Sodium hypochlorite (NaOCl) is the chemical agent most frequently used as irrigation solution during endodontic therapy. When extruded to periapical tissue, it is highly toxic. In endodontics, hemolysis caused by NaCOl has been proven using different models, nevertheless, there is little or no evidence of morphological alterations in the cellular membrane of erythrocytes.
ObjectiveTo propose an experimental model which might allow to assess morphological alterations suffered by erythrocytes when they are exposed to NaOCl used in the dental practice by means of high resolution scanning electron microscopy (SEM).
Materials and methodsIn the present study, 20mL of peripheral blood were obtained and deposited in tubes with EDTA (ethylenediaminetetraacetic acid) anticoagulant. Rinses were conducted with a phosphate buffer solution (Evan's solution). Several dilutions of the erythrocyte sample were prepared (1:1, 1:2, 1:4, 1:8 and 1:16); 100μL of each of these dilutions was obtained to be then confronted with 100μL of dental use 5.25% NaOCl (Viarzoni-T, Medental®); 0.5μL of these samples were taken to then be deposited in a sample holder made of Zn-Cu alloy which was subjected to a process of Cu ion metallization bath, following the old Spluttering method. Microphotographs were obtained with SEM.
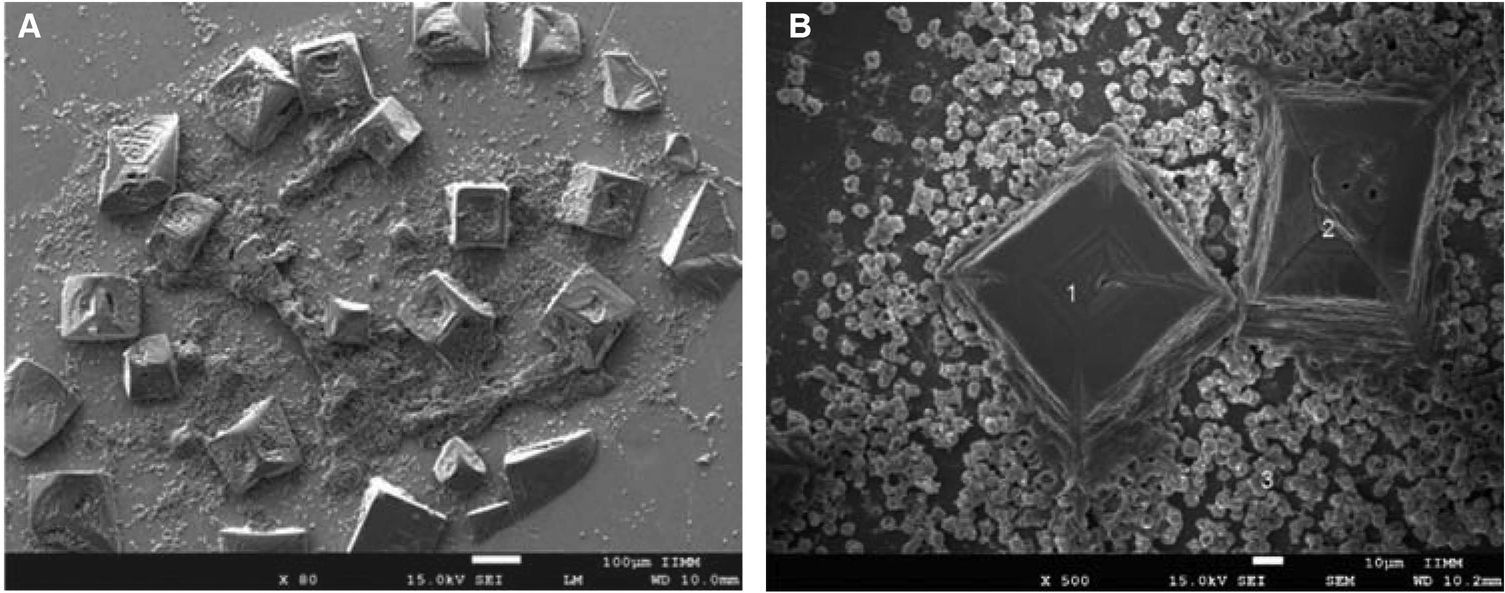
ResultsErythrocytes with alteration type anisocytosis and poikilocytosis (stomatocytes, elliptocytes and discocytes) were observed. Some structural characteristics of NaOCl crystals were equally observed.
ConclusionThis experimental model allowed assessment of morphological changes experienced by erythrocytes when exposed to 5.25% NaOCl.
El hipoclorito de sodio (NaOCl) es el agente químico más utilizado como solución irrigadora durante la terapia endodóntica. Es altamente tóxico cuando se extruye a tejido periapicales. En endodoncia la hemolisis causada por el NaOCl ha quedado demostrada utilizando diferentes modelos, sin embargo poca o ninguna evidencia se tiene de las alteraciones morfológicas en la membrana celular de los eritrocitos.
ObjetivoProponer un modelo experimental que permita evaluar las alteraciones morfológicas que sufren los eritrocitos cuando son expuestos a NaOCl utilizado en la práctica odontológica mediante microscopia electrónica de barrido de alta resolución (MEB).
Material y métodosSe obtuvieron 20mL de sangre periférica y se depositaron en tubos con anticoagulante EDTA (ácido etilendiaminotetraacético). Se realizaron lavados con solución amortiguadora de fosfatos (solución Evan's). Se prepararon diferentes diluciones de la muestra de eritrocitos (1:1, 1:2, 1:4, 1:8 y 1:16). Se obtuvieron 100μL de cada una de estas diluciones y se confrontaron con 100μL de NaOCl 5.25% de uso odontológico (Viarzoni-T, Medental®). Se tomaron 0.5μL de estas muestras para depositarse en un portamuestra de aleación Zn-Cu, el cual se sometió a un proceso de metalización de baño de iones de Cu por el método antiguo llamado Sputtering. Obteniendo microfotografías por MEB.
ResultadosSe lograron observar eritrocitos con alteración de tipo anisocitosis y poiquilocitosis (estomatocitos, eliptocitos, esferocitos y discocitos). También se observaron algunas características estructurales de cristales de NaOCl.
ConclusiónEste modelo experimental permitió evaluar los cambios morfológicos que sufren los eritrocitos cuando son expuestos a NaOCl 5.25%.
Sodium hypochlorite (NaOCl) is the agent most used as irrigating solution during endodontic therapy,1,2 this is due to its wide-spectrum antimicrobial activity3 and its ability to dissolve vital and necrotic tissue;4,5 it additionally exhibits low viscosity thus facilitating penetration into the root canal system.6 It is used at different concentrations ranging from 0.5% up to 6.0%.7 In scientific literature, the most recommended concentrations for irrigation use are 2.5% and 5.25%.8–10 Its antimicrobial action mechanism is explained by the reactions of aminoacid neutralization and chloramination, while the dissolution of organic matter is caused by saponification processes which are a product of the degradation of lipids and fatty acids.11
Contrary to its advantages, NaOCl by itself does not possess the ability to remove smear layers from the canal walls, moreover, it demineralizes dentin and induces corrosion of endodontic instruments.12–14 Nevertheless, NaOCl cytotoxicity is the property that requires greatest attention from the clinical operator. Extrusion of this irrigating material towards periapical tissue causes hemolysis, ulceration, inhibition of neutrophil migration as well as damage to endothelial cells and fibroblasts,15–17 which clinically manifests itself as pain, burning sensation, edema and hematoma.18
In endodontics, biocompatibility of material is determined by several parameters such as genotoxicity, mutagenicity, carcinogenicity, histocompatibility, antimicrobial effects and mutagenicity. For over 30 years, cell culture studies have been used to assess cytotoxicity reactions induced by endodontic materials.19
Among methods used to determine cytotoxicity we can count the following: determination of cell morphology alterations by means of light microscopy, confocal microscopy and scanning electronic microscopy (SEM).19 Evaluations conducted with SEM have been restricted to mainly showing alterations on periodontal and fibroblast cell lines.20–23
In other medical areas, scanning electronic microscopy has enabled evaluation of abnormalities of the red blood cell membrane.24 In endodontics NaOCl-caused hemolysis has been fully demonstrated using different models,25 nevertheless there is scarce or no evidence of morphological alterations in the cell membrane of erythrocytes when they come in contact with NaOCl solutions of dental use. Therefore, the aim of the present article was to propose an experimental model which might allow SEM assessment of morphological alterations experimented by erythrocytes when they are exposed to NaOCl used in dental practice.
MATERIAL AND METHODSCollection of biological sampleA 31 year old male was selected; he reported non-contributory pathological data; 20mL of peripheral blood were obtained and deposited in sterile glass tubes with EDTA anti-coagulant. Samples were centrifuged at 2,000rpm/5minutes, plasma was withdrawn with 100-1,000μL micro-pipettes (Spinreact®). For the cellular package, three washes were performed with Evan's solution (PBS phosphate buffer solution Allerstand®, registry 0027 R98 SSA, lot 407-360, Mexico DF) in a 2:1 proportion. A final red blood cell suspension of 2:1 was conducted with PBS.
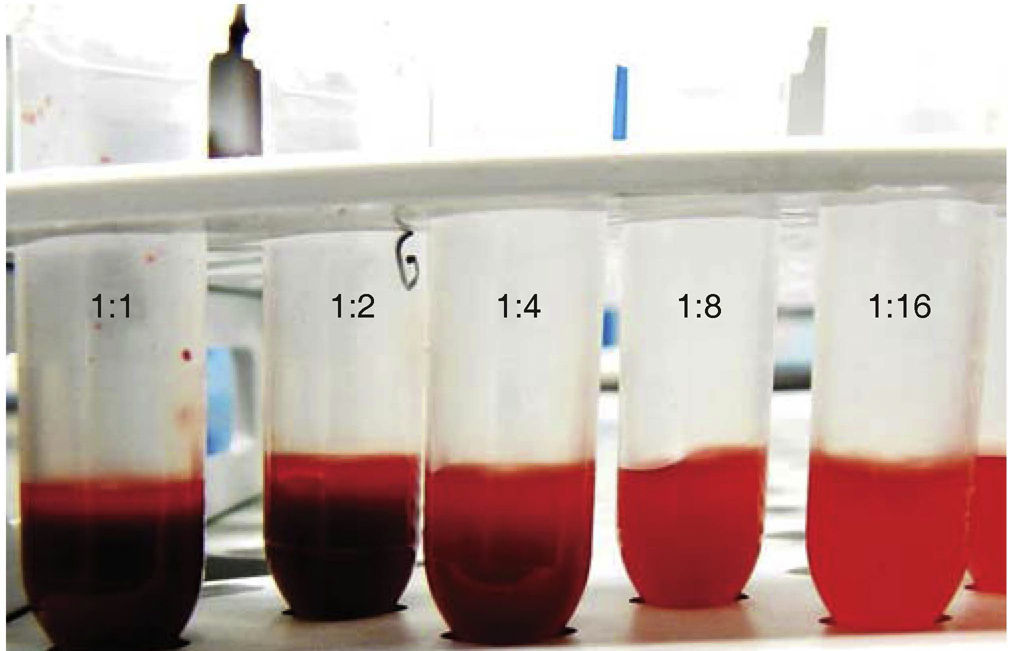
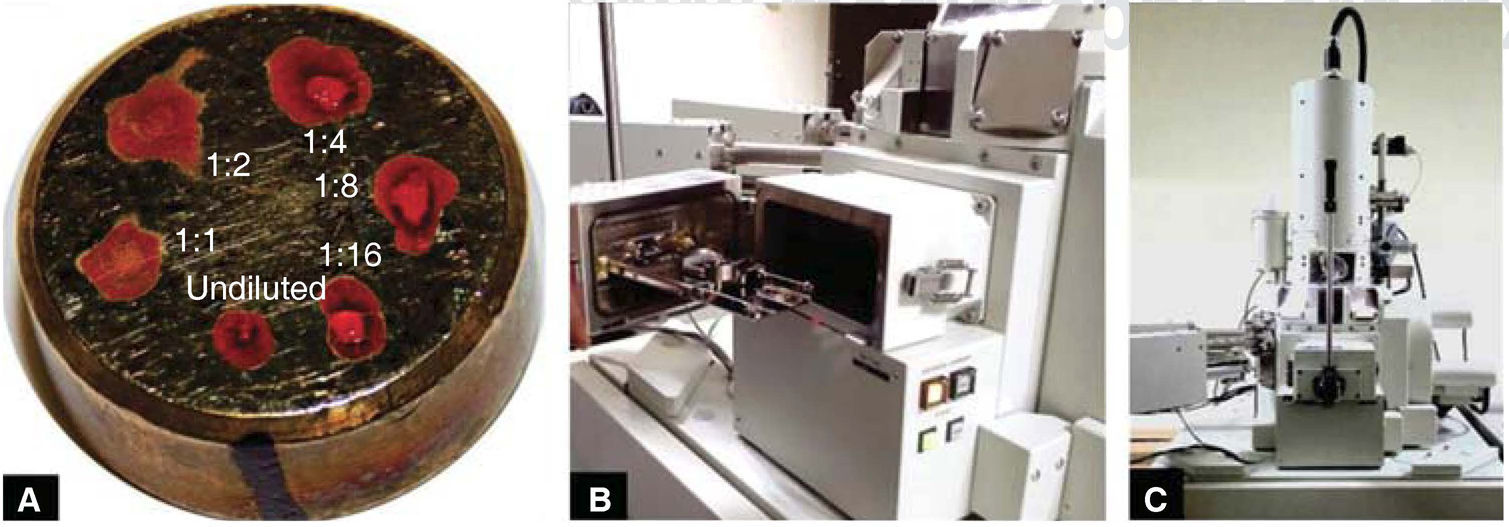
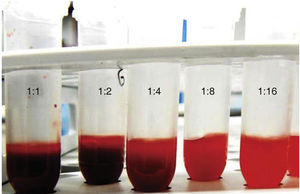
Red blood cell dilution preparationThe following different dilutions with PBS were conducted with the red blood cell suspension: 1:1, 12:2, 1:4, 1:8 and 1:16, in 1.5mL capacity propylene microtubes (Eppendorf®) (Figures 1 and 2). After this, 100μL of each of these solutions were obtained and confronted to 100μL of 5.25% NaOCl n (dental use); 5μL were taken and then deposited in a Zn-Cu alloy sample holder (1cm in diameter and height). Samples were tempered for 30 days.
After this, samples were subjected to a Cu ion metallization bath for 15minutes following the old Sputtering technique. This technique consisted on Cu ion bombardment on the surface of sample's blood red cells so as to achieve thin sheets, aiming at allowing the flow of electrons emanating from the filament beam to thus favor morphological analysis of red blood cells. To this effect equipment Vaccum coating model s150, Sputter Coating brand was used. This process of Cu ion metallization is a fundamental part of the proposed cellular experimental model development in order to evaluate the morphology of red blood cells exposed to dental use 5.25% NaOCl.
In order to analyze morphology of red blood cells, different magnitude images (110x, 250x, 500x, 1,000x, and 5,000x) were taken in the retro-dispersed secondary electron mode, a high resolution field electron scanning microscope JEOL JSM-7600F was used. Moreover, different spectrograms of the surface of different areas of the blood cells, Dispersive Energy Specters (DES), were obtained by means of Bruker Xflash 6130 Micro-analyzer (Figure 3) Likewise, weight percentage of elements forming the red blood cells was obtained. The aforementioned analyses were only determined at 1,000x images.
Physicians with expertise in the area of hematology (or better hematologically trained physicians) analyzed images obtained of all samples.
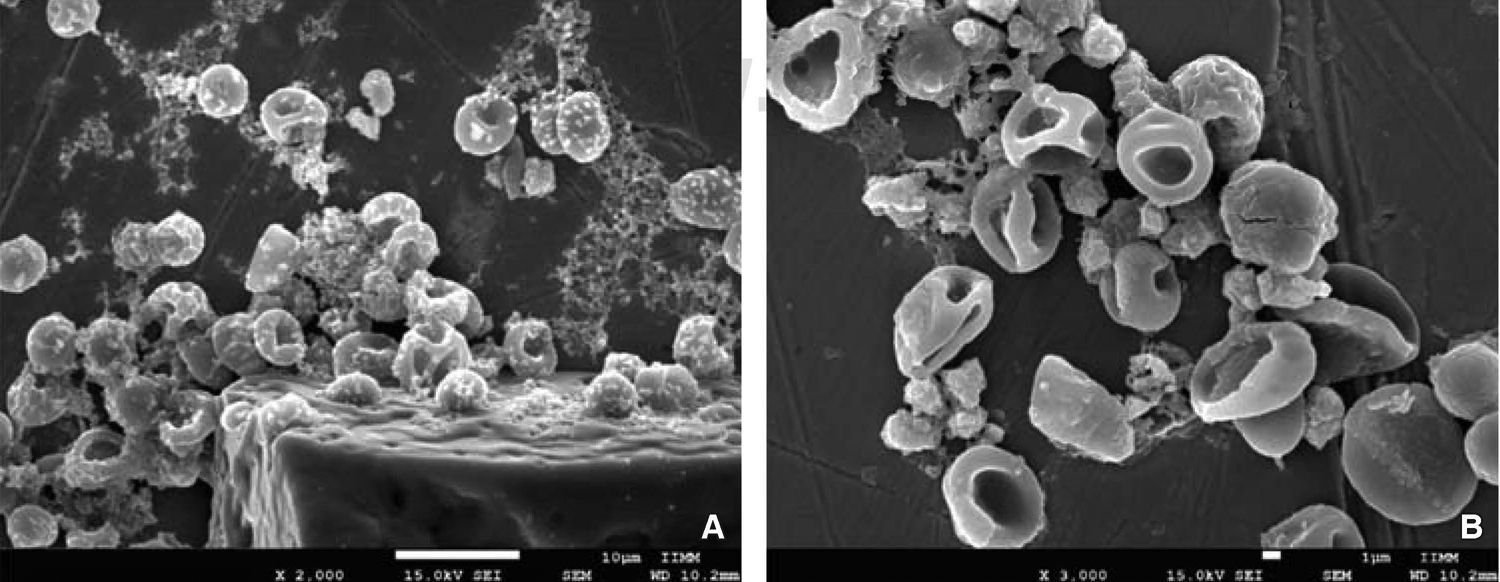
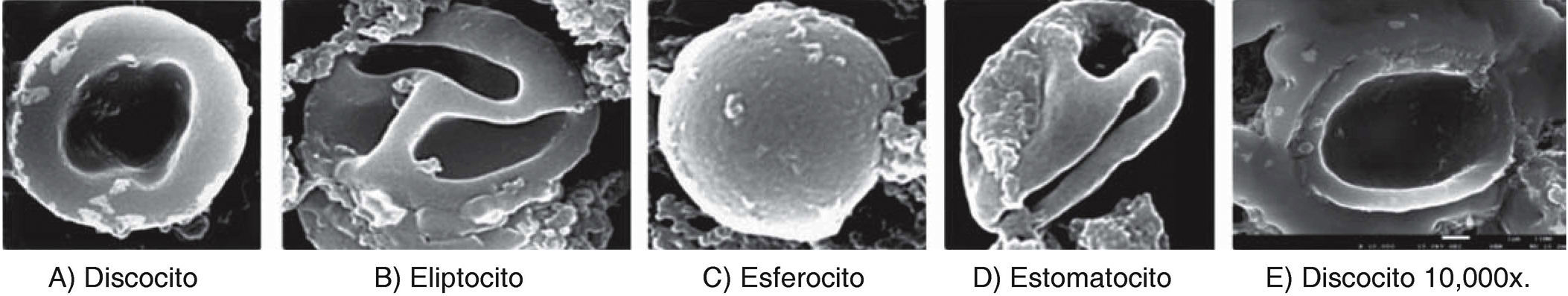
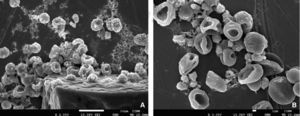
RESULTSWithin anomalies suffered by erythrocytes when exposed to dental use 5.25% NaOCl, red blood cells of different sizes were observed, which would indicate anisocytosis; abnormal shapes characteristics of poikilocytosis were equally observed (Figure 4). Among main poikilocytosis variations, stomatocytes, elliptocytes, spherocytes and discocytes were identified (Figure 5) Moreover, the present experimental model allowed identification of some structural characteristics of NaOCl crystals (Figure 6).
Morphological alterations of erythrocytes exposed to dental use 5.25% NaOCl, through images obtained with high resolution scanning electron microscopy. A) Discocyte: bi-concave dis lacking nucleus. B) Elliptocyte: elliptic form with rounded tips and small bulge in lateral walls. C) Spherocyte: spherical erythrocyte with dense hemoglobin content without central halo. D) Stomatocyte: erythrocyte with a mouth-shaped elongated central zone, E) Discocyte at 10,000x where structural damage can be observed at the level of the plasmatic membrane.
It has been observed that in vitro experimental models have provided valuable information allowing thus a better understanding of several biological phenomena. The present experimental model which used high definition scanning electronic microscopy permitted the assessment of morphological alterations of the membranes of erythrocytes exposed to dental use 5.25% NaOCl; it revealed greater frequency of anomalies characteristic of anisocytosis and poikilocytosis (stomatocytes, elliptocytes, spherocytes and discocytes). According to Wang et al., 26 erythrocytes are a relatively simple in vitro model to assess cytotoxicity of chemical substances. Studies conducted on erythrocytes during the last decades, have enabled the development of detailed knowledge of alterations in the function of red blood cells, as well as of disorders in their membrane due to either external, hereditary or pathological factors.27 For this reason, it is important to assess morphological alterations of erythrocytes when they are exposed to dental use NaOCl solutions.
Ionescu-Zanetti et al,28 and Bierbaum et al29 mention the lysis process experienced by erythrocytes through the saponification process, where it has been observed that lysophospholipids have shown to porate the membrane until causing its death when extra-cellular means are added. This poration allows small ions to permeate the membrane, thus, larger anionic proteins will be found in the cytoplasm. Small ions associated to water molecules, penetrate into the cell, creating thus positive osmotic pressure which will cause colloidal osmotic lysis; this leads to the transition of discocytes, and then of sperocytes, before finalizing in cell death. The aforementioned forms were equally reported in the present study. On the other hand, characteristics shapes of elliptocytes and stomatocytes were found. These erythrocyte anomalies could depend on the viscosity of the cytoplasmic fluid and the rigidity of its cellular membrane, which are affected by changes in the Redox potential and/or decrease of oxygenation in some of its cyto-skeletal proteins: spectrin and ankryn.30 These proteins are considered to be of the utmost importance to preserve membrane architecture.31
The present experimental model using high resolution scanning electron microscopy allowed identification of alterations experienced by the erythrocytes's plasmatic membrane, this model could be implemented to assess biocompatibility among several conventional irrigating solutions used for root canal treatment
CONCLUSIONThe present experimental model allowed the evaluation of morphological changes experienced by erythrocytes when they are exposed to 5% NaOCl. It is therefore considered that this model could be used to analyze biocompatibility of future chemical solutions which might be used in the field of endodontics as well as in other stomatological areas, since it can be considered a viable and easy to replicate model.
DDS, Endodontics Specialist, Type «B» Professor.
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam
Delivery (childbirth) physician, Specialist in Hematology, Type «B» Professor.