One of the most important issues within the practice is the cross infection control. We currently have enough technology to disinfect and sterilize instruments. Dental protection measures must be adopted to prevent infection and they are established in our country by the Mexican Official Norm (NOM-013-SSA) for epidemiologic surveillance, and for the prevention and control of hospital-acquired infections.
Material and methodsIn order to conduct the study, six different brands of packaged metal brackets were used. Three of them are packaged in Mexico: American Orthodontics (Protorque .018), Borgatta and GAC (Roth .022); and three are packaged in the US: TP Orthodontics, Inc. (MBT .022), ORMCO Corporation. (Roth .022), 3M Unitek (MBT .022).
ResultsOf the six Petri dishes, only one colony growth was observed and it corresponded to the brand Borgatta (Roth .022). It was determined by optical microscopy, the presence of bacilli GN and cocci (Staphylococcus).
ConclusionsOrthodontic attachments should be subjected to a disinfection process and/or sterilization prior to their placement to ensure the absence of microorganisms.
Uno de los problemas más importantes dentro de la consulta es el control de las infecciones cruzadas. Para ello disponemos en la actualidad de la tecnología suficiente para desinfectar y esterilizar el instrumental. En la práctica odontológica se deben adoptar medidas de protección para prevenir el contagio de las enfermedades y son establecidas para nuestro país por la Norma Oficial Mexicana (NOM-013-SSA) para la vigilancia epidemiológica, la prevención y el control de las infecciones nosocomiales.
Material y métodosPara llevar a cabo el estudio se emplearon seis diferentes marcas comerciales de brackets metálicos empaquetados. Tres de ellas corresponden a las marcas American Orthodontics (Pro-torque .018), Borgatta y GAC (Roth .022), empacados en México; y TP Orthodontics, Inc. (MBT .022), ORMCO Corporation. (Roth .022), 3M Unitek (MBT .022), empacados en USA.
ResultadosDe las seis cajas de Petri solo en una, que correspondió a la marca Borgatta (Roth .022), se observó el crecimiento de colonias, las cuales se determinaron por microscopia óptica y la presencia de cocos grampositivos (Staphylococcus).
ConclusionesConsideramos la necesidad de que los aditamentos ortodóncicos deberían someterse a un proceso de desinfección y/o esterilización previo a su colocación para con ello garantizar la ausencia de los microorganismos.
One of the most important problems in dental practice is cross-infection control. For that matter, we currently have at hand sufficient technology to disinfect and sterilize instruments.
Orthodontic appliances such as brackets have the possibility of being contaminated by microorganisms (MO).
MO may be transmitted in different ways, indirectly or directly. They can be transmitted from patient to patient through contaminated instruments and/or clinical procedures.
In dental movements that require the application and control of different kinds of forces intraoral appliances are bonded to teeth to achieve an adequate dental alignment.
In dental practice protective measures must be adopted to prevent disease transmission and these measures in our country are established by the Official Mexican Norm (NOM-013-SSA).1
The Emergency Mexican Official Norm (NOM-EM- 002-SSA2-2003) for epidemiological surveillance, prevention and control of hospital-acquired infections defines the mechanisms for infection:
- 1.
By direct transmission. It implies direct person-to- person contact and indirect transmission implies contact through a reservoir of contaminated objects or surfaces (i.e. needles, gloves, etc.).
- 2.
Transmission by infectious droplets. Theoretically, this is a form of transmission by contact, however the transfer mechanism from the pathogen to the host is through sneezing, coughing, speaking or by doing certain clinical procedures (bronchoscopies suction). The infective material is deposited on the host over the mucous membranes. It does not remain suspended so it does not require care in air handling or ventilation.
- 3.
Airborne transmission. It happens by the spread of particles suspended in the air. In this type of transmission care is needed in air handling and ventilation.
- 4.
Common vehicle transmission. Transmitted by food, water, medication and equipment.
- 5.
Vector-borne transmission. Mosquitoes, flies, mammals, etc.
The control of cross-infection should be considered a fundamental part of the dental practices.
In dental procedures, the transmission of the infection depends on four factors:
- 1.
Source of infection (patient/operator).
- 2.
Mode of transmission (blood, saliva).
- 3.
Mode of entry (inoculation: hepatitis, herpes simplex, HIV; inhalation: chicken pox virus, influenza virus, Mycobacterium tuberculosis).
- 4.
Individual susceptibility (nutritional status, inheritance, medication).
Therefore, the objective in infection control is to prevent transmission of MO.
The objective of this study was to determine if MO are present and what kind of MO may exist in different brands of metal brackets in their package of origin.
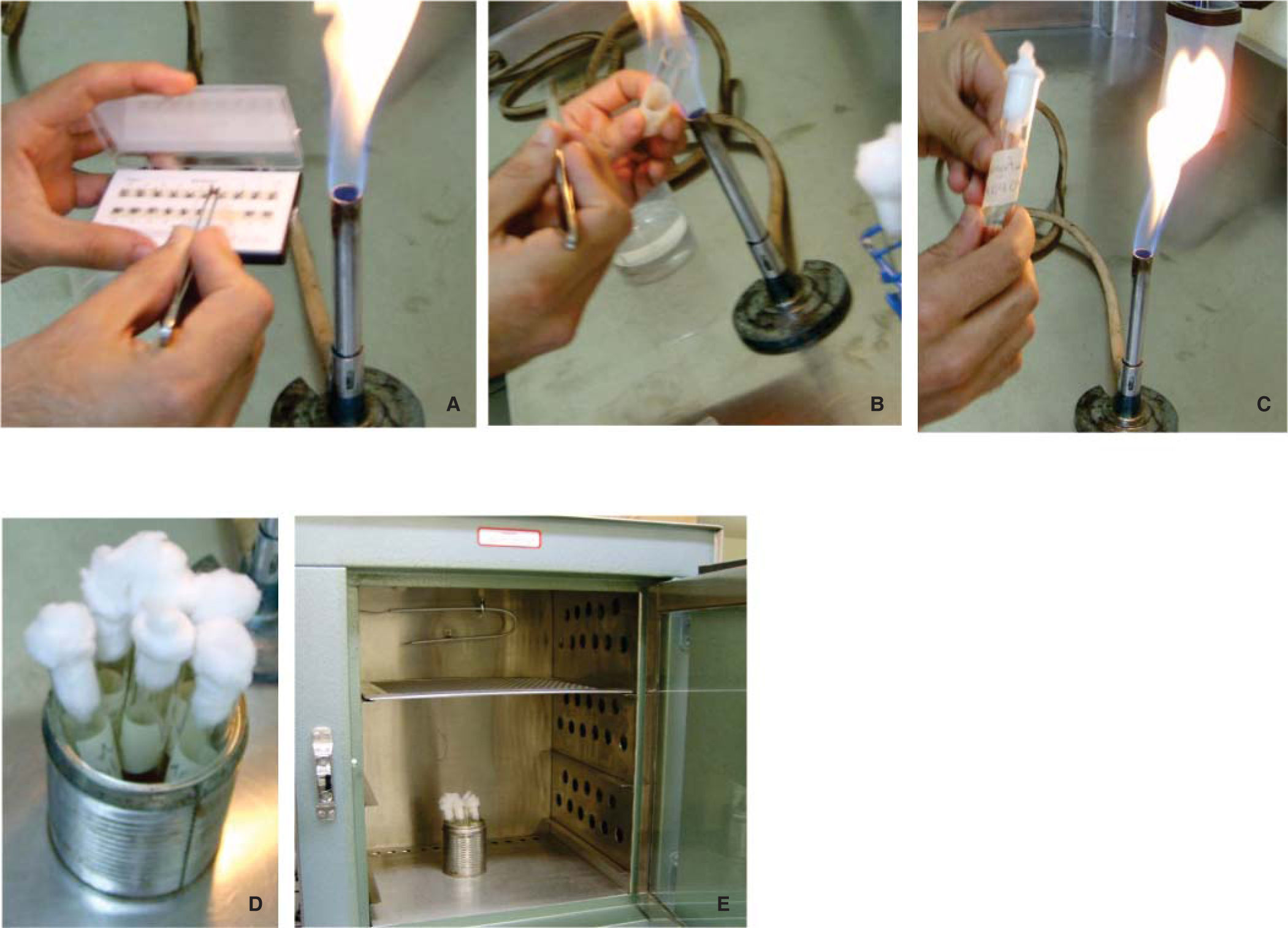
Material and methodsTo conduct the study 6 different brands of commercially available metal packaged brackets were used (Figure 1) Three of them correspond to the brands American Orthodontics (Pro-torque .018), Borgatta and GAC (Roth .022), which are packaged on national territory; the other brands were: TP Orthodontics, Inc., (MBT .022), ORMCO Corporation (Roth .022) and 3M Unitek (MBT .022), all packaged in the US. From each package, two brackets were taken randomly with dressing pliers previously flamed in a direct way.
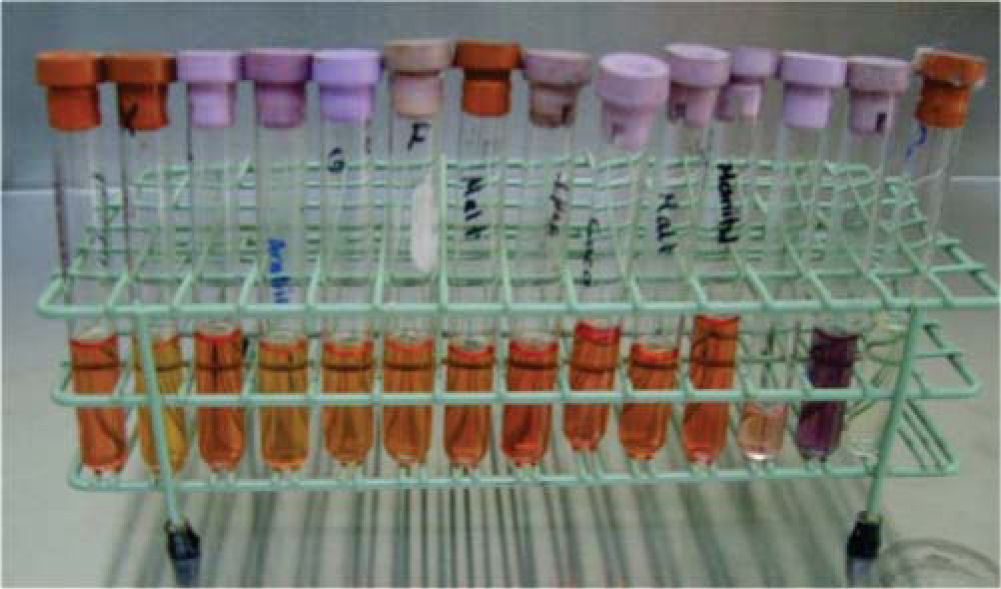
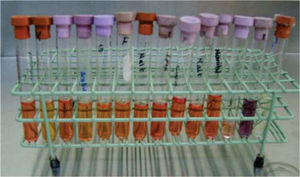
In six test tubes previously labeled with the names of the commercial brands, half filled with Brain-Heart Infusion Liquid (BHI),2,3 (Rosenow and Hayden, calf brain infusion 200 g, bovine heart infusion 250 g, peptone 10 g, sodium chloride 5 g, glucose 2 g, disodium phosphate 2.5 g, pH 7.4) two brackets from each package were introduced for incubation for 24 hours at 35 oC (Figure 2). The opening of the test tube was flamed previously.4
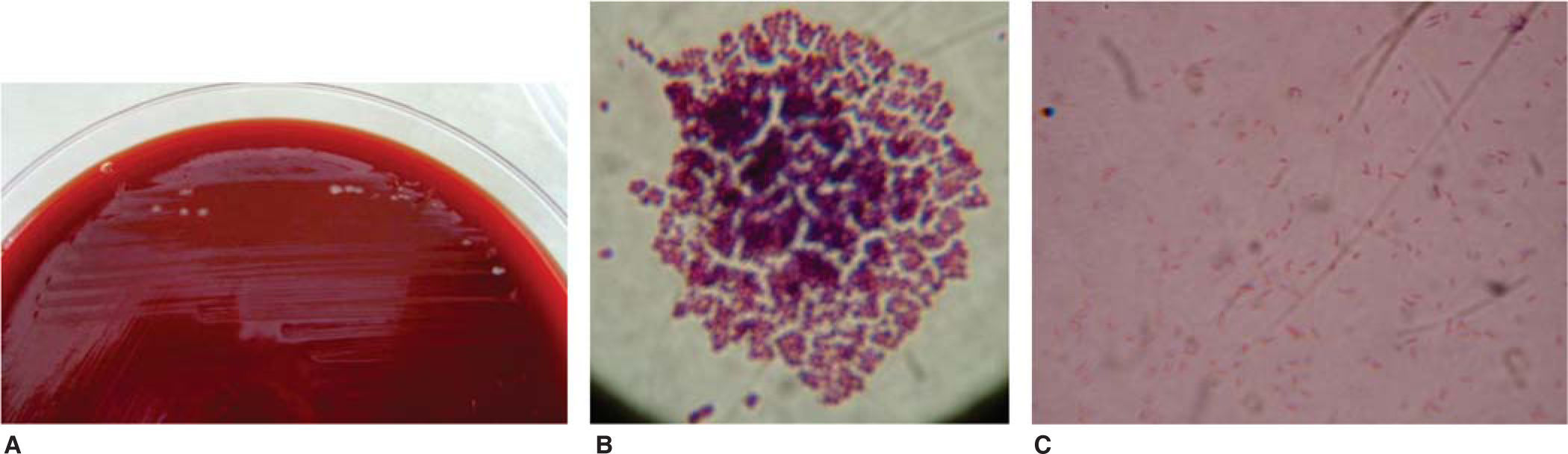
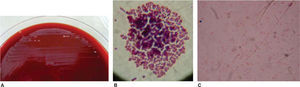
Afterwards, in six Petri dishes with Blood Agar Gel (that corresponded with the previous labeled test tubes) the MO obtained from the BHI were planted with the Streak Plate technique5 (inoculation and isolation of bacteria) (Figure 3). They were incubated for 24 hours at 35 oC to be observed under the microscope (Olympus, 100X) with Gram technique6 (Christian Gram 1884) and determine the taxonomy, a new culture method and from this determine the genus and species of the MO.
ResultsFrom the six Petri dishes only one showed colonial growth and it corresponded to the brand Borgatta (Roth .022). The presence of gramnegative bacilli7–9 and grampositive cocci was determined by optical microscope (Figure 4).
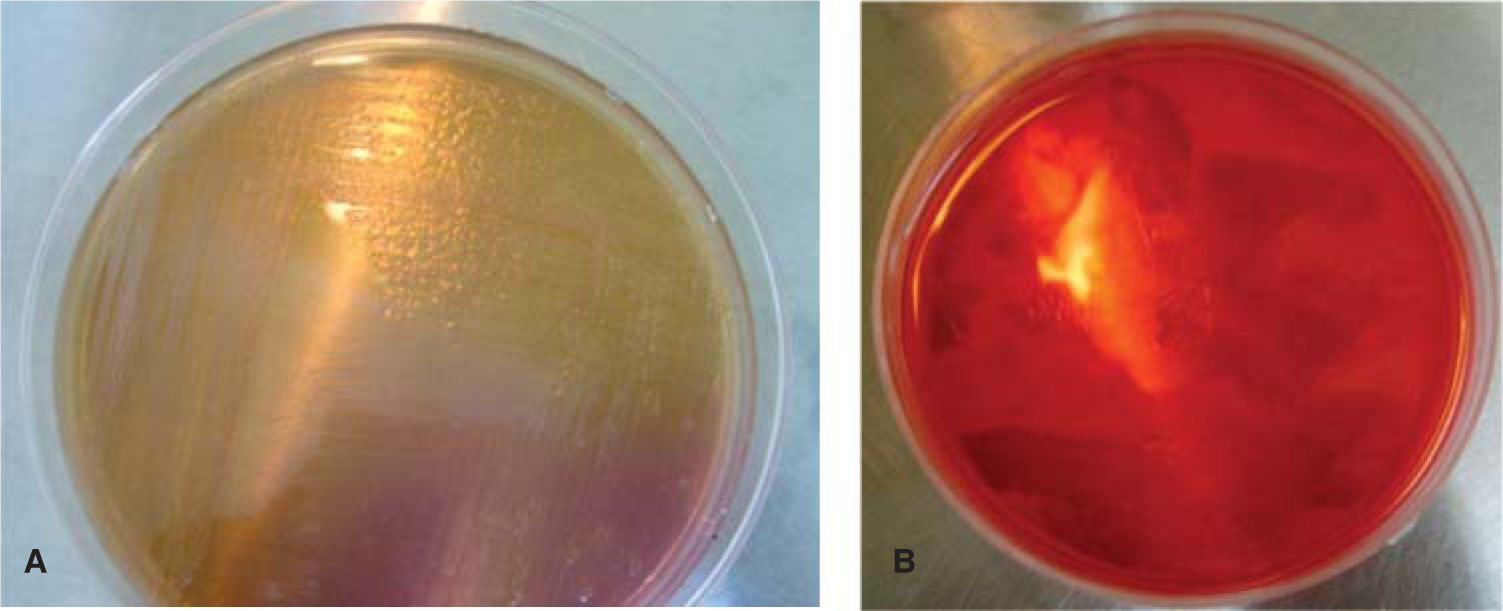
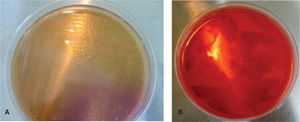
In an Agar MacConkey10 culture medium (peptone 17 g, polipeptone 3 g, lactose 10 g, biliary salts 1.5 g, sodium chloride, agar 13.5 g, neutral red 0.03 g, violet crystal 0.001 g, distilled water c.s.p. 1 L, pH 7.1) bacilli were planted and in an Agar Salt Mannitol culture medium (Chapman, agar, sodium chloride 7.5%, phenol red and mannitol)11 the Staphylococcus colony was planted (Figure 5). They were both incubated for a 24-hour period at 35 oC.
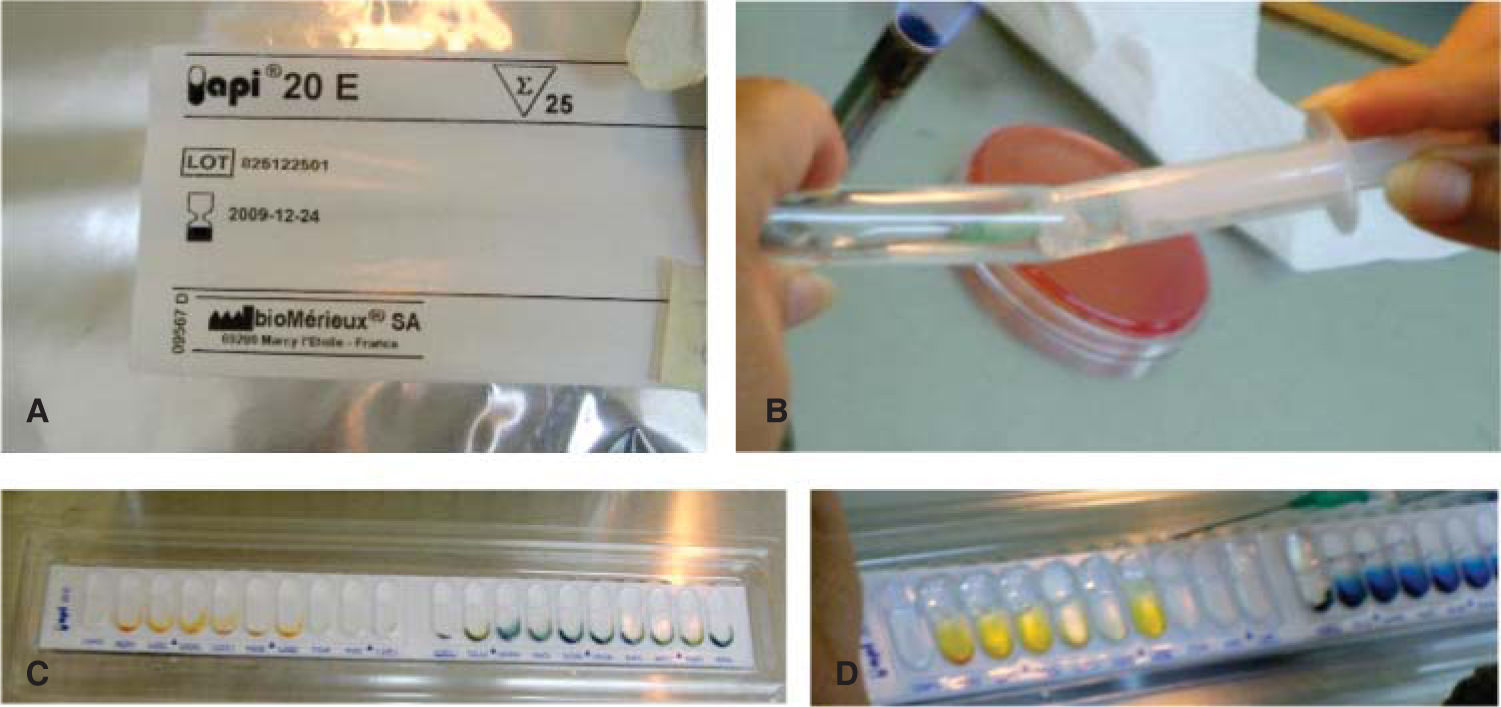
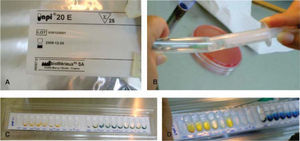
Negative oxidase and positive peroxidase tests were performed on the bacilli and the positive peroxidase test on the Staphylococcus. With the system for identifying Enterobacteriaceae and other undemanding gram negative bacilli (API 20E bioMerieux® SA) the presence of Shigella could be determined (Figure 6). The Staphylococcus were planted in biochemical tests: carbohydrates (maltose, fructose, sucrose, lactose, mannitol, mannose, xylose and trehalose), urea broth sulfanilic acid + α naphthylamine, negative MIO (ornithine indole motility) and coagulase-negative; with all of them the species Staphylococcus epidermidis was identified12–14(Figure 7).
Orthodontics in its daily practice is based on preventing, diagnosing and correcting any possible alterations in the position, relationship and function of the dentomaxilofacial structures, through the use of multiple appliances as well as techniques. Today orthodontics is supported by the use of elements that bond to teeth, with special emphasis on brackets.
These appliances, as well as arches, bands, elastic, and others, are produced and packaged by multiple commercial brands and in the daily practice are placed by most professionals without previously undergoing a disinfection process and/or sterilization. Many of them can be purchased individually, which contributes to their contamination.
Few bacteria are as ubiquitous as the Staphylococcus,15they are very resistant to environmental conditions and extremely difficult to eradicate.
It endures well extreme conditions, although it remains inactive at low temperatures and can be removed at high temperatures. In spite of not being sporulated it can be located on any surface. This bacteria is found in the skin and respiratory tract, causing a wide variety of infections, from minor skin infections (furuncles, blisters), and skin abscesses to life-threatening diseases (endocarditis, pneumonia, and toxic shock syndrome) as is the case of S. aureus. Another bacterium that belongs to this family is the S. epidermidis and is probably the most common species found in laboratory analysis. We found that in one of the samples there was growth of bacterial colonies. Through reactive tests the presence of S. epidermidis was determined and also that of a common enterobacteria belonging to the genus Shigella,16 a bacterium transmitted by fecal contamination and that causes gastrointestinal symptoms (diarrhea, fever, myalgia, vomiting).
The packaging of brackets usually takes place in an automatic fashion. This could reflect the absence of bacterial growth in the majority of the samples, except for one of them. However, none of them are a representative sample of bacterial absence or cross-contamination, and we can infer that the latter was contaminated during manual packaging or after packaging.
ConclusionsWe consider that orthodontic appliances should be subjected to a process of disinfection and/or sterilization prior to their placement so as to ensure the absence of MO and that it is necessary to take basic measures for the control of infectious risks during clinical procedures.
We suggest that future studies should be made with models and scientific analysis that provide more significant scientific evidence.