To compare the efficacy of two concentrations of heparin to clear the lumen of in vitro clotted neonatal peripherally inserted central catheters (PICCs).
MethodsThis is an in vitro, experimental quantitative study of 76 neonatal 2.0-Fr PICCs coagulated in vitro. The catheters were divided into two groups of 38 PICCs each. In both groups an infusion of low molecular weight heparin was administered with a dose of 25IU/mL for Group 1 and 50IU/mL for Group 2. The negative pressure technique was applied to the catheters of both groups at 5, 15 and 30min and at 4h to test their permeability. Kaplan–Meier survival analysis was used to verify the outcome of the groups according to time intervals.
ResultsThe comparison between both groups in the first 5min showed that more catheters from Group 2 were cleared compared to Group 1 (57.9 vs. 21.1%, respectively). Kaplan–Meier survival analysis showed that less time was needed to clear catheters treated with 50IU/mL of heparin (p<0.001).
ConclusionsThe use of low molecular weight heparin at a concentration of 50IU/mL was more effective in restoring the permeability of neonatal PICCs occluded in vitro by a clot, and the use of this concentration is within the safety margin indicated by scientific literature.
Comparar a eficácia de duas concentrações de heparina para a desobstrução por coágulo do cateter venoso central de inserção periférica (CCIP) neonatal in vitro.
MétodosEstudo experimental in vitro quantitativo que usou 76 CCIPs neonatais de tamanho 2 French coagulados in vitro. Os cateteres foram divididos em dois grupos com 38 CCIPs cada. Ambos os grupos receberam infusão de heparina de baixo peso molecular, com dose de 25UI/mL no Grupo I e de 50UI/mL no Grupo II. Os cateteres de ambos os grupos foram submetidos à técnica de pressão negativa com cinco, 15 e 30 minutos e com quatro horas e testou-se sua permeabilidade. Usou-se a análise de sobrevivência para verificar o desfecho dos grupos conforme os intervalos de tempo.
ResultadosA comparação dos dois grupos no intervalo de tempo de cinco minutos mostrou um número maior de desobstrução de cateteres no Grupo II (57,9%) em relação ao grupo 1 (21,1%). A análise de Kaplan Meier indicou menor tempo para desobstrução dos cateteres quando a heparina em maior concentração (50UI/mL) foi usada (p<0,001).
ConclusõesO uso de heparina de baixo peso molecular na concentração de 50UI/mL foi mais eficaz na restauração da permeabilidade de CCIPs neonatais ocluídos in vitro por coágulo e situou-se tal concentração dentro da margem de segurança indicada na literatura científica.
The peripherally inserted central catheter (PICC) has been shown to be safe for intravenous infusion of solutions in neonates.1,2 It has a lower incidence of complications when compared to other central venous catheters, supporting the thesis that it is a safe and useful device to be used in situations to be used when venous access is limited and difficult.3
Eventually, complications can occur, anticipating the unscheduled removal of the catheter.2 Among the main complications are obstructions, with rates that can vary from 11% to 50%, and catheter rupture.2–8 Obstruction may be caused by thrombus formation, a poorly positioned catheter tip or drug precipitation.9,10
These complications can be prevented and minimized through specific interventions. There are many practices related to maintaining the PICC permeability, although there is little scientific evidence on the best thrombolytic agent, as well as its safe and effective concentration that can support a single practice.11–13
Although heparin is almost universally used in clinical practice, its benefits have not been firmly established, as well as the effective and safe dose of this substance for arterial and venous catheters in neonatology.12,13 The lack of scientific evidence and standardization through protocols leads to the use of several heparin concentrations, which can often be abusive as well as be as underdoses, which can result in unknown side effects or failure in catheter clearance.14
In this context, the aim of this study was to compare two different concentrations of sodium low molecular weight heparin (SLMWH) regarding its efficacy to restore the permeability of neonatal PICC obstructed by a clot in the laboratory.
MethodThis is an experimental, in vitro study of quantitative approach, developed in the Analysis Laboratory of a university hospital in Londrina, state of Paraná, from July to December 2013. The study sample consisted of 76 PICC used in newborns admitted at the Neonatal Intensive Care Unit. In this unit, the indication for PICC use is the administration of antibiotics for longer than 7 days, start of vasoactive drugs, need for glucose infusion rate (GIR) >7, and parenteral nutrition; thus, all the catheters used in this study received all or some of these therapies. The catheters were obtained from the Neonatal Intensive Care Unit (NICU) of the above-mentioned hospital, after being removed from the newborns because of the end of the treatment. After they were removed, the catheters were immediately washed with a 10mL syringe, filled with distilled water, at least twice, until they were clean, and tested for integrity and permeability, after which they were stored in the original catheter plastic packaging. These catheters were stored in a cabinet away from light or moisture for approximately 6 months, the necessary time to obtain a sufficient number of catheters to start the study.
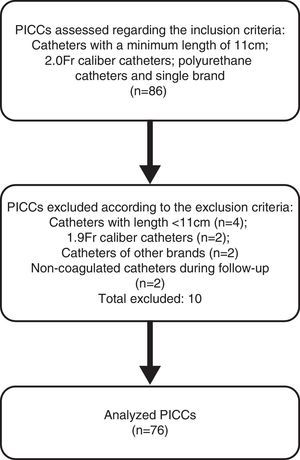
The criteria for catheter inclusion were minimum length of 11cm, 2.0-Fr caliber, made of polyurethane, single brand, and having remained in the newborn for a minimum of 1 week and a maximum of 30 days. The exclusion criterion was the impossibility of maintaining catheter permeability after its removal. For that purpose, the catheters were tested through the infusion of distilled water at their distal end using a 10mL syringe, and the visualization of the water output through the proximal end. Additionally, catheters that were not adequately stored after their removal were excluded.
Sample calculation was obtained through a file containing the record of all catheters used in newborns in the NICU, which had the patient's name, catheter brand, batch, caliber, reason for catheter installation, length of catheter introduced and reason for removal. The data spanned the last 6 months of the year prior to collection, i.e., July to December 2012. A total of 68 PICCs were removed during this period because of the end of treatment and/or obstructions. Considering a sampling error of 5%, confidence level of 95% and clearing difference between Groups I and II of 50%, the necessity of having 36 catheters in each group was verified.
In most newborns, the length of the introduced PICC ranges from 8 to 28cm, depending on the newborn's length and the puncture location. Consequently, for the study, the PICCs were cut with a scalpel at a specific marking on each catheter that indicated 11cm. Subsequently, the 76 PICCs were divided into two groups of 38 catheters each, randomly distributed, regardless of storage time, as they had already been tested for permeability, and were properly identified as Groups I and II, followed by the numeric sequence 1–38. The randomization was carried out through drawing lots. For that purpose, the researcher placed 76 catheters on the workbench and asked the biochemist to choose 38 catheters, which were called Group I, and the other 38 catheters left on the workbench were called Group II. These PICCs were coagulated in vitro using human blood obtained by venipuncture from two healthy employees, who accepted to donate the blood and gave written consent, both having blood type O Rh+, hematocrit 39.2% (donor 1) and 42.2% (donor 2) and platelets 410,000 (donor 1) and 400,000 (donor 2), respectively – that is, within the normal range. Immediately after collection, made with a 23G scalp vein needle and a 10mL syringe, blood was transferred to 1mL syringes, and a volume of 0.04mL was introduced into each of the catheters, which were arranged on a surgical field. This volume was necessary because a 10cm catheter obstruction was used, which corresponds to approximately 0.04mL of the internal volume capacity (priming). Catheter obstruction was performed by two professionals with proven capacity and dexterity, who were previously trained to prevent clotting of blood outside the catheter. The mean time between blood collection and the obstruction of each catheter was 2min. After filling the catheters with blood, the distal end was connected to a three-way tap, which was properly closed, whereas the proximal end was occluded with a Kelly clamp to prevent the escape of blood or air-drying. The technique used in this research to perform in vitro coagulation and the permeability testing of the catheters was based on another study, which aimed to test a new technique to restore permeability of PICCs occluded by clots in the laboratory.15
Then, each catheter was transferred to and immersed in a container with 100mL of saline heated to 37°C in order to simulate the body temperature of the neonate. Each container had 14 catheters from the same group. The containers with the catheters were placed in a water bath (Quimis Aparelhos Científicos Ltda.), keeping the solution between 36.5 and 37°C, with strict temperature control through thermometers immersed in the solution. The catheters were immersed for 6h and then removed and cut at the 10cm marking to eliminate any damage caused to the catheter by the preliminary clamping. Subsequently, their permeability was tested through a 1mL infusion of saline solution, considering as completely obstructed those catheters into which it was not possible to infuse the saline solution and from which the blood could not be drawn by the syringe. Then, the catheters were again placed in the water bath device for the start of the infusion of both heparin concentrations. The laboratory room was used exclusively for the procedure, and no professionals other than the researchers were allowed to enter. The room temperature was maintained between 22 and 24°C, and the period of time during which the catheters remained in the saline solution was chosen so as to not affect the results.
The SLMWH (5000IU/mL) was used due to its mechanism of action, it being the most often used heparin type in neonatal intensive care units, and having the lowest cost. SLMWH exerts anticoagulant action by activation of antithrombin III, which has its activity accelerated up to 1000 times in order to inhibit coagulation factors IIa and Xa and, to a lesser extent, IXa, XIa and XIIa. Heparin binding to antithrombin III depends on the presence of a single pentasaccharide sequence contained in approximately one-third of heparin molecules. The remaining two-thirds have minimal anticoagulant activity at usual therapeutic concentrations. This pentasaccharide sequence confers high affinity of the SLMWH to the antithrombin III. Any heparin or SLMWH molecule containing the pentasaccharide can inhibit Xa action simply by activating AT III. To inactivate thrombin (IIa), the SLMWH must bind to the antithrombin III and the factor IIa simultaneously, forming a ternary complex that only occurs with longer chains, with at least 18 saccharides. In addition to decreasing the anti-IIa effect, SLMWH has other pharmacokinetic advantages, such as decreased binding to plasma proteins and acute phase proteins, decreased binding to macrophages and endothelial cells, decreased binding to platelets and platelet factor 4 (PF4), and decreased binding to osteoblasts. These differences result in therapeutic advantages, such as more predictable anticoagulant response, longer plasma half-life, increased bioavailability, reduced heparin-induced thrombocytopenia and reduced osteopenia.16
Group I received an infusion of SLMWH at a concentration of 25IU/mL, diluted in saline solution, in a 10mL syringe; Group II received the same SLMWH at a concentration of 50IU/mL, also diluted in 10mL saline solution. The researcher was blinded to the choice of group to which the catheter belonged. Two nurses participated at the time of the experiment, one of whom was responsible for the clearing of Group I catheters, whereas the other nurse was responsible for Group II. Both nurses were trained to perform the experiment, and they had a time period of 3min to perform the technique for each catheter, which was controlled by the biochemist using a digital timer. The biochemist was responsible for delivering the two syringes, one with SLMWH with 25IU/mL and the other with 50IU/mL, and only he had knowledge of the concentrations used in each catheter. According to the literature, the recommended intermittent doses of heparin are 50–100IU/kg/dose every 4h, without causing significant changes in coagulation,17 which was not exceeded in this study, as the maximum concentration of heparin was 50IU/mL every 4h in the group with the highest concentration. The choice of using a concentration lower than that found in the scientific literature was made to maintain a safe dose and to allow lower doses to be used in the neonatal population.
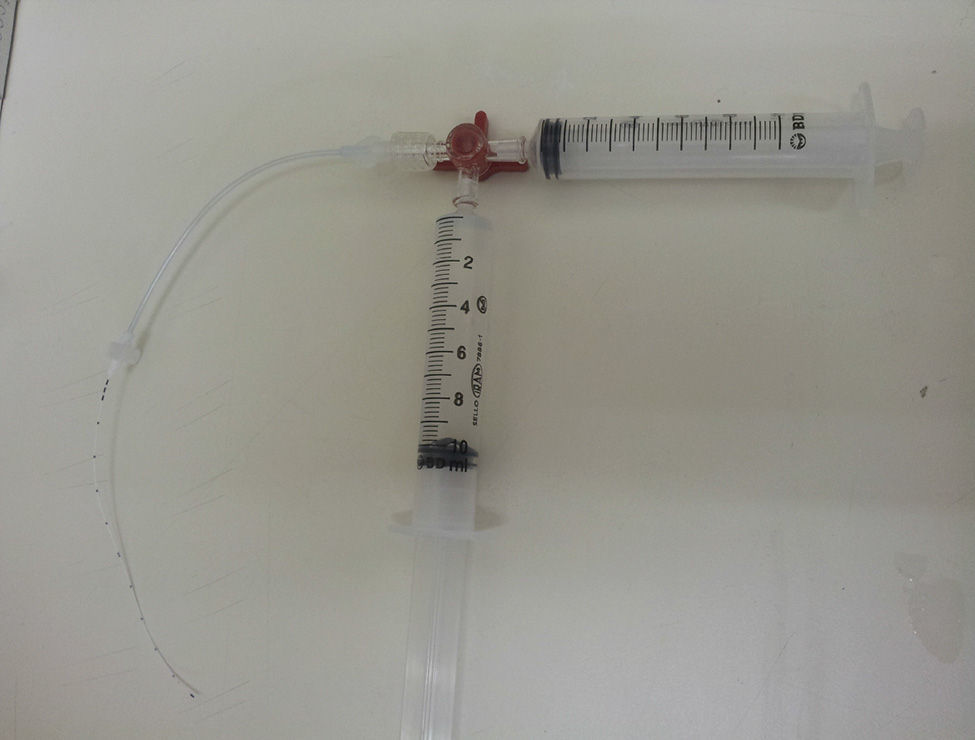
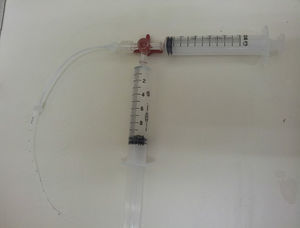
The negative pressure technique was used for the clearance attempts,18 as shown in Fig. 1. The technique involves the use of a three-way tap, connected to the catheter, with two 10mL syringes connected to the tap, one containing 10mL of heparin solution according to the group, and the other one empty. The content of the catheter was drawn to the 10mL graduation using the empty syringe, forming a vacuum inside, with this route being closed soon after. The tap route containing the solution was then opened, aiming to fill the catheter with just the volume aspirated by the vacuum. The system was thus blocked, and the negative pressure technique was repeated four times, with 5, 15 and 30min and 4h. The same heparin solution was used in the four intervals. Always prior to solution infusion, an attempt to aspirate the blood clot and evaluate catheter patency was made and, when cleared, subsequent tests were not performed.
At the end of each attempt in both groups, the heparin solution was totally aspirated, while also trying to aspirate the clot, and the permeability was tested again by using 1mL of saline solution infusion in a 10mL syringe. Catheter permeability was considered to be restored when clot suction capacity was possible, as well as the infusion of 1mL of saline solution from the distal end to the proximal end of the catheter. To ensure that catheter permeability had been restored, the saline solution was disposed off in a container with an accurate 0.1mL graduation, so that the researchers could verify whether the entire infused volume had been returned to the container. To ensure that the infusion rate among nurses was as accurate as possible, they used a digital timer manipulated by the biochemist, who controlled the time of 1min for the saline infusion by the professionals. The examiners responsible for the permeability test were the nurses responsible for groups I and II, who determined whether the catheter had recovered its permeability, whereas the biochemist was the second to confirm and record the result.
If catheter permeability was not achieved, the doses of heparin were repeated five more times at the two assessed concentrations, with new dilutions every 4h, due to the time of heparin stability. The maximum time for catheter clearance would be 24h after the infusion of the first heparin concentration. These intervals were used following the informal account of nurses of the above-mentioned NICU, who already used them in a protocol for clearing catheters with heparin concentration of 50IU/mL.
The results were recorded in a previously structured form and entered in a spreadsheet using Microsoft Office Excel 2007. Later, survival analysis was performed using Kaplan–Meier curve, and the log-rank (Mantel–Cox), Breslow (Generalized Wilcoxon) and Tarone–Ware tests were applied to verify the development of the groups according to the time intervals. The confidence interval was set at 95%.
The research was carried out after approval of the Institutional Review Board of Universidade Estadual de Londrina, process n. CEP/UEL: 066/2013, CAAE: 13890613.8.0000.5231, and was performed in accordance with the required ethical standards.
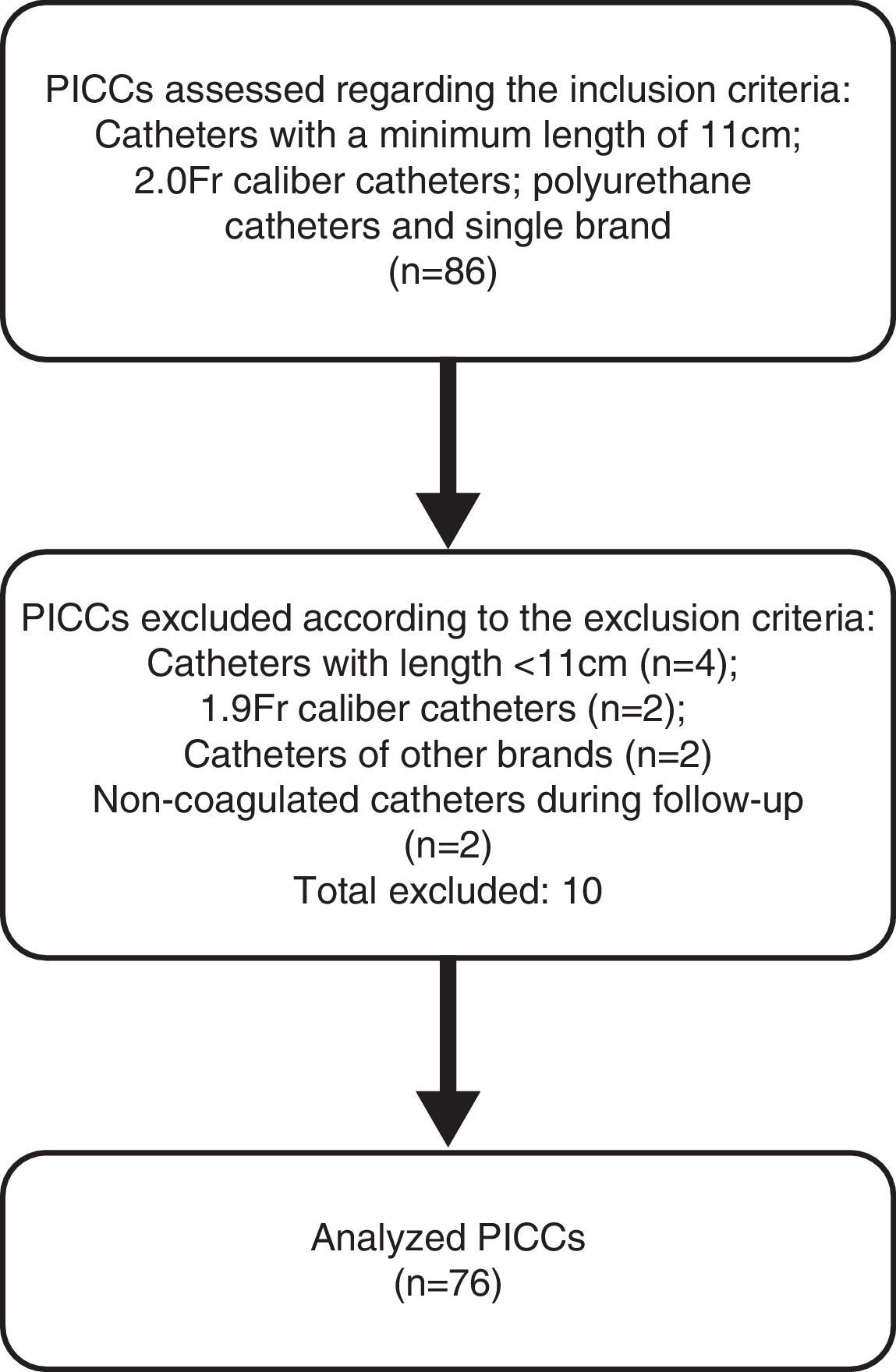
ResultsDuring the data collection period, the daily removal of PICCs at the NICU was followed, aiming to identify eligible catheters to be included in this study sample, as shown in Fig. 2. Finally, 76 catheters were included in the sample. Therefore, the study groups consisted of 38 catheters each from the same brand, 2.0-Fr caliber and 11cm long, with a mean time of use of 30 days. Of these, 35 (46%) were used exclusively in partial parenteral nutrition (PPN), and 41 (54%) were used for the infusion of antibiotics, glucose and electrolyte solutions.
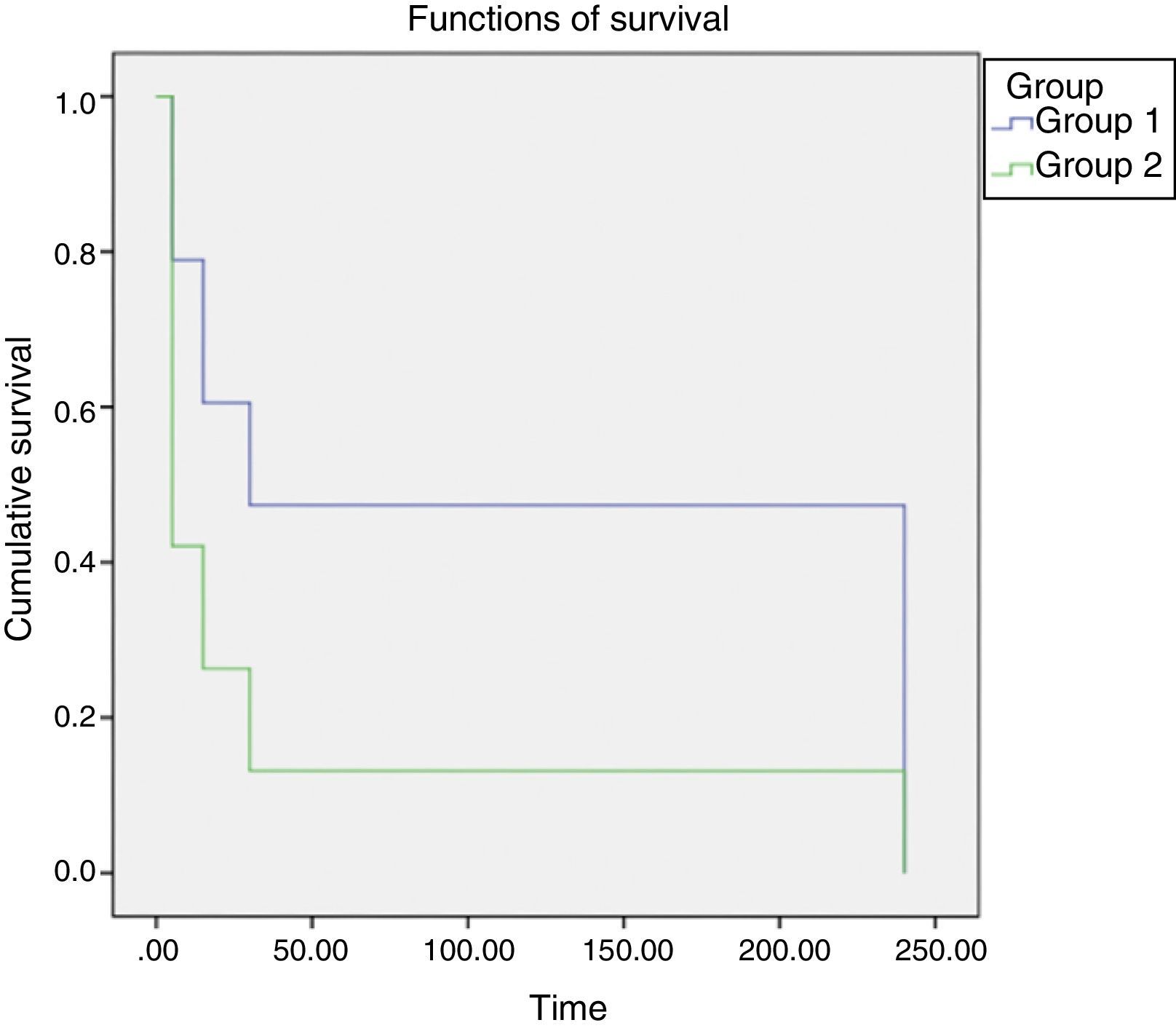
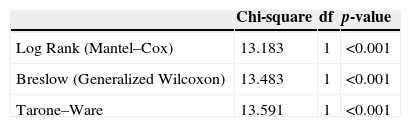
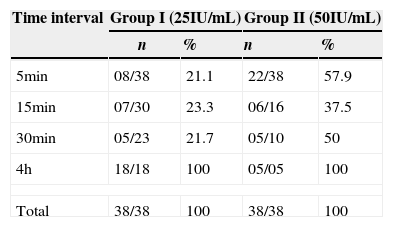
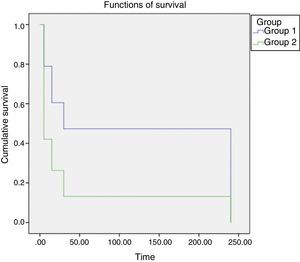
The behavior of groups according to the time intervals is depicted in Fig. 3, showing a significant difference between the behaviors of different concentrations, with less time of catheter clearance in Group II, with a higher concentration of heparin. After performing the test for survival distribution equality for the different group levels (Table 1), we obtained p<0.001, confirming that Group II, with the higher concentration, allows clearance in less time.
These data are reaffirmed in Table 2, which indicates that when comparing the results of the two groups during the 5-min time interval, a greater number of catheters was cleared in Group II (57.9%), when compared to Group I (21.1%), whereas in the 4-h time interval the inverse occurred, which allows us to conclude that the catheters from Group II, which received the concentration of low molecular weight heparin of 50IU/mL, showed faster clearance than those from Group I, which were treated with 25IU/mL of low molecular weight heparin.
Clearance of PICCs obstructed by clots in the laboratory according to two heparin concentrations, during the 4 days of the study. Londrina, 2013.
| Time interval | Group I (25IU/mL) | Group II (50IU/mL) | ||
|---|---|---|---|---|
| n | % | n | % | |
| 5min | 08/38 | 21.1 | 22/38 | 57.9 |
| 15min | 07/30 | 23.3 | 06/16 | 37.5 |
| 30min | 05/23 | 21.7 | 05/10 | 50 |
| 4h | 18/18 | 100 | 05/05 | 100 |
| Total | 38/38 | 100 | 38/38 | 100 |
Intraluminal obstruction of a central catheter may be more common in patients with PICCs because, as insertion occurs through peripheral vessels that have a smaller caliber at the introduction, the catheter occupies most of the luminal diameter, hindering blood flow, allowing clot formation and, consequently, obstruction. Another factor responsible for thrombus formation is the inappropriate catheter positioning, which can lead its tip to lean against the wall of the vessel, allowing obstruction to occur. In addition, the selected limb may contribute to this complication, as limb movement can cause catheter dislocation, facilitating the formation of clots and obstruction.19 Thus, there is an option to perform maneuvers for clot clearance, as they can occur even with adequate catheter handling and maintenance, unlike the obstruction due to crystal formation caused by incompatible drugs with low solubility, which is considered an iatrogenic event by nursing care.
To simulate what occurs in vivo, it was decided, during the catheter coagulation process, to immerse them in a solution heated at 36–37°C, in order to mimic the body temperature of the newborn, stipulating a 6-h period of time to subsequently test their permeability. This time interval was determined taking into account one of the most widespread recommendations to maintain PICC permeability: flushing the catheter at pre-established intervals, usually every 8h.20 Considering the nursing care process, the catheter is usually flushed at every shift. For this reason, during the practice of nursing care, it is an important step to clean the catheter before and after drug administration, when the interval for the administration of these drugs exceeds 6–8h; that is, the team should be able to identify and start the clearing maneuver of an obstructed catheter as early as possible after the start of the event.
When considering that the obstruction concept also includes the slowed flow along the catheter,9 one questions whether the clearance procedure by means of negative pressure technique with heparin concentration should also be used in these cases. It is known that the catheter may rupture if syringes with volumes <10mL are used, since the lower the volume of the syringe, the greater the pressure exerted on the catheter. Therefore, in this study, we only used 10mL syringes with the negative pressure technique, and at the time of the procedure, all the plungers were pulled up to the mark of 10mL. Thus, the pressure was controlled and it was assured that all catheters received the same pressure during the clearance process.
The action mechanism of the heparin depends on its binding to a plasma cofactor, the antithrombin, thereby inactivating thrombin (factor IIa) and the activated coagulation factor X, preventing the conversion of prothrombin into thrombin, and of fibrinogen into fibrin.21 The advantages of SLMWH include: no interference of diet or medications on its action mechanism and minimum monitoring; when compared to unfractionated heparin, it does not show increased risk of bleeding, and it reduces the incidence of heparin-induced thrombocytopenia when used alone.22
The concentrations of heparin (25 and 50IU/mL) chosen for comparison were based on clinical and scientific practice regarding their clearance efficacy and at the maximum safe dose, to prevent adverse effects and other complications that may occur during their use in neonates.17 Among the studies using different concentrations of continuous or intermittent heparin in neonates, no adverse events were found.12,13,23 This study decided to consider the lowest concentration of heparin considered safe, as it was carried out in vitro and it was not possible to verify the occurrence of adverse events in newborns.
This study, however, tested two different concentrations of heparin associated with the negative pressure technique. Two catheter clearance techniques were found in the literature: the negative pressure technique,18 described earlier, and the standard operating procedure (SOP) technique.15 In the SOP technique, the syringe plunger is pulled back and released at regular intervals in the catheter, sending a shock wave that displaces the clot from the catheter lumen, allowing the thrombus to be aspirated.15 Because the latter technique has been tested in 3.0 and 4.0-Fr catheters, not used in neonatology, it was decided to use the negative pressure technique. It is noteworthy that only 10mL syringes were used in all procedures performed with catheters, according to published recommendations, as lower-volume syringes have greater intravascular pressure, increasing the occurrence of adverse events, such as the catheter rupture.4,24 Catheter clearance attempts made by means of positive pressure, even with the infusion of thrombolytic agents, can cause catheter rupture due to high resistance. Therefore, the negative pressure technique was used, which provides contact of the thrombolytic agent with the clot without causing positive pressure, which facilitates clot removal.13
It should be emphasized that, in Group II, heparin at a higher concentration was more effective in catheter clearance, as the latter occurred within a shorter period of time when compared to Group I. Perhaps, the interventions performed resulted from the association of heparin with the negative pressure technique; however, one cannot make this claim, as the study was not designed to test the negative pressure technique for catheter clearance. During the experiment, however, the criteria related to the negative pressure technique were maintained, such as using only 10mL syringes, which was considered safer, attempting to remove the clot using the empty syringe before the solution infusion, maintaining the obstructed catheter immersed in solution for at least 5min before attempting suction, which is the minimum time for heparin to act, and detaching the clot from the lumen catheter. It is worth mentioning that the clot is not diluted by the heparin action, but the latter allows the clot to detach itself from the catheter wall – hence the importance of aspirating it to prevent further obstruction or the introduction of the clot into the newborn's bloodstream.
The fact that the clots from all catheters were aspirated into the syringe before the permeability test does not allow us to affirm the efficacy and safety of the experiment. The negative pressure technique associated with the tested heparin concentrations led to the restoration of catheter permeability and thrombus aspiration visualization, preventing it from being introduced into the bloodstream, which can lead to severe consequences for the neonate. Although no clot was visualized after the saline infusion during the permeability test, it is not possible to affirm the total absence of blood clots, as the solution was not filtered and analyzed microscopically.
One must remember that the measures used to maintain PICC permeability are essential for appropriate intravenous therapy. However, when catheter obstruction eventually occurs, many damages to the newborns are identified, such as the need for repeated punctures, which are painful and can be difficult, discontinuation of drug therapy or continuous parenteral nutrition, more catheter handling, which predisposes to infection, and increased stress levels for the newborn and the staff.
It should be emphasized that, although the study sought to simulate the clinical condition of the catheter into the newborn's vessel, it was carried out in a laboratory, which brings some limitations due to the absence of factors present in the bloodstream, such as the turbulence of blood within the vessels. Another study limitation was the impossibility of verifying microclot detachment from the catheter after the saline infusion to verify the catheter's permeability, and thus, other studies are necessary to allow this solution to be filtered and analyzed microscopically, as the presence of microclots could trigger new obstructions or embolization into the bloodstream. Another limitation of the study is related to previous use of the catheters, as several of them might be more likely to have clots more or less adhered to them, as they were used in different neonates for infusion of PPN, antibiotics, and glucose and electrolyte solutions, which would not depend on the type of heparin used.
For the nursing staff to obtain successful catheter implantation, they should be aware of the risks involved in its use. Nursing care is essential to maintain the PICC, and the identification of possible complications related to its use becomes a necessity for professionals who work directly with catheter handling.10 Thus, scientific evidence research is essential for the decision-making process of optimizing the PICC, particularly regarding what is the most effective solution in preventing catheter obstruction.25
It can be concluded that the use of low molecular weight heparin at a concentration of 50IU/mL was more effective in restoring permeability of neonatal PICCs (2.0Fr) occluded in vitro by a clot than the 25IU/mL concentration, emphasizing that these concentrations are still within the safety margin indicated in the literature.
FundingThis study did not receive funding.
Conflicts of interestThe authors declare no conflicts of interest.