The aim of the present study was to characterize components of commercial cements used in dentistry MTA Angelus® White (Angelus Lodrina, Parana Brazil) and BiodentineTM (Septodont, Saint-Maur-des Fosses, France). Techniques used for said characterization were Scanning Electron Microscope, X-Ray Diffraction, X Ray Fluorescence, Electron Dispersion Spectrometry, and Infrared Spectroscopy. Both cements were mixed according to manufacturer's instructions. A study of surface texture was conducted with Scanning Electron Microscope (SEM), and X Ray Diffraction (XRD) analysis, and X Ray fluorescence analysis (XRF), an analysis of Dispersive Energy Spectrometry (DES), as well as an Infra Red Spectroscopy (IRS) in order to determine functional groups.
ResultsIn XRD analysis, a difference was found: Biodentine exhibited Na2 O and ZrO2. These elements were absent in MTA. MTA presented Cr2 O3 and BiO2 which in turn were absent in Biodentine. EDS analysis revealed that differences were found in the radio-opacifying agent, and that Biodentine presented CaCl2 differing in this from MTA. Statistical analysis conducted revealed statistically significant percentages in contents, even though components were found to be practically the same. SEM analysis revealed marked differences: MTA presented irregular and porous surface whereas Biodentine exhibited irregular and filament form.
ConclusionThere is a great similarity in the chemical components of MTA Angelus and Biodentine, with the exception of chemical components providing radioopacity, the size and form of the grain, and, in Biodentine presence of calcium chloride.
El propósito de este estudio fue caracterizar los componentes de los cementos comerciales para uso en odontología MTA Angelus® Blanco (Angelus, Lodrina, Paraná Brasil) y de BiodentineTM (Septodont, Saint-Maur-des Fosses, Francia) mediante Microscopia Electrónica de Barrido, difracción de rayos X, fluorescencia de rayos X, espectrometría de dispersión de electrones y espectroscopia infrarroja. Los dos cementos se mezclaron según las indicaciones del fabricante. Se les practicó un estudio de textura de superficie mediante el microscopio electrónico de barrido (MEB), un análisis de difracción de rayos X (DRX), un análisis de fluorescencia de rayos X (FRX), un análisis de espectrometría de energía dispersiva (EDS) y un análisis de espectroscopia infrarroja (IR), para determinar los grupos funcionales.
ResultadosSe presentó una diferencia en el análisis XRD entre Biodentine presentó Na2O y ZrO2 mientras que están ausentes en el MTA. El MTA presentó Cr2O3 y BiO2 ausentes en el Biodentine. En el análisis EDS las diferencias fueron en el agente radiopacador y que el Biodentine presentó Cl a diferencia del MTA y en el análisis estadístico realizado a pesar de que prácticamente se presentaron los mismos componentes los porcentajes en los contenidos de éstos fueron estadísticamente significativos. En el análisis de MEB hay una gran diferencia, el MTA presenta una superficie porosa e irregular, el Biodentine una forma fibrilar e irregular.
ConclusiónExiste una gran similitud en los componentes químicos entre el MTA Angelus y Biodentine con excepción de los componentes químicos para proporcionarles radioopacidad, el tamaño y la forma del grano y en el caso del Biodentine el cloruro de calcio.
Dental materials have been evolving alongside dentistry due to technological advances, which have assisted these materials to possess better physical, chemical and biological properties.
Retro-filling materials are commonly used in endodontic surgical procedures. An ideal endodontic reparation material should be radioopaque, biocompatible, with anti-bacterial effect, dimensionally stable, easy to manipulate and not be contaminated or affected by blood. Other desirable characteristics for the selected material would include for it to be osteo-inductor, provide suitable sealing against bacteria and fluids as well as being able to avoid filtrations when placed in humid environment and possessing sufficient resistance to compression and hardness.1
Many materials have been used to perform retrograde filling. Among them we can count amalgam, zinc oxide-eugenol, polycarboxylate cements, glass ionomer cements, composite resin, epoxy-resin, guttapercha and mineral trioxide aggregate (MTA) type cements based on Portland cement.
Main disadvantages of the aforementioned materials include micro-leakage, varied degrees of toxicity, as well as sensitivity to presence of humidity.2,3 Among these MTA has been recognized as a bioactive material,4 hard tissue conductor5 hard tissue inductor as well as biocompatible.6
MTA is a material commonly used for retrograde filling procedures, apex formation and perforation repairs, nevertheless its handling is less than ideal due to its long setting time and difficulties in preserving mix consistency.7
Calcium silicate cements, especially those derived from Portland cement, such as mineral trioxide aggregate (MTA) and others have been designed and are used in clinical dental applications.
Self-adjusting properties of calcium silicate cements are due to the progressive hydration reaction of orthosilicate ions (SiO4).
When calcium silicate particles react to water a hydrated calcium silicate nanoporous amorphous gel is formed (HCS gel) in the cement particles, while calcium hydroxide (Ca(OH)2) (portlandite) forms nuclei and grows in available gaps and spaces of the pores. With time, HCS gel polymerizes and hardens, forming thus a solid net which is associated to greater mechanical resistance. HCS gel is soluble in Ca(OH)2, released by the cement surface and increases alkalinity of surrounding environment.8
The purpose of the present study was to explore the components of MTA Angelus® White cement (Angelus, Lodrina, Paraná Brazil) and BiodentineTM (Septodont, Saint-Maur-des Fosses, France) by means of X-ray diffraction and electron dispersion spectrometry, X-ray fluorescence, as well as observing the surface with scanning electron microscope and infra red spectroscopy.
MATERIALS AND METHODSBoth cements used for the present project were divided into two groups:
- •
Group 1 MTA Angelus® White (Angelus, Lodrina, Parana, Brazil).
- •
Group 2: BiodentineTM (Septodent, Saint-Maur-des-Fosses, France).
One gram of the powder provided by the manufacturer was used for XRD and XRF analyses. For DES, SEM and IRS analyses all products were mixed using powder and liquid provided by the manufacturer. Manufacturer's instructions were strictly followed. One 8mm diameter x 4mm thickness sample was manufactured for each group. Five points were randomly taken for the analysis.
X ray diffraction analysis was conducted with a diffractometer Phillips Mod 1130/96 (generator) and pw1050/24 (goniometer) using CuKα at angular intervals ranging from 4° to 70°.
X ray fluorescence analysis (XRF): An X ray fluorescence quantitative chemical analysis was conducted with a Siemens SRS 3000 spectrometer, gauged with Geochemical Reference materials. This analysis was conducted with the sample in dry base, and loss by calcination (LBC) was determined by calcinating 1g of the sample at 950°C during one hour.
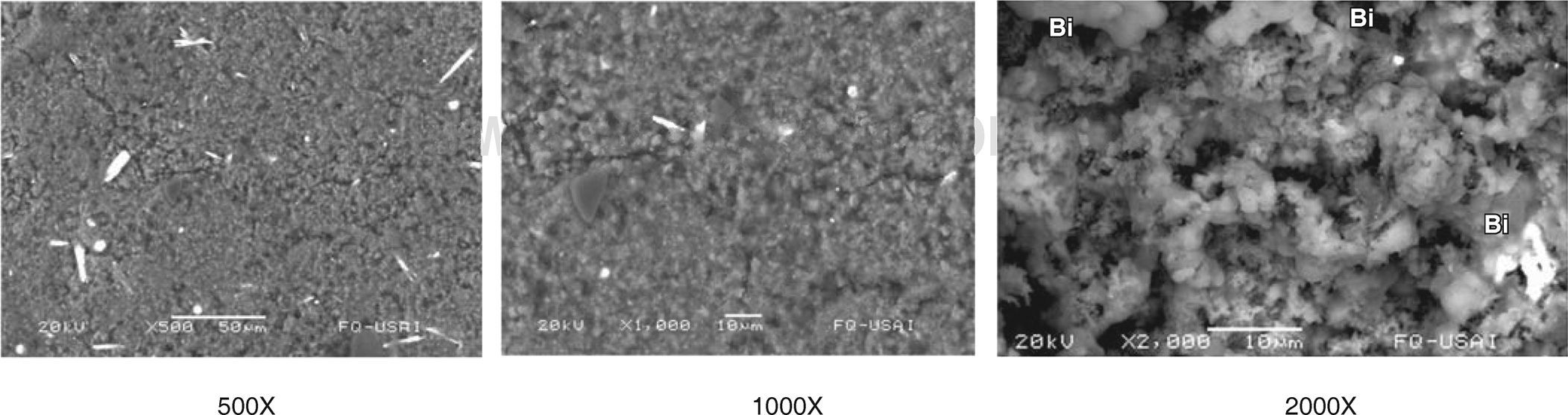
Dispersive energy spectrometry (DES) and scanning electron microscopy (SEM): Once hardened, the samples were placed on the sample holder with a carbon film to which they adhered. Observations were made with Scanning Electron Microscope (leol model 5900 LV, Tokio, Japan). Used magnifications were 500X, 1000X and 2000X.
For the dispersive energy spectrometry analysis (DES) an elemental chemical analysis was conducted with an Oxford device, ISIS model, with 133eV resolution, with carbon to uranium element detection. For the present study amplifi cations of 500X, 1000X and 2000X were used in all samples at four predetermined points.
Infrared spectroscopy (IRS): Samples were analyzed with attenuated total refl ectance technique (ATR) in a Bruker Brand spectrometer, model Vector 33 with 32 scanning and resolution of 4cm-1. Samples to be analyzed were directly placed on the diamond crystal for analysis, since this technique did not require previous preparation.
RESULTSXRD results of MTA and Biodentine were the following:
- •
In both cements presence of calcite and larnite was detected.
- •
In MTA there was presence of bismite, vesuvianite, and bassanite. These materials were absent in Biodentine.
- •
In Biodentine there was presence of hatrurite and baddeleyite. These materials were absent in MTA.
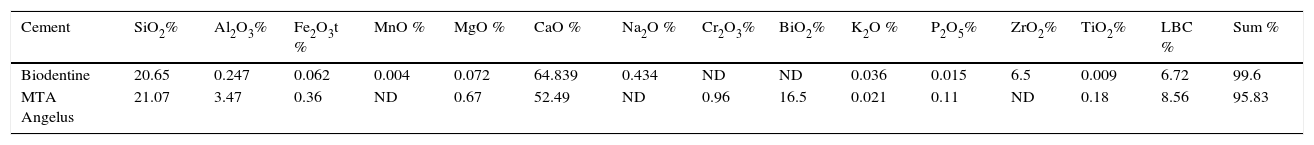
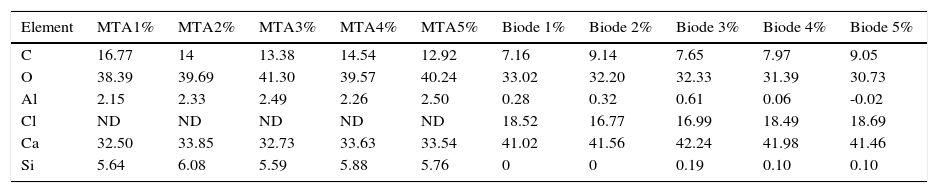
In XRF analysis, the following were identified in both cements: SiO2, Al203, Fe2O3t, MgO, CaO, K2O, P2O5, TiO2. MTA exhibited Cr2O3 and BiO2, these materials were not detected in Biodentine. Biodentine exhibited Na2O and ZrO2; which were absent in MTA Angelus (Table I).
Percentages and components found in Group 1 (MTA Angelus) and Group 2 (Biodentine) through ray diffraction procedures.
| Cement | SiO2% | Al2O3% | Fe2O3t % | MnO % | MgO % | CaO % | Na2O % | Cr2O3% | BiO2% | K2O % | P2O5% | ZrO2% | TiO2% | LBC % | Sum % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biodentine | 20.65 | 0.247 | 0.062 | 0.004 | 0.072 | 64.839 | 0.434 | ND | ND | 0.036 | 0.015 | 6.5 | 0.009 | 6.72 | 99.6 |
| MTA Angelus | 21.07 | 3.47 | 0.36 | ND | 0.67 | 52.49 | ND | 0.96 | 16.5 | 0.021 | 0.11 | ND | 0.18 | 8.56 | 95.83 |
ND = Not detected; LBC = Loss by calcination.
SEM analysis of samples revealed Biodentine structure with fibrillar and irregular shape, with crystal appearance (Figure 1) whereas MTA samples exhibited irregular and porous structure, loose granules were observed and identified as Bismuth (Figure 2).
DES results revealed presence of Bi in Group 1 (MTA Angelus). Bi wasn’t present in any other group. In Group 2 (Biodentine) CI presence was observed, which was absent in Group 1.
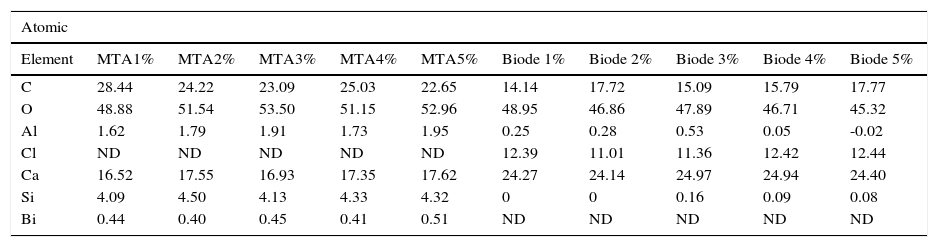
C, O, Al, Si, Ca were regularly detected in both groups (Tables II and III).
Percentages of elements found in the five samples of each cement.
| Element | MTA1% | MTA2% | MTA3% | MTA4% | MTA5% | Biode 1% | Biode 2% | Biode 3% | Biode 4% | Biode 5% |
|---|---|---|---|---|---|---|---|---|---|---|
| C | 16.77 | 14 | 13.38 | 14.54 | 12.92 | 7.16 | 9.14 | 7.65 | 7.97 | 9.05 |
| O | 38.39 | 39.69 | 41.30 | 39.57 | 40.24 | 33.02 | 32.20 | 32.33 | 31.39 | 30.73 |
| Al | 2.15 | 2.33 | 2.49 | 2.26 | 2.50 | 0.28 | 0.32 | 0.61 | 0.06 | -0.02 |
| Cl | ND | ND | ND | ND | ND | 18.52 | 16.77 | 16.99 | 18.49 | 18.69 |
| Ca | 32.50 | 33.85 | 32.73 | 33.63 | 33.54 | 41.02 | 41.56 | 42.24 | 41.98 | 41.46 |
| Si | 5.64 | 6.08 | 5.59 | 5.88 | 5.76 | 0 | 0 | 0.19 | 0.10 | 0.10 |
Percentages of elements found in the five samples of each cement.
| Atomic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | MTA1% | MTA2% | MTA3% | MTA4% | MTA5% | Biode 1% | Biode 2% | Biode 3% | Biode 4% | Biode 5% |
| C | 28.44 | 24.22 | 23.09 | 25.03 | 22.65 | 14.14 | 17.72 | 15.09 | 15.79 | 17.77 |
| O | 48.88 | 51.54 | 53.50 | 51.15 | 52.96 | 48.95 | 46.86 | 47.89 | 46.71 | 45.32 |
| Al | 1.62 | 1.79 | 1.91 | 1.73 | 1.95 | 0.25 | 0.28 | 0.53 | 0.05 | -0.02 |
| Cl | ND | ND | ND | ND | ND | 12.39 | 11.01 | 11.36 | 12.42 | 12.44 |
| Ca | 16.52 | 17.55 | 16.93 | 17.35 | 17.62 | 24.27 | 24.14 | 24.97 | 24.94 | 24.40 |
| Si | 4.09 | 4.50 | 4.13 | 4.33 | 4.32 | 0 | 0 | 0.16 | 0.09 | 0.08 |
| Bi | 0.44 | 0.40 | 0.45 | 0.41 | 0.51 | ND | ND | ND | ND | ND |
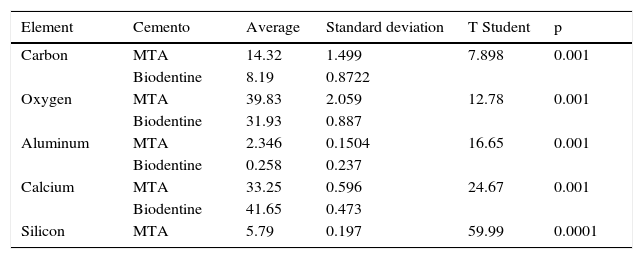
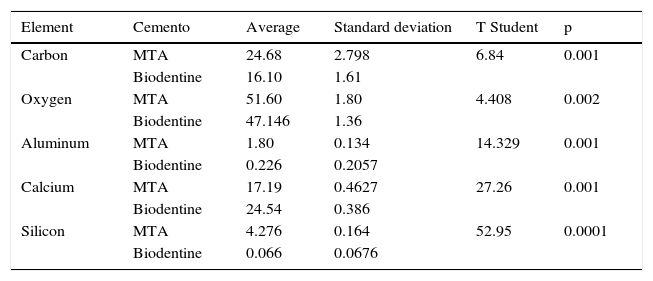
T Student test was used for statistical analysis. Results revealed that points from where samples were taken were homogeneous, and there were statistically signifi cant differences in the percentages of the varied detected components in both cements with DES tests (Tables IV and V).
Statistical analysis conducted with Student t-test.
| Element | Cemento | Average | Standard deviation | T Student | p |
|---|---|---|---|---|---|
| Carbon | MTA | 14.32 | 1.499 | 7.898 | 0.001 |
| Biodentine | 8.19 | 0.8722 | |||
| Oxygen | MTA | 39.83 | 2.059 | 12.78 | 0.001 |
| Biodentine | 31.93 | 0.887 | |||
| Aluminum | MTA | 2.346 | 0.1504 | 16.65 | 0.001 |
| Biodentine | 0.258 | 0.237 | |||
| Calcium | MTA | 33.25 | 0.596 | 24.67 | 0.001 |
| Biodentine | 41.65 | 0.473 | |||
| Silicon | MTA | 5.79 | 0.197 | 59.99 | 0.0001 |
Statistical analysis conducted with Student t-test.
| Element | Cemento | Average | Standard deviation | T Student | p |
|---|---|---|---|---|---|
| Carbon | MTA | 24.68 | 2.798 | 6.84 | 0.001 |
| Biodentine | 16.10 | 1.61 | |||
| Oxygen | MTA | 51.60 | 1.80 | 4.408 | 0.002 |
| Biodentine | 47.146 | 1.36 | |||
| Aluminum | MTA | 1.80 | 0.134 | 14.329 | 0.001 |
| Biodentine | 0.226 | 0.2057 | |||
| Calcium | MTA | 17.19 | 0.4627 | 27.26 | 0.001 |
| Biodentine | 24.54 | 0.386 | |||
| Silicon | MTA | 4.276 | 0.164 | 52.95 | 0.0001 |
| Biodentine | 0.066 | 0.0676 |
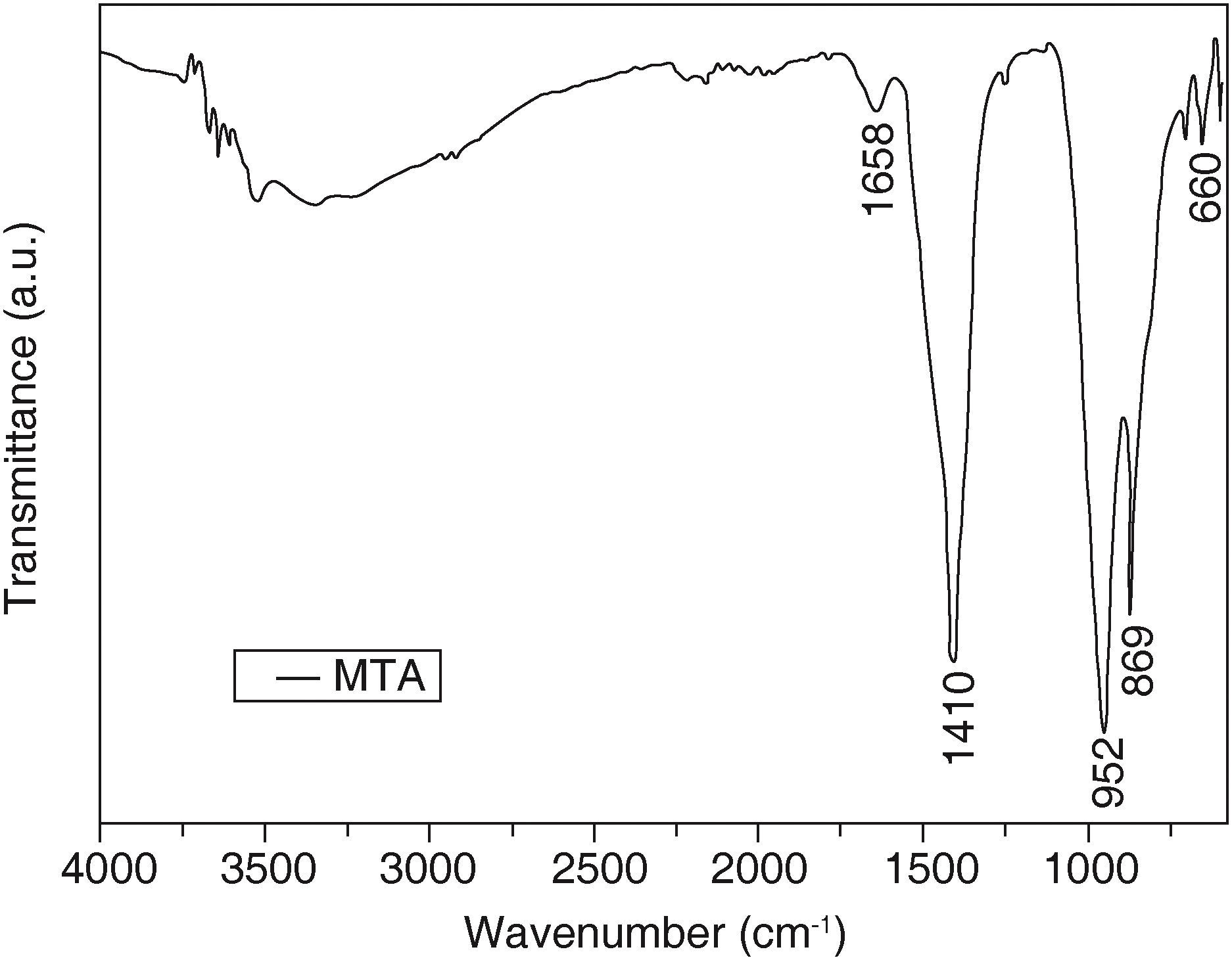
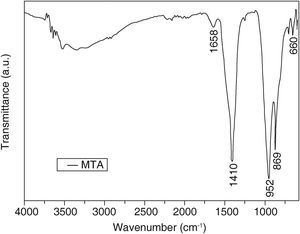
Results obtained from MTA sample (Figure 3) revealed calcium carbonate characteristic groups in the absorption bands in 1410,869 and 660cm-1 in the C-O group. The band 952cm-1 corresponded to calcium chloride (CaCl2).
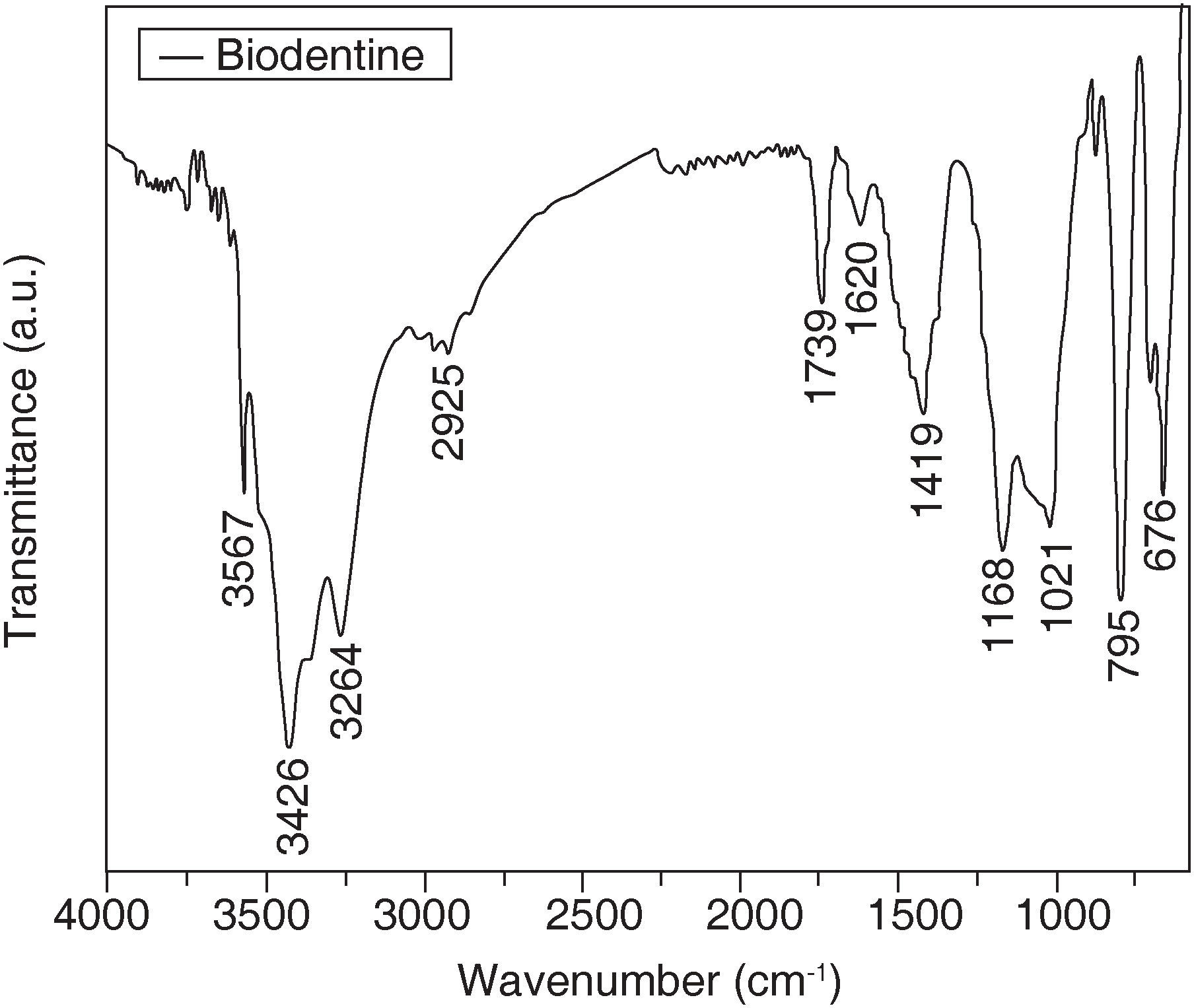
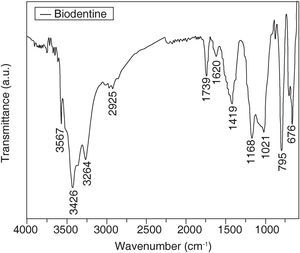
Figure 4 shows the Biodentine sample spectrum. It can be observed that bands at 3567 7 3426cm-1 correspond to groups N-H. The band at 2925cm-1 corresponds to methylene groups (CH2) The band at 1739cm-1 is assigned to C=O of a carboxylic acid, this is corroborated with bands at 1558 and 1419cm-1 with the group COO of carboxylic acids. The band at 1620 can correspond to an aromatic ring or a secondary amine. The aromatic ring can be corroborated in the region of 1410 to 1500cm-1 and the amine group at the 795cm-1 band. The 1168cm-1 band corresponds to the CN group. Bands between 702 and 676cm-1 correspond to monosubstituted groups (in the aromatic ring) C-CH2 and C-H respectively.
DISCUSSIONHydroxyapatite as such and other materials which contain Ca exhibit excellent biocompatibility, which is expressed in minimal tissue toxicity and reaction to foreign body, osteoconductivity and osteogenicity (LeGeros, 1991).9
The composition of Biodentine powder is tricalcium silicate, calcium carbonate and zirconium oxide (Koubi et al., 2011).10
Calcium chloride is one of the most efficient accelerators for Portland cement hydration and confi guration (Camilleri et al., 2006).11
Calcite (CaCO3), which was observed in both cements, has two different functions: 1. as active agent involved in the hydration process and 2. as filling which improves the cement's mechanical properties. (Garrault et al., 2006).12
Hydration of tricalcium silicate (3CaO.SiO2) leads to the formation of a hydrated calcium, gel silicate hydroxide (gel CSH) and calcium hydroxide (Ca(OH)2) (Taylor 1997).13
Biodentine surface examined with SEM reveals crystals of varied sizes in the shape of hexagonal plates of Ca(OH) 2 (Taylor 1997)13 which differs from the present study which observed crystals of fi lament shape.
Asgary14 reported in his XRD analysis of white MTA the presence of CaO, SiO2, Bi2O3Al2O3, MgO, SO3 Cl, FeO, P2O5, TiO2. In the present study, no Cl was found in MTA and contrary to Asgarty's reports presence of Cr2O3, K2O was observed.
White MTA is composed of a variety of oxides typically of SiO2, CaO and Al2O3. Among oxides, aluminum (Al), a neurotoxin, is harmful to human health due to its ability to alter cellular calcium homeostasis, and promote cellular oxidation (Zatta 2002).15
Gandolfi 16 reported that in his DES analysis of MTA he obtained the following elements: Ca, Si, Cl, Bi, and O. No Cl was found in the analysis we conducted, and C and Al were found in addition to results reported by Gandolfi.
Song17 in an DES analysis of MTA reported presence of Ca, Si, C, O, Mg, Al, S and Bi. Our analysis differed from Song's inasmuch as S and Mg were not present.
MTA has a proportion of 4:1 of bismuth, radioopacity oxide (Camilleri 200718, Torabinejad 199519) In the present study proportion of bismuth oxide was 16.5% as shown in Table I.
Asgary S et al20 reported in DES analysis the highest peaks of calcium, silica and bismuth contents. Nevertheless, aluminum, magnesium and especially iron peaks were significantly lower in white MTA, this could explain color differences. We concur with results obtained in our study.
CONCLUSIONChemical components of MTA Angelus and Biodentine are very similar, the exception would be in the chemical components which provide opacity, size and shape of grain, and in the case of Biodentine, calcium chloride.