The aim of the present study was to determine which oral alterations can be found in patients with head and neck cancer treated with radiotherapy as well as to explore dentist's involvement in treatment of these patients. An exploratory study was conducted in 52 patients who had previously received over 1,000 cGy radiation. A survey was undertaken as well as oral examination of each participant, in order to assess stimulated salivary flow and flavor tests. Results revealed high prevalence of oral alterations in patients with accumulated radiation of 3,001-5,000 cGy. Dry mouth (xerostomia) was the most frequently reported alteration (78.8%). Estimated total salivary secretion rate confirmed a state of hyposalivation in 82.7% of all patients. A statistically significant association was found between cancer location (p < 0.01) and type of tumor with presence of trismus (p < 0.05). Hyposialia was more frequently present in patients with stage IV tumors (50%) in those subjected to combined treatments (p < 0.05). Periodontal assessment was possible in 50% of all patients, Of this proportion, 92% exhibited periodontitis with mainly moderate to severe insertion loss; 84.6% of all participants reported not to have been remitted to dentists either before or after treatment. Findings support high frequency of oral alterations in patients subjected to radiotherapy treatment and dental care inappropriateness to prevent or treat these effects. An alert is raised with respect to the compulsiveness to follow treatment protocols for cancer patients, which should include dental evaluation before, during and after respective treatment.
El objetivo del estudio fue determinar las alteraciones bucales en pacientes con cáncer de cabeza y cuello tratados con radioterapia y explorar la participación del Odontólogo en la atención de estos pacientes. Se realizó un estudio exploratorio en 52 pacientes que habían recibido más de 1,000 cGy de radiación. Se encuestó y realizó examen bucal a cada participante, tasa de flujo salivar estimulado y prueba de sabores. Los resultados muestran alta prevalencia de alteraciones bucales en pacientes con radiación acumulada entre 3,001 y 5,000 cGy. La boca seca (xerostomía) fue la alteración más sentida (78.8%). La tasa de secreción salivar total estimulada confirmó hiposalivación en el 82.7% de los pacientes. Se encontró una asociación estadísticamente significativa entre el lugar del cáncer (p < 0.01) y el tipo de tumor con la presencia de trismus (p < 0.05). La hiposialia se presentó más en los pacientes con tumores en estadio IV (50%) y en aquellos sometidos a tratamientos combinados (p < 0.05). Fue posible realizar la valoración periodontal al 50% de los pacientes, el 92% de ellos presentó periodontitis con pérdida de inserción principalmente severa y moderada. El 84.6% de los participantes manifestaron no haber sido remitidos a odontólogo antes o durante el tratamiento. Los hallazgos ratifican una alta frecuencia de alteraciones bucales en pacientes sometidos a tratamiento de radioterapia e inoportunidad de atención odontológica para prevenir o tratar estos efectos. Se alerta sobre la obligatoriedad de seguir protocolos de manejo del paciente oncológico, incluyendo valoración odontológica antes, durante y después del tratamiento respectivo.
Over 650,000 subjects are annually diagnosed with cancer of the head and neck (paranasal sinuses, nasal cavity, nasopharynx, oropharynx, mouth, hypopharynx and larynx). Treatments for patients afflicted with this type of malignant lesions are commonly radical surgery, radiotherapy and chemotherapy, in single or combined interventions which are decided upon depending on the anatomical location of the tumor, tumor stage as well as compromise of adjacent structures. It is widely acknowledged that these treatments cause early or late oral alterations in soft and hard tissues which compromise patient's welfare and quality of life. In cases of radiotherapy treatment, onset and severity of these alterations depend on radiated area, total radiation dosage, exposition time as well as the patient’ s personal characteristics.1
Among oral effects related to radiotherapy treatment, the following are reported in scientific literature: periodontitis, xerostomia, mucositis, dysgeusia, hyposalivation, trismus, candidiasis, other infections and osteoradionecrosis, which are considered debilitating effects for subjects thus afflicted. Alterations of pH and salivary flow are caused by damage to gland tissue, and generate fibrosis, degeneration, acinar atrophy and cell necrosis, causing a sensation of dry mouth (xerostomia); clinically, we can observe non-reversible and chronic hyposalivation which can derive in deleterious effects for the patient such as fungi and bacteria-caused local disease, halitosis and great discomfort when wearing dentures, which in turn favor mucositis, taste alterations (dysgeusia) functional alterations in deglutition, speech and other complications in hard tissues.2
Mucositis is characterized by erythema, edema, epithelial detachment, ulcers and formation of pseudomembranes. Patients’ symptoms can be so severe as to, in certain cases, prompt the patient to abandon treatment (11% of cases).3 Candida infection is compounded in this lesion, thus increasing symptoms and fostering the fact of impossible clinical distinction to presence of independent candidiasis or mucositis.3
Dysgeusia signifies an alteration of the sense of taste due to damage of lingual gustatory corpuscles; it is fostered by mucositis and hyposialia. Trismus is the inability to properly open the mouth, and is the result of fibrotic changes in the muscles and temporomandibular articulation; this is one of the late effects which can appear 3 to 6 months after treatment completion.
In Colombia, according to a tracking conducted by the authors, no publications were found on studies of prevalence of these alterations in the population treated with ionizing radiation. Therefore, the aim of the present research project was to determine the aforementioned alterations in patients afflicted with malignant tumors in the head and neck, previously treated with radiotherapy administered at the Oncology service of the City of Medellin and the Metropolitan Area in 2013. An additional target was to explore the participation of general dentists or specialists in the patients’ treatment team in order to contribute to prevention or treatment of oral alterations which, due to radiotherapy might appear or worsen.
MATERIAL AND METHODSAn exploratory study was conducted with a convenience sample of 52 patients treated at three Health Provider Institutions (HPI) of the city of Medellin and Metropolitan Area, Antioquia, Colombia. Patients had received diagnosis of cancer in the head and neck and were under radiotherapy treatment, with over 1,000 cGy received.
The study was approved by the Ethics Committee of the School of Dentistry, University of Antioquia, the Committee for Research Development (CRD) of the same University and the Ethics Committees for Research of the HPI where participant patients received radiotherapy treatment. Patients were informed on the study's target, procedures to be undertaken, risks incurred, compensation for their participation as well as subjects responsible for the study, among other matters contemplated in Resolution 8,430, 1993, Health Ministry, Colombia. After being advised, patients were requested to sign an informed consent form. For underage patients, responsible adult or companion's assent as well as the minor's compliance were procured.
Contact with study participants was established at the time they attended their radiotherapy session. Data harvesting was conducted by means of a structured survey. This survey was composed of questions concerning patient's socio-demographic and clinicalpathological characteristics, treatment to which he was subjected and a clinical oral examination (soft tissues and periodontium). These procedures were achieved before a pilot test conducted by five dental students, a stomatologist and a periodontist, who were standardized for field work. Additionally, a sample of stimulated salivary flow was taken from all patients and they were subjected to a test of flavors. These examinations were conducted to track existence of mucositis, candidiasis, xerostomia, hyposialia and dysgeusia. Laboratory exams were processed and analyzed at the Microbiology and Histopathology Laboratory of the School of Dentistry of the University of Antioquia, using values for the stimulating salivary secretion rate according to Per Axelsson,4 values suggested by Epstein5 were used to count Candida albicans.
Mucositis was classified according to agreement NCI-CTC V 2.0,6 where Grade 0 represents absence, grade 1 erythema in the mucosa, grade 2 irregular, noncontiguous pseudo-membranous lesions measuring 1.5cm, grade 3: confluent pseudomembranous lesions larger than 1.5cm and grade 4: necroses or deep alterations with bleeding induced by minor trauma. In the case of trismus, the patient was requested to open his mouth as much as possible, then measurement was taken with a millimetric gauged ruler placed between incisal edges of teeth 41 to 11 and from 21 to 31. In edentulous patients measurements were taken from the edge of the maxillary gingiva to the ridge of the mandibular gingiva. With respect to trismus, measurement of 35mm or less was considered positive, and higher measurements were considered negative (healthy), which was equally the case when the patient informed that he had always had a small opening. Dysgeusia was recorded through identification test performed by the patient with respect to sweet, salty, acid and bitter flavors prepared in dilutions of sugar 60 mL/L, table salt 60 mL/L, hydrochloric acid 15 mL/L and urea 200 mL/L. The patient placed in his mouth 5mL of the solution, without swallowing, for three seconds, he then discarded it and rinsed his mouth with distilled water for one minute. Non recognition of at least one flavor indicated positive dysgeusia. Flavor called Umami was not assessed in this study due to difficulties encountered in its procurement, and because literature reports greater affectation in the four basic flavors, especially bitter and acid.7
Hyposalivation was confirmed through rate of stimulated salivary flow with positive value of lesser than 0.7 mL/min. Patients were asked to sit down, and a 2 × 2cm piece of paraffin was provided to be held in the mouth until it was softened (30seconds approximately) and were then allowed to swallow saliva accumulated during that time. Patients were then requested to chew the paraffin for one minute at normal speed and asked to expectorate into a trial tube for fourt minutes. Finally, volume was measured and expressed in mL/min. An agar culture for candida was achieved from this same sample, so as to count UFC/mL; reference value was > 4 × 102 UFC/mL.
Periodontal examination was conducted in patients older than 12 years of age, with over 10 teeth present in the mouth, using periodontal millimeter probe (UNC- 15) in six locations per tooth. Measurement of probing depth (PD) was recorded in mm, as well as clinical attachment level (CAL) and bleeding on probing (BOP%). After this, average ± SD was calculated. Diagnosis of chronic periodontitis and gingivitis according to severity and extension was determined following indications of the American Academy of Periodontics (AAP 1999). Loss of insertion severity was classified as: mild, 1-2mm, moderate 3-4mm and severe ≥ 5mm. Periodontitis extension was defined as amount of affected sites and was classified as: localized ≤ 30% and generalized > 30%.
Validation of information provided by the patient, with respect to responses with high probability of error or forgetfulness was conducted through data search in the clinical history. When some information provided by the patient did not match data included in the clinical history, the latter were taken as valid.
A previous process of standardization of research team participants was conducted for all procedures of field work. A pilot test was conducted to refine instruments and techniques to be used.
Statistical analysisData of all questionnaires were introduced and stored in Microsoft Excel 2007, previously conducting a process of manual cleansing, codification and digitalization, Later this information was exported to SPSS, version 19.0; through this software and with the support of Epidat 3.1 statistical data corresponding to univariate and bivariate analysis were calculated. In order to evaluate and describe behavior of sociodemographic variables and clinical and lesion characteristics of the patients with radiotherapy, proportion of these variables were estimated with their respective CI 95%, according to each lesion. In order to analyze association of aforementioned variables in recording time, statistical χ2 of association was applied.8 Finally, to assess correlation among periodontal parameters and radiation, Kendall test was applied.8
RESULTSOut of the 52 patients who met inclusion criteria, 36 were male (69.2%) and 16 female (30.8%). When analyzed according to age and socioeconomic status it was observed that adults over 60 years of age were predominant (61.5%; CI95% = 47.4-75.5) mainly belonging to middle class, that is to say levels 3 and 4 (44.2%, CI95% = 29.8-58.7). Most of these patients were in a partner's relationship (59.6%, CI95% = 45.3- 73.9) either living together or married, have studied secondary school, (32.7% CI95% = 19.0-46.4) were of mixed race (78.8%, CI95% = 66.8-91.0), were affiliated to the contributive regime (59.6%, CI95% = 45.3-73.9) and did not possess another health preventive measure (88.5%; CI95% = 78.8-98.1). The majority of these patients were involved in elemental occupations (23.1% IC95% = 10.7-35.5) such as housewife, messenger, miner, varied occupations or street sellers.
With respect to diagnoses characterizing patients, clinical history evidence revealed that cancer's most frequent location was the larynx (34.6%; CI95% = 20.7-48.5). Generally, these tumors were found at stages 3 and 4, with respective percentages of 32.7% and 46.2%. Likewise, data revealed that for every 10 patients treated with radiotherapy, approximately 7 suffered some type of systemic disease.
When conducting the study, minimum dosage received by a patient was 1,476 cGy and maximum dosage was 7,000 cGy, out of which 48.1% of all patients had received from 3,001 cGFy to 7,000 cGy of accumulated radiotherapy doses When assessing treatment received by patients, it was found that in addition to radiotherapy, 32.7% were also treated with chemotherapy, 30.8% had been subjected to surgery and were receiving chemotherapy and 23.1% had previously been subjected to only surgery. Only 13.5% were receiving radiotherapy only. Out of all 52 patients, 94.2% were treated with teletherapy, and 5% with IMRT (Intensity Modulated Radio Therapy); 60.0% of all patients subjected to some type of combined treatment exhibited the disease at stage II.
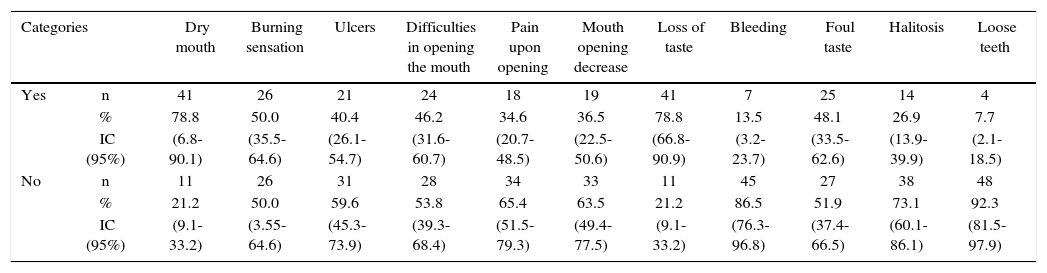
Most frequent discomfort in the mouth suffered by patients were dry mouth and loss of taste (78.8%; IC95% = 66.8-90.1), followed by burning sensation (50.0% CI95% = 35.5-64.6), whereas the less frequent were presence of loose teeth (7.7%; CI 95%=2.1-18.5) and bleeding (13.5% CI95% = 3.2-23.7) (Table I).
Most frequent mouth alterations reported by head and neck cancer patients subjected to radiotherapy. Medellin 2013.
| Categories | Dry mouth | Burning sensation | Ulcers | Difficulties in opening the mouth | Pain upon opening | Mouth opening decrease | Loss of taste | Bleeding | Foul taste | Halitosis | Loose teeth | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | n | 41 | 26 | 21 | 24 | 18 | 19 | 41 | 7 | 25 | 14 | 4 |
| % | 78.8 | 50.0 | 40.4 | 46.2 | 34.6 | 36.5 | 78.8 | 13.5 | 48.1 | 26.9 | 7.7 | |
| IC (95%) | (6.8-90.1) | (35.5-64.6) | (26.1-54.7) | (31.6-60.7) | (20.7-48.5) | (22.5-50.6) | (66.8-90.9) | (3.2-23.7) | (33.5-62.6) | (13.9-39.9) | (2.1-18.5) | |
| No | n | 11 | 26 | 31 | 28 | 34 | 33 | 11 | 45 | 27 | 38 | 48 |
| % | 21.2 | 50.0 | 59.6 | 53.8 | 65.4 | 63.5 | 21.2 | 86.5 | 51.9 | 73.1 | 92.3 | |
| IC (95%) | (9.1-33.2) | (3.55-64.6) | (45.3-73.9) | (39.3-68.4) | (51.5-79.3) | (49.4-77.5) | (9.1-33.2) | (76.3-96.8) | (37.4-66.5) | (60.1-86.1) | (81.5-97.9) | |
n = 52 patients.
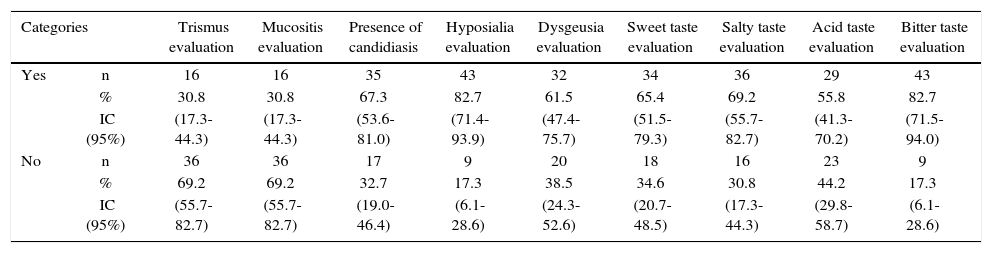
The main finding revealed by clinical and complementary examinations conducted in radiotherapy treated patients was hypo salivation, which was found in 82.7% of patients, average salivary flow was 0.54 mL/min, varying from 0.17 to 1.24 mL/ min, with a laboratory reference value of 1-3 mL/min (Table II).
Oral clinical characteristics of patients with head and neck cancer treted with radiotherapy. Medellin 2013.
| Categories | Trismus evaluation | Mucositis evaluation | Presence of candidiasis | Hyposialia evaluation | Dysgeusia evaluation | Sweet taste evaluation | Salty taste evaluation | Acid taste evaluation | Bitter taste evaluation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | n | 16 | 16 | 35 | 43 | 32 | 34 | 36 | 29 | 43 |
| % | 30.8 | 30.8 | 67.3 | 82.7 | 61.5 | 65.4 | 69.2 | 55.8 | 82.7 | |
| IC (95%) | (17.3-44.3) | (17.3-44.3) | (53.6-81.0) | (71.4-93.9) | (47.4-75.7) | (51.5-79.3) | (55.7-82.7) | (41.3-70.2) | (71.5-94.0) | |
| No | n | 36 | 36 | 17 | 9 | 20 | 18 | 16 | 23 | 9 |
| % | 69.2 | 69.2 | 32.7 | 17.3 | 38.5 | 34.6 | 30.8 | 44.2 | 17.3 | |
| IC (95%) | (55.7-82.7) | (55.7-82.7) | (19.0-46.4) | (6.1-28.6) | (24.3-52.6) | (20.7-48.5) | (17.3-44.3) | (29.8-58.7) | (6.1-28.6) | |
n = 52 patients.
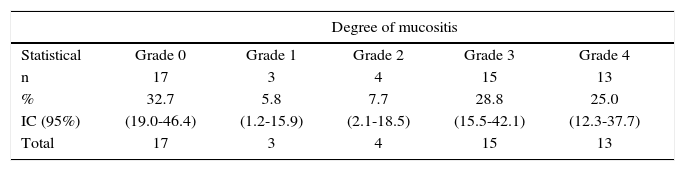
According to mucositis degree, 28.8% of all patients exhibited grade 3 mucositis; 25.0% presented grade 4 mucositis and 13.5% showed mucositis grades 1 and 2 (Table III). Candidiasis was found in 67.3% of all patients. This clinical finding was assessed by means of candida culture in saliva sample. Average count was 2.78 × 10s with deviation of ± 7.39 × 10s. When considering 4 × 102 CFU (colony forming units) as reference value, candidiasis was confirmed in 59.6% of all patients (Table II).
Degrees of mucositis in patients with head and neck cancer treated with radiotherapy. Medellin 2013.
| Degree of mucositis | |||||
|---|---|---|---|---|---|
| Statistical | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| n | 17 | 3 | 4 | 15 | 13 |
| % | 32.7 | 5.8 | 7.7 | 28.8 | 25.0 |
| IC (95%) | (19.0-46.4) | (1.2-15.9) | (2.1-18.5) | (15.5-42.1) | (12.3-37.7) |
| Total | 17 | 3 | 4 | 15 | 13 |
With respect to taste alterations (dysgeusia) it was found present in 7 out of every 10 patients treated with radiotherapy; acid taste was the less recognized (44.2%, CI95% = 29.8-58.7) and sour taste was the best identified flavor (82.7%, CI95% = 71.5-94.0). With respect to trismus, 30.8% (CI95% = 17.3-44.3) of all patients were diagnosed with this alteration. Maximum mean opening expressed in millimeters was 41.9mm with deviation of ± 13.11mm. It was observed that opening value was very disperse, due to the fact that mean value varied from 28.83 and 55.05 millimeters.
A statistically significant association was found between location of cancer lesion (p value < 0.01) and type of tumor with presence of trismus (p value < 0.05).
Oral alterations were more frequent in patients with accumulated radiation dosage varying from 3,001 to 5,000 cGy. Patients diagnosed with malignant tumor in the nasopharynx exhibited greater trismus (25.0%) (p < 0.01). Hyposialia was more frequent in patients with stage IV tumors (50.0%) and in patients subjected to combined treatments such as chemotherapy and a combination of surgery, radiotherapy and chemotherapy, both cases exhibited percentages of 34.9% (p < 0.05).
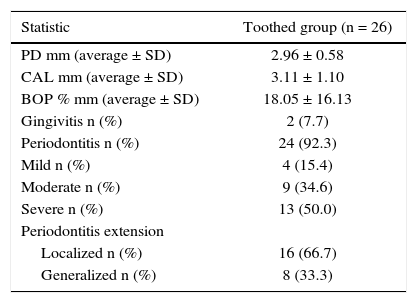
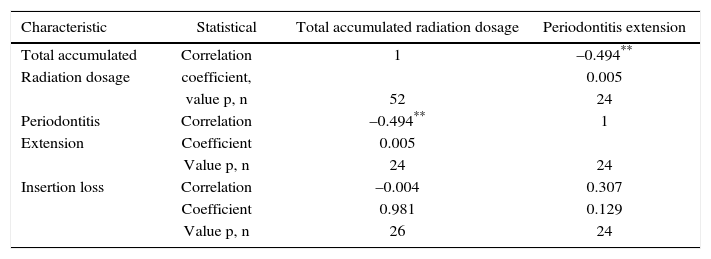
Periodontal examination was conducted on 26 patients which met requirements of remaining teeth, that is to say 50% of the sample Out of these patients, 92% exhibited periodontitis with mainly severe and moderate loss of attachment (Table IV). In average, patients exhibited 22 teeth present in the mouth and 6 absent teeth. Accumulated radiation dosage was negatively related to periodontitis extension (Table V).
Periodontal conditions of patients with head and neck cancer treated with radiotherapy. Medellin 2013.
| Statistic | Toothed group (n = 26) |
|---|---|
| PD mm (average ± SD) | 2.96 ± 0.58 |
| CAL mm (average ± SD) | 3.11 ± 1.10 |
| BOP % mm (average ± SD) | 18.05 ± 16.13 |
| Gingivitis n (%) | 2 (7.7) |
| Periodontitis n (%) | 24 (92.3) |
| Mild n (%) | 4 (15.4) |
| Moderate n (%) | 9 (34.6) |
| Severe n (%) | 13 (50.0) |
| Periodontitis extension | |
| Localized n (%) | 16 (66.7) |
| Generalized n (%) | 8 (33.3) |
PD = Probing depth, CAL = Clinical attachment level, BOP = Bleeding on probing, SD = Standard deviation.
Correlation between periodontal parameters and radiation dosage in patients with head and neck cancer treated with radiotherapy. Medellin, 2013.
| Characteristic | Statistical | Total accumulated radiation dosage | Periodontitis extension |
|---|---|---|---|
| Total accumulated | Correlation | 1 | –0.494** |
| Radiation dosage | coefficient, | 0.005 | |
| value p, n | 52 | 24 | |
| Periodontitis | Correlation | –0.494** | 1 |
| Extension | Coefficient | 0.005 | |
| Value p, n | 24 | 24 | |
| Insertion loss | Correlation | –0.004 | 0.307 |
| Coefficient | 0.981 | 0.129 | |
| Value p, n | 26 | 24 |
Oral alterations found in participants was evident, 84.6% of them reported they had not been remitted to dental assessment or treatment either before or during radiotherapy treatment. Patients who were remitted to this assessment (15.4%, CI95% = 4.6-26.2) indicated that remittance was mainly directed by the treating physician. Thus, at the time of the present study, 98.1% of all patients were not under dental treatment or in any dental follow-up program. When interrogating the patients with respect to whether they had been informed about oral alterations which might be present during radiotherapy, 86.5% informed that they had indeed received that information, approximately 50.0% of all patients had received that information from treating physicians, radiotherapy operators and nurses, 28.0% had been informed by media and only one patient had received it at a dental appointment.
The professional health team, which all patients identify as close and important during the treatment process from diagnosis onwards is composed of an oncologist and radiotherapist, in a lesser proportion patients identified other professionals such as nutritionist, psychologist, speech therapist and physiotherapist. For this group of patients, need and importance of dentist participation was not so evident.
When research was conducted with professionals implicated in the patient care, with respect to the reasons why patients had not been remitted to a dentist, it was pointed out that there were difficulties with these remissions, mainly caused by delays to access a dental appointment, as well as mobility barriers of the patient within the network of health services. In a hospital environment, presence of a dentist is very occasional or even non-existent. Another aspect subject to question was the training of a dentist to provide care to patients with complex health status as found in patients with this type of diagnosis. On the other hand, reasons related to the general circumstances of the patient which might hinder frequent trips to the dentist were equally highlighted.
DISCUSSIONEven though head and neck cancer represents 5.0% of all cancer cases, this disease and its treatment exert a huge, perhaps even disproportionate impact on all aspects of the patient's quality of life. Worldwide, approximately 40% of patients suffering cancer of the head and neck are at an advanced stage by the time the disease is diagnosed;1 that is to say, they are at stages III and IV; 30% will suffer local recurrence, 25.0% will exhibit distance metastasis with local and regional recurrence, and will reach global five years survival of 30.0-40.0% in spite of technological advances in radiotherapy and innovative efforts of chemotherapy agents and surgical techniques.9–12
It must equally be taken into consideration that Health Services suffer access barriers, and services are every day more saturated due to care and treatment demands; this causes delays in attention time given to the patients, and, when treatment is finally available, the condition has reached more advanced levels. Additionally health personnel exhibit limitations to recognize symptoms, suspect a diagnosis, confirm the suspicion, conduct necessary inter-consultations and might even cause therapeutic and diagnostic deviations of the patient to inappropriate attention levels.
Considering this situation of advanced-stage tumors at the time of first contact, treatment must be immediately initiated, before the patient had access to evaluation and dental treatment recommended in protocols, increasing thus risk of complications, discomfort and disability. In the present study, the situation found was one of urgency for oncologic treatment in patients lacking initial dental evaluation.
Based on statistics of the Colombian National Cancer Institute, in the period comprised 2002 and 2006, cancer incidence in lip, mouth and pharynx was 1,566 cases, out of which 242 were found in Antioquia, 123 in males, and 119 in females13 in this region. It can be stated that malignant tumors of the head and neck are diagnosed at advanced stages (78.9% of all patients were diagnosed at stages III and IV) situation caused by the own patient, the Health Social Security System and health personnel. Most frequently found factors related to patient's tardiness in the identification of his own symptoms, the probability of developing a disease or to detect its onset in order to timely attend in order to attend a health provider facility are the following: education level, socioeconomic situation and cultural characteristics.
Findings of the present study concur with other worldwide reports: a 2:1 male/female relationship was found for head and neck malignant tumors, with average age of 60 years, in subjects belonging to middle class, and having completed secondary school education level. The most frequent cancer was located at the larynx. It is important to note that subjects participating in the present study (except one) were attached to some Health Social Security Regime, this fact would then facilitate treatment and care of the disease.
In general terms, head and neck cancer at early stages is easy to treat with only one modality, be it surgery or radiotherapy. A more extended tumor is frequently treated with a combination of surgery and radiotherapy, or with radiotherapy combined with adjuvant chemotherapy. In the present study it was found that over 90% of all patients were subjected to combined treatments; in patients with tumors at stage II (60%) the most frequent was a combination of surgery, conventional radiotherapy (teletherapy) and chemotherapy. This treatment is not common for tumors at those stages, nevertheless, exceptional cases might exist according to tumor type and location in which a different protocol might have been followed according to medical criterion. The complexity of the disease, and the enormous emotional burden of suffering a cancer lesion, as well as treatment required for this disease, generate in patients organic, psychological and social effects which require a multidisciplinary approach involving concerted decision making as well as sequencing among different treatment types, which, besides being timely, must be continuous and suitable.
Like for patients in the present study, radiotherapy for head and neck tumors varies in dosages of 5,000 to 7,000 cGy on the lesion and surrounding area. It is administered in fractions of 150-250 cGy per day, for five days a week, during 6-7 weeks, until reaching the desired amount.14 At the aforementioned dosages, presence of different effects in the mouth have been well recognized, like in 82.69% of studied patients. This figure is somewhat below other figures reported in scientific literature, where reports are made of 90% to 100%, with alterations which become more evident at doses between 3,000 and 5,000 cGy as, previously reported.15
In a study of post-radiated patients conducted by Fisher in Brazil,16 the most frequent alteration reported by studied patients was dry mouth (xerostomia), which concurs with findings in the present study, where total stimulated saliva secretion confirmed presence of hyposalivation. Prevalence of both alterations (xerostomia 78.8% and hyposalivation 82.7%) were similar to those reported worldwide; these figures vary from 73.5-93.0%.17 Bearing this finding in mind, it is important to acknowledge that saliva decrease and pH alterations bring about and aggravate presence of other complications such as dysgeusia and mucositis. A positive correlation was found between presence of hyposialia and combined treatments composed of surgery, radiotherapy and chemotherapy (p < 0.05), which leads us to think that these combined treatments possibly affected salivary glands. On the other hand, 7 out of 10 patients were afflicted with some sort of chronic systemic disease such as diabetes, cardiovascular disease and medication intake which might also cause xerostomia (such as anti-hypertensive , anxiolythics or antidepressant drugs among others).18
Dysgeusia was the third most frequent alteration in subjects participating in the present study, after dry mouth (xerostomia) mucositis and candidiasis. After undertaking laboratory tests, candidiasis was observed in 59.6% of the sample, representing a very high figure when compared to other studies where frequency was reported between 17.0-29.0%.14 This difference is probably related to the clinical bias in differential diagnosis between candidiasis and different stages of mucositis. This was the reason that prompted the present study to verify candidiasis diagnosis with laboratory cultures. Some studies report a candidiasis increase of 62% and 80% in cases when clinical diagnosis is confirmed with a culture; difference in results depends on weeks of candidiasis treatments.19–21
With respect to mucositis, a systemic literature review comprising 33 studies showed that incidence was 97.0% with conventional radiotherapy;22 it is considered the most debilitating alteration during head and neck cancer treatment.23 In our study, results of mucositis presence showed lower proportion, nevertheless, they were more frequent in advanced stages, according to used system (grade 3 and 4); it was reported as the third most frequent discomfort (burning sensation) experienced by patients. No significant relationship was found between different degrees of mucositis and implemented treatments.
With respect to dysgeusia, studies report prevalence of 70-90%,24,25 pointing out that this alteration persists during the duration of treatment,26 and at high radiation levels total lack of taste is experienced. In most patients, this situation is reversible during the first year, although some might delay up to seven years to experience recovery.27 Taste alterations elicit very important effects in the nutritional status of the patient, they are associated to weight loss since the patient alters his nutritional patterns.
In a lesser proportion, literature reports trismus as a radiotherapy complication, with variations of 5.0% to 38.0%.28 In the present study a frequency of 30.8% was found.29,30 Even though reports show that normally it appears from 3 to 6 months after having completed radiotherapy and is irreversible,16 our findings were gathered during the course of treatment, therefore, preventive action was the better conduct to follow.
Periodontal circumstances of patients who still preserved some teeth was not favorable. Although association between attachment level and probing depth was not statistically significant, a trend to worse periodontal status was observed. With severe insertion loss and abundant tooth loss, patients experience compromised oral function, which can help to aggravate their general health circumstances. When analyzing correlation between accumulated radiation dosage and periodontitis extension, it was observed that it was mainly localized (66.7%). This is probably due to previous loss of teeth with poor prognosis and more severe insertion loss which decreases generalized periodontitis frequency. Nevertheless, poor periodontal circumstances predisposes patients to suffering recurrent infections such as candidiasis, ulcerative mucositis and even represent a risk factor for pulmonary and cardiovascular status.31–36
It must be mentioned that periodontitis prevalence in the studied sample was higher than that reported in the National Study of Oral Health (Estudio Nacional de Salud Bucal (ENSAB IV) which informed of a 61.8% in its different stages of severity.37 This supports the claim that these patients with special health circumstances are particularly sensitive to development of this type of periodontal alterations, and thus merit timely care in order to intervene in pre-existing risks, or that might be instated during the disease developmental stages of cycles of cancer treatment.
In spite of the fact that there is ample scientific evidence of oral alterations which might appear during radiotherapy treatment (supported by our study’ s results) and the fact that active participation of the dentist in the care of these patients might help to decrease severity of these secondary effects, it is worth noting that there is no clear process to achieve that this dental care be effective and be provided with monitoring or follow up of circumstances and changes in oral health, which patients might exhibit during the course of their cancer treatment. Thus, it is possible to obtain information on pre-existing conditions which might be aggravated by treatment, such would be the case of periodontal disease, or finally, infection risk might be enhanced. Clinical pictures associated to immunodeficiency induced by chemotherapy are: necrosis of the tumor induced by radiotherapy, bacterial proliferation, mechanical or thrombotic obstruction of the venous system, physical weakness, exaggerated growth of resistant pathogens, hospital infection, nutritional deficiencies and poor hygiene.38
Patients who are going to be treated with radiotherapy require dental evaluation before treatment, so as to conduct a comprehensive examination of all structures and tissues of the mouth (stomatologial, dental, endodontic, periodontal and articular examination), a radiographic assessment (set of panoramic and periapical X rays), a saliva test (measurement of saliva volume), a microbiological test (presence of pathogenic flora). All the aforementioned tests target procurement of final diagnoses to include in comprehensive medical history, establishing the relationship existing between prognosis and palliation, date determination as well as the decision to combine radio-therapeutic treatment with chemotherapy.39 In cases of severe oral morbidity, the patient might not be able to continue with the cancer treatment, in which case it is habitually interrupted. This dosage-related disorders caused by oral complications can directly affect patient's survival.
Moreover, importance of preventive dental care has clearly been shown. This care should focus on patient's education about oral hygiene habits to control alterations in oral soft tissues and tooth damage after cancer treatment. In any case, in cases the patients were to be already under chemotherapy or radiotherapy, dental control and supervision can be conducted during treatment, during treatment intervals, or even after treatment. To this effect, there are several varied publications of dental performance patterns.30,40–47
Although several studies deal with oral complications resulting from cancer treatment, there is a lack of comprehensive research projects where effectiveness of many preventive and/or treatment oral/dental protocols is assessed. In Colombia, Legislation 1384 (Sandra Ceballos Law) regulates and establishes actions for comprehensive cancer treatment, from comprehensive control for the disease in the population so as to decrease mortality and morbidity caused by adult cancer, as well as improving quality of life of cancer patients. This latter aspect can be achieved when the State and actors playing a role in the General System of Health Social Security guarantee all services necessary for cancer prevention, early detection, comprehensive treatment, rehabilitation and palliative care.
With respect to guides and protocols to this effect in the aforementioned law, article 7 paragraph 1 states that: the Social Protection Ministry, assisted by the National Cancer Institute and Scientific Clinical and/ or Surgical Societies directly related to oncology subjects, along with a representative of duly organized patient associations, will elaborate and adopt Guides of Clinical Practice in a six-month term after permanent instauration of the present law. In addition the following aspects will be equally and compulsorily adopted: protocols for handling diagnosis, treatment, rehabilitation and palliative care of neoplasms and related conditions in cancer patients.48
Although the aforementioned recommendations exist for dentists, and are based on scientific evidence, in order to comprehensively handle cancer patients (as well as oncological therapyinduced secondary effects in the mouth as specified at the Health District Ministry of Bogota),2 all recommendations must be permanently studied, reviewed, updated and divulged with participation of the issuing institutions and scientific societies. Moreover, necessary resources and conditions in health services are needed in order to compulsory comply to these measures and see them frequently monitored by controlling authorities.
It must be acknowledge that these patients require a multi-disciplinary approach and care coordination including informative, emotional and clinical support.49 Dentists should be involved in this support team as a full member of the cancer treatment team, they should be able to provide dental care during radiotherapy as well as after its completion, and even, as reported by Silvestre-Donat,39 months or years after cancer therapy completion, patients should still be subjected to oral hygiene care, observation and preservation of oral health, since some of the most severe complications have late manifestations. Such would be the case of trismus or osteorradionecrosis which might appear after no definite period of time.
Not all dentists will have the opportunity or exert the choice of being part of a cancer patient care team, nevertheless, as a health professional he can and must play a key role in prevention of catastrophic diseases, in a society where statistical figures on oral cancer are in the rise. A general dentist must leave university fully prepared to conduct, at least, unprejudiced observation and comprehensive clinical history, in order to devise health educational strategies wherever he might practice his profession.
In many areas of the world there have been studies on levels of awareness and knowledge on head and neck cancer (early detection and prevention) possessed by students and professionals of general medicine, internal medicine and family medicine, as well as general dentists and specilists.50–54 Results are consistent, identifying many voids and the clear necessity to review study plans in order to provide sufficient clinical training so that future general and specialized dentists become competent in early detection and cancer prevention at undergraduate and graduate levels. Researchers recommend compulsory incorporation of diagnostic tools used for oral cancer in training programs, since this has been one of the greatest weaknesses observed in students, and therefore, in future professionals.
CONCLUSIONSFindings of the present study ratify a high frequency of alterations in soft oral and periodontal tissues in head and neck cancer patients subjected to radiotherapy. This evidence shows the need to establish an effective follow-up of cancer patients handling protocols. These protocols include dental treatment to achieve comprehensive evaluation of the patient before, during and after treatment (surgery, radiotherapy or chemotherapy). Described complications can be reduced, therefore, the dentist's role the prevention and treatment of these alterations is important, since he can propose the most appropriate dental therapeutic care patterns. Any treatment alternatives must always be discussed with the patient. All dental professionals play thus a relevant role in the prevention and healing or control of oral complications in patients afflicted with head and neck cancer who are subjected to radiotherapy, since, by providing relief and eradication of symptoms they greatly contribute to improve these patients’ quality of life. Radiotherapy services must compulsorily remit patients to the dentist and conduct a follow-up of the patient's attendance to those visits.
Moreover, it is important that, from the training process onwards, the general dentist be prepared to achieve excellent clinical history of his patients. This will allow, in many cases, early detection of oral cancer, as well as increase in life expectancy and quality of life of patients who have been timely treated.