 37% phosphoric acid gel (ORMCO) during 15 seconds according to the manufacturer’s instructions; they were subsequently washed with water for 10 seconds and dried with oil- free air and water for 10 seconds.
In the first (control group) and second set of teeth, ORMCO adhesive was placed according to the manufacturer’s instructions. The brackets were adhered with resin Enlight and light-cured for 30 seconds.
In the third (control group) and fourth set of teeth, 3M Adhesive was placed according to the manufacturer’s instructions. The brackets were attached with Transbond resin and light-cured for 30 seconds.
After adhesion, all teeth were placed in physiological saline at 37 °C for 24 hours.
After 24 hours, the groups were subjected to thermal cycling in cold water (5 °C) and hot water (55 °C) during 500 cycles.
At the end of thermal cycling, the second and fourth groups were submerged in the alcoholic drink for 12 days at 37 °C. The control group remained submerged 12 days in physiological saline.
To perform the debonding test, teeth were placed horizontally in acrylic blocks up to the level of the clinical crown; they were later tested in a universal machine for mechanical testing (INSTRON model 5567) with an upload speed of 1 mm/min (Figure 1) to measure the debonding strength and calculate the resistance by dividing force between bracket area (11.5 mm2).
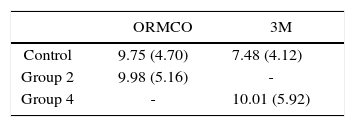
ResultsThe table I shows the average values of bond strength in MPa.
When comparing between 3M control group, it was found that the alcoholic beverage (group 4) increased their bond strength 34%. ORMCO experienced a 2% increase in relation to the control group.
The analysis of variance showed no statistically significant differences for any group with a p < 0.05. However, there was a considerable difference between the resins submerged in serum.
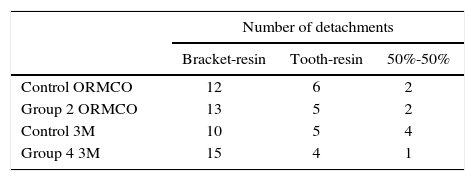
The presence of resin in the bracket or on the tooth was visually examined to determine the site of failure. The table II shows the frequency of the events. The 50%-50% ratio means that regardless of the amount of resin bonded to the tooth or bracket, this occured in both.
The site where most detachments occurred was between bracket and resin with the latter on the surface of the tooth. The next site of higher frequency of failure was between tooth and resin with the latter on the bracket. These results show that we need to apply more force to debond resin from the tooth surface which means that the adhesive helps increase bond strength.
DiscussionIn orthodontics, a large number of brackets suffer from adhesion failures during treatment.15,16 Under these circumstances it is common for a bracket to be re-bonded with a re-preparation of the enamel surface.9The different conditions that brackets undergo inside the mouth can influence or change bracket bond strength and be detrimental for treatment.
The results of this study demonstrate that ORMCO had superior values than 3M when both were in contact with physiological saline; it can be theorized that there are different components between the two resins or between heir adhesives.
When both resins were immersed in the alcoholic beverage, 3M significantly increased its bond strength compared to ORMCO. Information provided by Chalgren17 and Arhun18 indicates that both the adhesives and resins from 3M and ORMCO are formulated with different dimethylacrylates where Transbond uses monomers of lower molecular size. This may be the explanation of the behavior of the materials before ethyl alcohol. It could be said that these reactions to alcohol are beneficial to the adhesive system since the bracket is notdebonded soeasily. On the other hand, it is a disadvantage for people who frequently consume this drink; one might think that the higher consumption, the more bond strength there will be and therefore will cause less bracket debonding, but more alcohol would be consumed to the detriment of their health.
These results motivate further research to know which components of the organic resin or adhesive chemically react with the alcoholic group causing the resin to adhere more to the tooth surface.
ConclusionsAlcoholic beverages increase the bond strength of the 3M system when compared to the ORMCO system. The ORMCO system presented higher bond strength than 3M when both were in contact with physiological saline.