The increasing resistance to quinolones has led to the consideration of other antibiotic options for the prevention of infectious complications in prostate biopsy. We present our experience using a single dose of piperacillin/tazobactam as prophylaxis.
MethodsA retrospective study of transrectal prostate biopsies performed at our institution from 2008 to 2013 was conducted. All patients received enemas before biopsy and a single 4.5g dose of piperacillin/tazobactam was administered i.v. a few minutes before the biopsy. Clinical and microbiological variables were analyzed to find out risk factors for complications.
ResultsA total of 543 biopsies were included. Ninety-two complications (16.9%) were reported in 74 (13.6%) patients, 4.2% of which were infectious complications. In these patients, the associated risk factors were a previous history of positive urine cultures within a 3-year period before biopsy, the presence of a transurethral indwelling catheter at the time of the biopsy, hospital admission within a month before biopsy, and a preoperative positive urine culture despite antibiotic therapy selected according to the resistance pattern.
ConclusionsPhysicians and patients should be aware of the risk for complications, particularly if risk factors are present. A single dose of piperacillin/tazobactam is a reasonable option for prophylaxis, especially in countries with a high prevalence of quinolone-resistant pathogens.
El objetivo de este estudio es analizar el desempeño de piperacilina/tazobactam dosis única como profilaxis para biopsias de próstata transrectales, así como conocer los factores de riesgo asociados a complicaciones en una región con alta tasa de resistencia a quinolonas.
Materiales and MétodosEstudio retrospectivo que incluyó todas las biopsias de próstata realizadas en nuestro instituto de 2008–2013. Todos los pacientes recibieron 2 enemas antes de las biopsias y una dosis de 4.5gr de piperacilina/tazobactam I.V. antes de la biopsia. Se incluyó múltiples variables y se hizo énfasis en las complicaciones presentadas. Se realizó un análisis estadístico descriptivo y se buscó asociaciones de posibles factores de riesgo.
ResultadosSe incluyó 543 biopsias. Se reportó 92 (16.9%) complicaciones en 74 (13.6%) pacientes. Veintitrés (4.2%) pacientes tuvieron una complicación infecciosa. Los factores que se asociaron al desarrollo de complicaciones infecciosas fueron la historia de cultivos de orina positivos en los 3 años previos a la biopsia, la presencia de sonda transuretral al momento de la biopsia, hospitalizaciones en el ultimo mes antes de la biopsia, así como un cultivo de orina positivo en los laboratorios pre-biopsia, incluso después de haber recibido tratamiento dirigido por antibiograma.
ConclusionesLos pacientes deben estar conscientes del riesgo de complicaciones, particularmente si presentan los factores de riesgo mencionados. Piperacilina/tazobactam en dosis única es una buena opción de profilaxis pre-biopsia particularmente en regiones con alta tasa de resistencia antimicrobiana.
Currently, prostate biopsy (PB) is the standard method for confirming the diagnosis of prostate cancer (PCa). The implementation of widespread screening with prostate-specific antigen (PSA) has increased the number of PBs over the last decades.1 A rising PSA and/or suspicious digital rectal exam (DRE) are the most frequent indications for a PB.2 Other indications include persistently elevated PSA with a low PSA free/total ratio, increased PSA velocity, PSA duplication time or PSA density, the histopathologic finding of atypical small acini proliferation (ASAP), prostatic intraepithelial neoplasia (PIN), and active surveillance.3,4
PB can be performed either transrectally or transperineally under ultrasound or magnetic resonance guidance. The number of cores taken depends on prostate size, history of previous biopsies, and patient age. In our center, we usually perform ultrasound-guided transrectal prostate biopsies (TRPB) with 12–18 cores at initial biopsy.5
The reported complication rate of TRPB is variable: bleeding occurs in up to 50% of cases, although it is usually clinically irrelevant.6 Nevertheless, serious infectious complications develop in 2–6% of cases, of which nearly 4% require hospitalization and treatment within 30 days after the procedure. Given the severity of infectious complications, some strategies have been recommended in order to lower their frequency, including the use of prophylactic antibiotics.7 However, there is still controversy regarding the adequate type and dosage of antibiotic. Quinolones are the most widely recommended, because they have good penetration into prostatic tissue.2 Unfortunately, quinolone resistance rates have increased significantly.8 In Latin America and other developing areas around the world, the resistance rates to quinolones and cephalosporins have reached alarming figures. For this reason since 2008, we have found it necessary to use an alternative prophylactic strategy based on a single i.v. dose of piperacillin/tazobactam (P–T).9 In this study we described the complications of TRPB, emphasizing infectious complications, with the use of this prophylactic regime.
MethodsWe performed a retrospective study on men that underwent TRPB from January 2008 to June 2013 at our department. Patients without complete clinical records were excluded. We analyzed clinical, laboratorial, and pathological data, as well as follow-up information. Previous evaluation in all patients included PSA, complete blood cell count, blood chemistry, coagulation tests, urinalysis, and urine culture.
As preparation, all patients received the indication to apply a micro-enema the night before and the morning of the procedure. Prophylaxis with a single i.v. dose of P–T 4.5g was administered 30–60min before biopsy. After measuring prostate and transition zone volumes, core biopsies were obtained with semi-automatic equipment (BARD® MAGNUM® Reusable Core Biopsy System) and an 18-Gauge thru-cut needle in a systematic fashion (12–18 cores for the first TRPB or extended protocol for subsequent procedures).
Perioperative complications were registered during the procedure and stated in the surgical report. Outpatient visits were scheduled 1 week and 1 month after biopsy. All patients were advised about possible symptoms and to go to the emergency room (ER), if necessary, in the case of further complications. Perioperative and early complications were classified according to the Clavien–Dindo system.10
We intentionally reviewed the past urologic history of the patients, looking for positive urine cultures within 3 years before biopsy and its association to urinary tract infection (UTI) or resistant strains after TRPB. A univariate analysis was performed to find variables related to the development of infectious complications, and statistical analysis was done using the IBM® SPSS® v. 20 package.
Because of the retrospective nature of the study, ethics committee approval was not necessary. Nevertheless, patient data confidentiality was maintained and there was no violation of the Helsinki Declaration.
ResultsA total of 543 men that underwent TRPB were included in the study. Mean age was 65±7.51 years. Median PSA was 7.49 (0.22–3000)ng/ml. Lower urinary tract symptoms (LUTS) were reported by 68% of the patients and 66% were under α-blocker therapy. The most frequent comorbidities were diabetes mellitus in 165 men (30.4%) and high blood pressure in 258 (47.4%). Thirty-seven patients (6.8%) had a transurethral indwelling catheter at the time of TRPB and 34 (6.3%) had a previous hospital admission within a month before TRPB.
A total of 436 men (80.3%) underwent first-time TRPB, whereas 107 (19.7%) underwent subsequent biopsy. Indications for TRPB included high PSA in 341 cases (62.7%), suspicious DRE in 33 (6.1%), both an elevated PSA and suspicious DRE in 143 (26.3%), ASAP in 11 (2%), active surveillance in 6 (1.1%), and not specified in 9 (1.6%). PSA value was <10ng/ml in 246 (45.3%) patients; between 10 and 20ng/ml in 81 (14.9%); >20ng/ml in 31 (5.7%); and missing in 185 cases. Mean prostate volume was 51.46±29.1cc. Twelve-core biopsy was performed in 300 men (55.8%), more than 12 in 234 (43.5%) patients, fewer than 12 in 4 cases (0.8%), and not specified in 5.
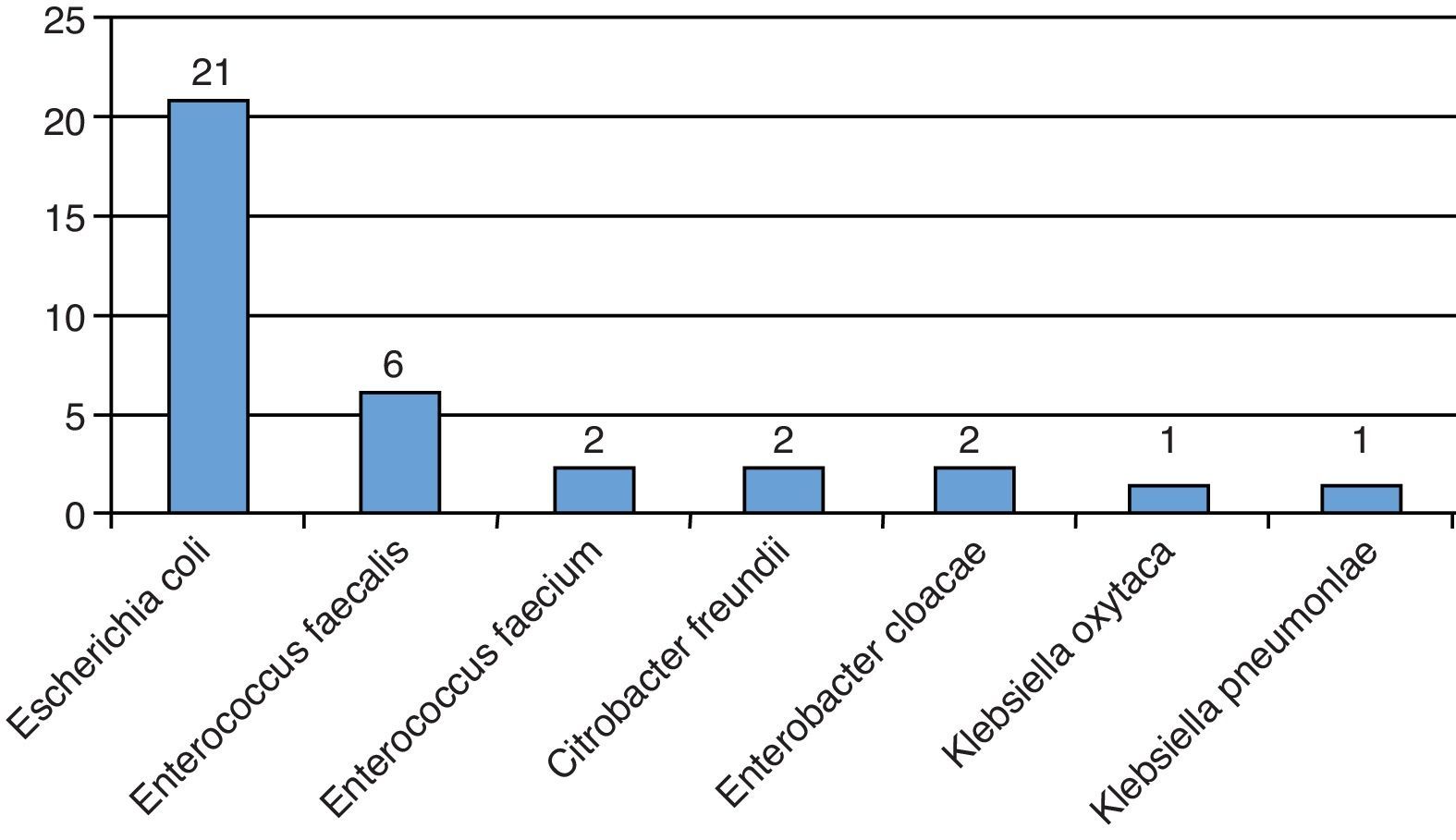
All patients had a urine culture before TRPB, of which 35 (6.4%) were positive. Treatment was completed according to resistance pattern (Fig. 1). We found that 217 (39.9%) of the patients had 2 or more urine cultures within 3 years before the procedure; 48 (22.1%) of them had at least 1 positive urine culture within that period.
Only 3 patients had intraoperative complications: 1 patient had transient bradycardia, 1 had transient hypotension (both required interruption of the procedure) and 1 patient presented with rash presumably due to P–T allergic reaction, in whom the procedure was completed safely.
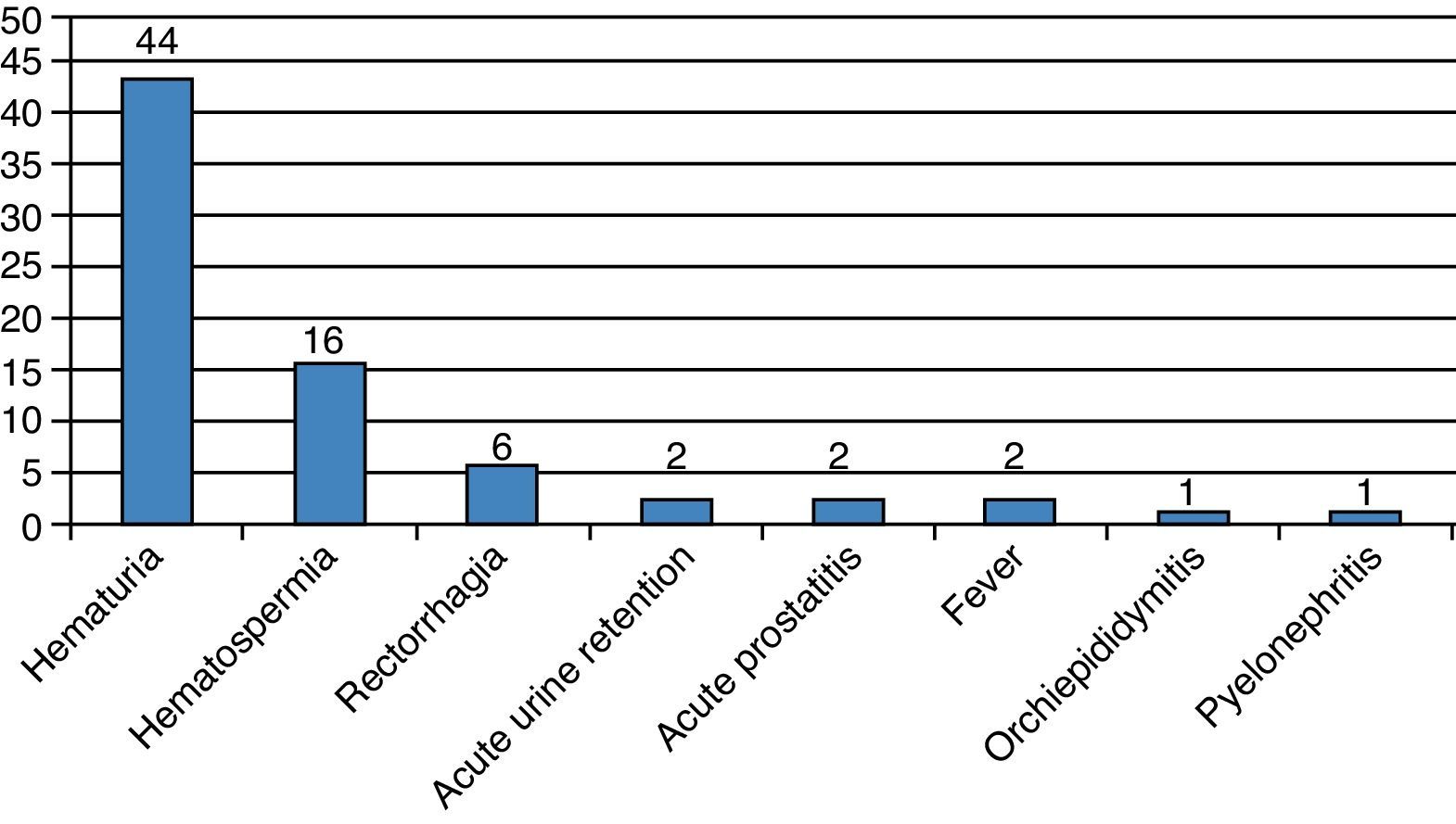
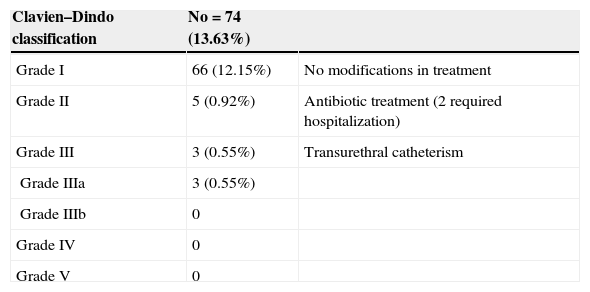
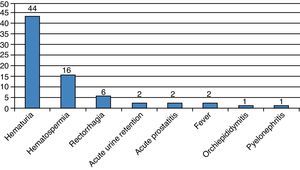
During the first month, 74 patients (13.6%) reported at least one complication, for a total of 92 events (Fig. 2). The Clavien–Dindo classification is reported in Table 1. Two patients had acute urinary retention (0.36%) and 23 (4.2%) had infectious complications including acute prostatitis, orchiepididymitis, pyelonephritis, or fever. Sixty-nine (12.8%) patients had a urine culture within the first month, of which 19 were positive (27.5%). Two (0.36%) patients were admitted to receive i.v. antibiotics.
Clavien–Dindo classification of complications within a month after biopsy.
| Clavien–Dindo classification | No=74 (13.63%) | |
|---|---|---|
| Grade I | 66 (12.15%) | No modifications in treatment |
| Grade II | 5 (0.92%) | Antibiotic treatment (2 required hospitalization) |
| Grade III | 3 (0.55%) | Transurethral catheterism |
| Grade IIIa | 3 (0.55%) | |
| Grade IIIb | 0 | |
| Grade IV | 0 | |
| Grade V | 0 |
The univariate analysis showed that a previous history of positive urine cultures within 3 years, the presence of transurethral indwelling catheter at the time of the procedure, and preoperative positive urine culture despite antibiotic therapy selected according to resistance pattern were factors associated with infectious complications (Table 2).
Univariate analysis of factors related to infectious complications.
| Variables | HR | 95% CI | p |
|---|---|---|---|
| History of at least one positive urine culture within 3 years prior to the biopsy | 4.061 | 1.48–11.13 | 0.003 |
| Transurethral catheter at the time of biopsy | 6.235 | 2.42–16.09 | 0.001 |
| Hospitalization within a month prior to the biopsy | 3.051 | 0.99–9.46 | 0.05 |
| A positive urine culture immediately before the biopsy, even after guided treatment | 5.007 | 1.87–13.42 | 0.001 |
PCa was diagnosed in 172 cases (31.8%), affecting both prostatic lobes in 91 (52.9%) and 2 or more cores in 93 (54%). A Gleason score of 6 was diagnosed in 47% of cases.
According to the D’Amico risk group classification, 59 (34.7%) cases were low risk tumors, 61 (35.9%) were intermediate risk, and 50 (29.4%) were high risk. Classification could not be completed in two patients due to insufficient data. Other diagnoses included chronic prostatitis in 331 (61.3%) patients; atrophy in 283 (52.4%); benign prostatic hyperplasia in 391 (73.1%); PIN in 15 (2.8%); and ASAP in 10 (1.8%).
DiscussionIn this retrospective analysis of TRPB, we found that the overall rate of early complications was low (13.6%). Although hematuria was frequently reported, it did not require therapeutic intervention. Other well-known complications included hematospermia and rectorrhagia.
Infectious complications are particularly relevant, given their life-threatening potential. The prophylactic regime with P–T allowed us to control this type of sequel (4.2%) during the first month. All patients were successfully treated and only two patients required hospital admission to complete their treatment. Sanders and Buchan analyzed 1421 TRPBs and reported a hospitalization rate of 2.8% (40 cases) within the first month after biopsy. They used a 4-day course of antibiotics as prophylaxis, mostly with ciprofloxacin. More than 50% of isolated Escherichia coli strains after biopsy were resistant to quinolones.11 Another retrospective study performed in two hospitals in London, UK, reported 2.1% of infectious complications requiring admission to the ER. They used a 5-day prophylactic course of ciprofloxacin and a single i.v. dose of amikacin before the procedure. The majority of isolated microorganisms, particularly those causing bacteremia, were ESBL-producing strains that were also resistant to quinolones.12
We and others have previously reported the experience with a single dose of PT as antimicrobial prophylaxis9,13 or in combination with quinolones.14 The use of a single dose of antibiotic prophylaxis is an appealing strategy,15 particularly in countries with a higher prevalence of resistant microorganisms. In recent years, our institutional resistance rate has risen, with 57% of E. coli strains being resistant to quinolones.16,17
Our results demonstrate that past medical history with a focus on infectious disease and urological issues is of the utmost relevance. As previously shown, the presence of a transurethral indwelling catheter at the time of biopsy was strongly associated with the development of infectious complications. Therefore, if possible, a TRPB should be avoided while patients are using this device or deferred until completion of proper antibiotic therapy. Other non-modifiable risk factors for infectious complications include a previous positive urine culture or UTI within a 3-year time period before biopsy. Modifiable risk factors are positive urine culture immediately before TRPB or recent hospital admission. The former requires properly selected antibiotic therapy based on an antibiotic susceptibility test and the delay of TRPB until a negative urine culture is obtained. Moreover, it would be advisable to perform biopsy at least 1 month after discharge from previous hospital admission. On the other hand, some authors have demonstrated the role of rectal swabs in providing targeted antimicrobial prophylaxis, thus decreasing the incidence of infectious complications.18
Overall, the infection rate we found was consistent with rates in the literature. However, our figure is of paramount relevance, considering that with this strategy we achieved infectious complication control similar to that in the developed countries, where the use of quinolones or cephalosporins is still safe for PB prophylaxis. Even though these data suggest that a single i.v. dose of P–T does not provide better prophylaxis than other regimens, we believe it is an excellent option for clinical practice in world regions other than the US and Europe, where resistance rates make the use of quinolones prohibitive. Furthermore, besides having a broader-spectrum, in comparison with cephalosporins, P–T induces to a lesser extent the expression of ESBL-producing strains.19
Our study has some limitations, mostly due to the retrospective nature of the investigation. Moreover, the inclusion of a comparative group receiving a different antibiotic would be desirable. However, the strength of this investigation is the fact that all patients underwent a homogenous evaluation, including a urine culture with an antibiotic susceptibility test prior to biopsy and all received the same antibiotic regime. Moreover, other advantages include the close follow-up based on electronic records and the processing of all urine cultures at our institution, as well as the description of risk factors associated with infectious complications.
We believe a single i.v. dose of piperacillin–tazobactam is an alternative for TRPB in an environment with a high prevalence of quinolone resistance. Being a single dose treatment with a broad spectrum and lower induction of resistant strains, complication rates remain low. Before performing a TRPB, it is advisable to acknowledge the following risk factors for infectious complications: a previous history of positive urine cultures within 3 years, the presence of transurethral catheter at the time of the procedure, hospital admission within the previous month, and preoperative positive urine culture despite antibiotic therapy selected according to resistance pattern. Future research should aim to analyze these factors in a prospective fashion. Patients with a positive culture should confirm urine sterility after antibiotic treatment.
ConclusionsProstate biopsies are a necessary procedure for the diagnosis of PCa, but urologists and patients must be aware of the risks. If possible, patients should wait if they have had recent hospitalization and transurethral catheters should be taken out. It is recommendable to have a confirmatory urine culture, even if patients have taken proper antibiotic treatments. Patients with a history of previous urine infections must be aware of the higher risk for infectious complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestDr. Herrera-Cáceres, Dr. Villeda-Sandoval, Dr. Ruiz-Quiñones, Dr. De La Rosa-Leiva Dr. Feria-Bernal, and Dr. Galindo-Fraga have nothing to declare.
Dr. Castillejos-Molina reports personal fees as a speaker from Lilly and personal fees as a speaker from GSK, outside the submitted work.
Dr. Rodriguez-Covarrubias reports personal fees as a speaker from GSK and personal fees as a speaker from Ferring Pharmaceutical, outside the submitted work.