Papillary thyroid microcarcinoma (PTMC), a tumor that measures 1cm or less, according to World Health Organization (WHO) histological classification of tumors, is the most common form of papillary thyroid carcinoma (PTC) comprising much more than half of all PTCs if one includes the so-called incidentalomas. Although PTMC has an excellent prognosis, a minority of cases were found to be clinically aggressive. We decided to perform a review of the literature on records on PTMC in attempt to find which molecular markers might be used as predictors of the clinical behavior of PTMC. This review article aims to summarize the molecular mechanisms that were associated to PTMCs described in the last 10 years, with a particular focus on the clinical importance of genetic alterations (BRAF mutation, RET/PTC rearrangement, NAD(P)H and NRH polymorphisms and TERT mutation) and anomalous expression of several molecules (P53, P27, COX-2, EGFR, ki-67, S100A4, cyclin D1, galectin-3, HMWK, CK-19, HBME-1, HGF, c-MET, membrane mucins and cell adhesion molecules).
MethodsWe made a systematic search in the PubMed database using the keywords papillary thyroid microcarcinoma and reviewed all the articles published in the last 10 years, in English, addressing issues related to PTMC.
ResultsUnfortunately, all genetic alterations and biomarkers reported to date have little potential per se to differentiate between indolent and aggressive PTMCs. Further studies using the aforementioned markers and, most likely, others are needed in order to try to find a combination of several markers that may be used for increasing the probability of identifying PTMC cases with more aggressive behavior, thus allowing the establishment of a more appropriately targeted treatment.
O microcarcinoma papilar da tiroide, um tumor que mede 1cm ou menos, de acordo com a classificação histológica dos tumores da Organização Mundial da Saúde, é o tipo mais comum de carcinoma papilar da tiroide e, se incluirmos os chamados incidentalomas, corresponde a muito mais de metade de todos os carcinomas papilares da tiroide. Embora o microcarcinoma papilar da tiroide tenha um excelente prognóstico, uma minoria dos casos são clinicamente agressivos. Decidimos realizar uma revisão da literatura, na tentativa de encontrar marcadores moleculares que possam ser utilizados como preditores do comportamento clínico dos microcarcinomas papilares da tiroide. Este artigo de revisão pretende resumir os mecanismos moleculares que foram associados ao microcarcinoma papilar da tiroide, descritos nos últimos 10 anos, com um particular ênfase na importância clínica das alterações genéticas (mutação BRAF, rearranjo RET/PTC, polimorfismos NAD[P]H e NRH, e mutação TERT) e da expressão anormal de várias moléculas (P53, P27, COX-2, EGFR, ki-67, S100A4, cyclin D1, Galectin-3, HMWK, CK-19, HBME-1, HGF, c-MET, mucinas de membrana e moléculas de adesão celular).
MétodosFoi feita uma pesquisa sistemática na base de dados PubMed usando as palavras-chave papillary thyroid microcarcinoma, com subsequente revisão de todos os artigos relacionados com os microcarcinomas papilares da tiroide publicados nos últimos 10 anos, em inglês.
ResultadosInfelizmente, todas as alterações genéticas e biomarcadores reportados até à data têm, per se, pouco potencial para distinguir entre microcarcinomas papilares da tiroide indolentes e agressivos. Estudos adicionais utilizando os marcadores acima mencionados e, muito provavelmente, outros são necessários, no sentido de tentar encontrar uma combinação de vários marcadores que possam ser utilizados para aumentar a probabilidade de identificar os casos de microcarcinoma papilar da tiroide com um comportamento mais agressivo, permitindo assim estabelecer um tratamento mais apropriado e direcionado.
Papillary thyroid microcarcinoma (PTMC) is defined, by the World Health Organization (WHO), as a small papillary thyroid carcinoma (PTC) measuring 10mm or less in its greatest dimension.1
Thyroid cancer is the most frequent endocrine malignancy, representing 2% of all malignant diseases and is responsible for almost 90% of neuroendocrine cancers.2,3 Eighty to 90% of thyroid cancers are PTCs and almost half of those are clinically evident PTMCs.3,4
The increasing incidence of small thyroid cancers was suggested to be caused by the use of sensitive screening imaging tools capable of identify subclinical disease in a way that, in countries with access to technology, 90% of incidental cases are due to low risk thyroid cancer. Besides that, there is no evidence of any clinical impact on mortality, so this increased incidence is probably an effect of overdiagnosis, reflecting our capacity to detect occult and indolent cancer.5–7
PTMCs usually have a benign behavior and do not affect patient survival.8 Considering their excellent prognosis and low mortality rate, one would expect that PTMCs were an indolent disease.9 However, in some cases, PTMCs have an aggressive behavior leading to loco-regional recurrence, distant metastasis and mortality.10
The predictive factors for this aggressively behavior have not been completely recognized, but clinicopathological factors such as age greater than 45 years, male gender, tumor size bigger than 5mm, multifocality, lymph nodes metastasis (LNM) and extrathyroidal extension (ETE) have been reported as predictors of poor prognosis.4,11 On the other side, many studies are trying to find the relationship between some molecular characteristics of PTMCs and their behavior. BRAF mutation, RET/PTC rearrangement, NAD(P)H and NRH polymorphisms, TERT mutation, and many molecular markers may play a role in PTMC behavior.
The uncertainty of the risk associated to PTMCs is probably responsible for the controversial management of these small tumors. It is not always easy to define the best way to manage these patients in terms of treatment and follow-up.
To estimate the prognosis and to find a marker or a combination of markers able to stratify the clinical risk in PTMC became an important issue due to the need of tools that may assist in defining the best therapeutic approach for patients with this kind of cancer.
In this review, we analyzed the molecular biology behind PTMC to contribute for the understanding of the influence of genetic alterations, molecular pathways and other biomarkers in PTMC behavior, having as an ultimate goal the identification of prognostic markers in this setting.
MethodsThe literature was retrieved using PubMed and aided by manual searching. The terms papillary thyroid microcarcinoma were used as keywords connected by the Boolean operator AND. Inclusion criteria were: published in English literature and during the last 10 years.
The query obtained through the database was: papillary[All Fields] AND (“thyroid gland”[MeSH Terms] OR (“thyroid”[All Fields] AND “gland”[All Fields]) OR “thyroid gland”[All Fields] OR “thyroid”[All Fields] OR “thyroid (usp)”[MeSH Terms] OR (“thyroid”[All Fields] AND “(usp)”[All Fields]) OR “thyroid (usp)”[All Fields]) AND microcarcinoma[All Fields] AND english[Language] AND (“2004/11/30”[CRDAT]: “2014/11/30”[CRDAT]) AND (“2004/11/31”[PDAT]: “2014/11/31”[PDAT]) AND English[lang].
This research provided 410 potentially relevant articles. The articles that did not seem focused on molecular biology of PTMCs were excluded through title and/or abstract review. After that, 46 potentially relevant articles remained, which were evaluated in detail. Forty-five of them were selected, and the remaining one excluded.
Finally, automatic alert up to February 2015 provided one more article eligible for this review and 13 more articles were also manually included through bibliographic references from review articles, resulting in total of 59 articles.
ReviewBRAFV600E mutationB-type Raf kinase (BRAF), a serine/threonine-selective protein kinase, is involved in the mitogen-activated protein kinase (MAPK) pathway.12 This signaling pathway is involved in the regulation of cell growth, division, and proliferation.13 When constitutively activated, causes abnormal cell proliferation, adhesion, migration and invasion, leading to carcinogenesis.8,12
BRAFV600E, the consequence of a unique thymine-to-adenine transversion, represents more than 90% of all the mutations found in the BRAF gene and is a very specific sign for PTC.12,14 Besides enhancing the capacity of BRAF mutated cells to proliferate and transform and the association with an increase in matrix metalloproteinases and desmoplastic stromal reaction, there are many roles attributed to BRAFV600E, namely: up-regulation of tumor promoting genes, down-regulation of tumor suppressor genes, angiogenesis, promotion of tumor growth, tissue invasion and extracellular matrix remodeling.15–18
The BRAFV600E mutation is the most common genetic alteration in PTC and has been associated with poor prognostic factors.19 However, literature remains controversial in this question. With regard to PTMC, the utility of BRAFV600E mutation, detected in 15.8–52% of PTMC cases, as a prognostic factor is unclear.13,14 Therefore, many efforts have been made to understand the role of this mutation.
Sedliarou et al. analyzed 46 PTMCs from 31 Russian and 15 Japanese patients.20 Mutated BRAF was found in 13 cases (28.2%), 9 in Russian (29.0%) and 4 in Japanese patients (26.6%). Presence of the BRAF mutation did not significantly correlate with any of the evaluated parameters, namely, gender, age at presentation, LNM and distant metastases.
Kim et al. detected BRAFV600E mutation in 31 of 60 Korean patients (52%) with PTMCs.19 Despite no significantly association was found with prognostic factors like age, gender, tumor size, multifocality, ETE and staging, some trends were observed. Tumors harboring BRAF mutation had higher probabilities of ETE, multifocality and LNM. In that study, the four tumors with lateral neck node metastases, including both patients with higher stages and the two patients with cervical neck recurrence during the follow-up, were BRAFV600E positives.
Min et al. analyzed the occurrence of the BRAFV600E mutation in relation to LNM.4 They detected BRAFV600E mutation in 32 of 60 PTMC cases (53%) and its occurrence was not associated to any clinicopathological features including LNM.
Kwak et al. evaluated, in a retrospective study, the relationship between BRAF mutation and poor prognostic factors and ultrasound (US) features.13 The BRAFV600E mutation was detected in 213 of 339 (62.8%) of PTMC cases and was significantly associated with tumor size, ETE, and high TNM stage in PTMC, but it was not significantly associated with any US features, although there was an extremely strong trend toward the feature of marked hypoechogenicity.
Lee et al. focused their attention in establishing the correlation between BRAFV600E and PTMCs with aggressive behavior. BRAFV600E mutation was detected in 24 of 64 PTMC cases (37.5%) and a significant association was observed with prognostic indicators, namely, T3 and T4 stage, LNM and ETE.21
Basolo et al., through an analysis of a sample of 578 PTCMs, showed a statistically significant association between BRAFV600E, present in 39.4% of the cases, and age at diagnosis, absence of tumor capsule, ETE, LNM and higher stages of disease.22 The mutation was not significantly associated with multifocality.
Lin et al. analyzed the utility of BRAF mutation screening of fine needle aspiration biopsy (FNAB) samples in predicting aggressive characteristics of PTMC.23 The BRAFV600E frequency in the PTMC was 34% (21 of 61) and was significantly associated with multifocality, ETE, lateral LNM, and advanced disease stages. Moreover, BRAF mutation was an independent predictive factor for lateral LNM.
Marchetti et al. did not find a significant relationship between BRAFV600E mutation, detected in 63 of 85 PTMCs (74%), and clinical features as multifocal disease, ETE and LNM.24
Zhou et al. analyzed 100 patients with clinically unilateral PTMC in order to investigate risk factors associated with occult contralateral carcinoma with emphasis on BRAF mutation on FNAB samples.25 Thirty-one in 100 patients (31%) were BRAF positive. Of the 20 patients identified with occult contralateral carcinoma (20%) preoperative BRAF mutation positive status was found in 12 (60%). The poorer outcome of bilateral PTC patients may be in part explained by the high incidence of BRAFV600E mutation and the fact that bilateral PTC often arises from a single clone with concordant BRAF status. Therefore they hypothesized and confirmed that preoperative BRAF mutation is an independent predictive factor for occult contralateral PTMC, suggesting that in these patients a total thyroidectomy should be considered.
Kurtulmus et al. observed the BRAFV600E mutation in 19 of 64 Turkish patients with PTMC (29.7%) and found that LNM rate significantly increased when the mutation was present and also that mutation was more frequent in classic type.26 Multifocality and ETE did not significantly differ according to BRAF status.
Rossi et al. evaluated a sample of 50 patients with PTMCs in which 35 had the BRAF mutation (70%) and found a significant association between BRAF mutation and bilateral disease and LNM, but not with ETE.27
Zheng et al. analyzed the clinical characteristics and BRAFV600E mutational status of 977 PTMCs in a Chinese population, 40.1% (392/977) were BRAFV600E positive.10 Their results showed that BRAFV600E mutation was significantly associated with ETE, LNM and male gender but not associated with age, multifocality, TNM staging, and distant metastasis. Their analysis also showed that tumor recurrence was not associated with BRAFV600E mutation.
Choi et al. observed the BRAFV600E mutation in 72 of 101 Korean patients that underwent surgery for PTMC (71.3%) and did not find a significant relationship between BRAFV600E mutation and prognostic factors such as older age, gender, TNM staging, nodal metastasis, multifocality, ETE, and distant metastasis.14
Mussazhanova et al. examined a small sample of 13 PTMCs of which BRAFV600E mutation was present in six cases (46%), and found no significant association between BRAFV600E and tumor size, ETE or LNM.28
Walczyk et al. performed a retrospective analysis in a population of 113 patients with PTMC with the least aggressive stage (T1aN0/Nx).29 The BRAFV600E mutation was found in 78 of the 113 patients (69.0%). During the 12 years of the study (2001–2012), there was no persistence, locoregional recurrence, lymph node or distant metastases or mortality. In this study, there was no relation between BRAF positive primary focus of PTMC and more aggressive or recurrent disease.
Yang et al. in a retrospective study of 291 patients treated for PTMC tried to identify predictive factors of central compartment LNM, namely, BRAF mutation, among others. BRAFV600E mutation was found in 124 of 291 (42.6%) patients and was independently associated with central compartment LNM.30
Despite their mostly benign behavior, Piana et al. recently reported three cases in which PTMCs, managed with optimal treatment, recurred, metastasized and caused the death of the patients.31 BRAF mutation was absent in all cases, either at primary tumor or in metastatic lesions.
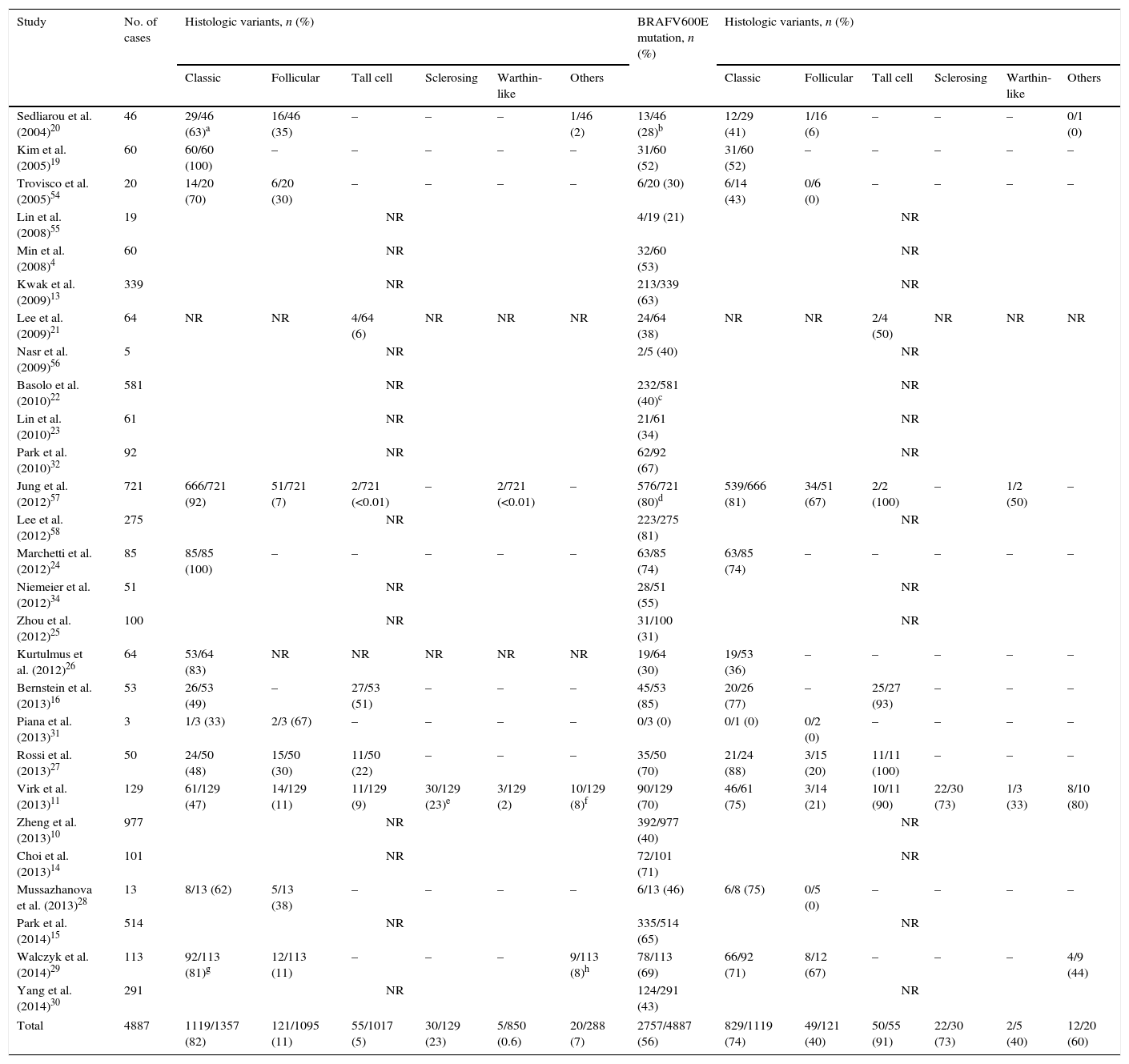
In Table 1, we summarize the results of published studies on BRAFV600E mutation in PTMC.
Summary of published studies of BRAF mutation in papillary thyroid microcarcinoma.
| Study | No. of cases | Histologic variants, n (%) | BRAFV600E mutation, n (%) | Histologic variants, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Classic | Follicular | Tall cell | Sclerosing | Warthin-like | Others | Classic | Follicular | Tall cell | Sclerosing | Warthin-like | Others | |||
| Sedliarou et al. (2004)20 | 46 | 29/46 (63)a | 16/46 (35) | – | – | – | 1/46 (2) | 13/46 (28)b | 12/29 (41) | 1/16 (6) | – | – | – | 0/1 (0) |
| Kim et al. (2005)19 | 60 | 60/60 (100) | – | – | – | – | – | 31/60 (52) | 31/60 (52) | – | – | – | – | – |
| Trovisco et al. (2005)54 | 20 | 14/20 (70) | 6/20 (30) | – | – | – | – | 6/20 (30) | 6/14 (43) | 0/6 (0) | – | – | – | – |
| Lin et al. (2008)55 | 19 | NR | 4/19 (21) | NR | ||||||||||
| Min et al. (2008)4 | 60 | NR | 32/60 (53) | NR | ||||||||||
| Kwak et al. (2009)13 | 339 | NR | 213/339 (63) | NR | ||||||||||
| Lee et al. (2009)21 | 64 | NR | NR | 4/64 (6) | NR | NR | NR | 24/64 (38) | NR | NR | 2/4 (50) | NR | NR | NR |
| Nasr et al. (2009)56 | 5 | NR | 2/5 (40) | NR | ||||||||||
| Basolo et al. (2010)22 | 581 | NR | 232/581 (40)c | NR | ||||||||||
| Lin et al. (2010)23 | 61 | NR | 21/61 (34) | NR | ||||||||||
| Park et al. (2010)32 | 92 | NR | 62/92 (67) | NR | ||||||||||
| Jung et al. (2012)57 | 721 | 666/721 (92) | 51/721 (7) | 2/721 (<0.01) | – | 2/721 (<0.01) | – | 576/721 (80)d | 539/666 (81) | 34/51 (67) | 2/2 (100) | – | 1/2 (50) | – |
| Lee et al. (2012)58 | 275 | NR | 223/275 (81) | NR | ||||||||||
| Marchetti et al. (2012)24 | 85 | 85/85 (100) | – | – | – | – | – | 63/85 (74) | 63/85 (74) | – | – | – | – | – |
| Niemeier et al. (2012)34 | 51 | NR | 28/51 (55) | NR | ||||||||||
| Zhou et al. (2012)25 | 100 | NR | 31/100 (31) | NR | ||||||||||
| Kurtulmus et al. (2012)26 | 64 | 53/64 (83) | NR | NR | NR | NR | NR | 19/64 (30) | 19/53 (36) | – | – | – | – | – |
| Bernstein et al. (2013)16 | 53 | 26/53 (49) | – | 27/53 (51) | – | – | – | 45/53 (85) | 20/26 (77) | – | 25/27 (93) | – | – | – |
| Piana et al. (2013)31 | 3 | 1/3 (33) | 2/3 (67) | – | – | – | – | 0/3 (0) | 0/1 (0) | 0/2 (0) | – | – | – | – |
| Rossi et al. (2013)27 | 50 | 24/50 (48) | 15/50 (30) | 11/50 (22) | – | – | – | 35/50 (70) | 21/24 (88) | 3/15 (20) | 11/11 (100) | – | – | – |
| Virk et al. (2013)11 | 129 | 61/129 (47) | 14/129 (11) | 11/129 (9) | 30/129 (23)e | 3/129 (2) | 10/129 (8)f | 90/129 (70) | 46/61 (75) | 3/14 (21) | 10/11 (90) | 22/30 (73) | 1/3 (33) | 8/10 (80) |
| Zheng et al. (2013)10 | 977 | NR | 392/977 (40) | NR | ||||||||||
| Choi et al. (2013)14 | 101 | NR | 72/101 (71) | NR | ||||||||||
| Mussazhanova et al. (2013)28 | 13 | 8/13 (62) | 5/13 (38) | – | – | – | – | 6/13 (46) | 6/8 (75) | 0/5 (0) | – | – | – | – |
| Park et al. (2014)15 | 514 | NR | 335/514 (65) | NR | ||||||||||
| Walczyk et al. (2014)29 | 113 | 92/113 (81)g | 12/113 (11) | – | – | – | 9/113 (8)h | 78/113 (69) | 66/92 (71) | 8/12 (67) | – | – | – | 4/9 (44) |
| Yang et al. (2014)30 | 291 | NR | 124/291 (43) | NR | ||||||||||
| Total | 4887 | 1119/1357 (82) | 121/1095 (11) | 55/1017 (5) | 30/129 (23) | 5/850 (0.6) | 20/288 (7) | 2757/4887 (56) | 829/1119 (74) | 49/121 (40) | 50/55 (91) | 22/30 (73) | 2/5 (40) | 12/20 (60) |
Abbreviation: NR, not reported.
Other studies compared PTC with PTMC in order to understand if PTMCs are an early stage of PTC or if they are different entities.
Park et al. compared a group of 278 PTMCs and 868 PTCs and their results suggest that PTMC is not an indolent cancer and can behave more aggressively like PTC.32 In that report, clinicopathological and prognostic features were not different between PTMC and PTC and the frequency of BRAFV600E mutation was similar in both groups, suggesting that their development biology may be the same and that BRAF mutation may be important in the beginning of PTC development.
Another study searched for some association between the BRAF mutation and US features.15 A group of 688 patients with PTCs were divided by BRAF mutation status and tumor size. There were no differences between PTMC (514) and PTC (178) groups, in what concerns US features. PTMCs with BRAF mutation were significantly larger than BRAF negative ones (8.0±3.8 vs 7.1±2.7mm; p=0.004) but no significant differences in other US features, according to the BRAF status, in either the PTMC or PTC group were found.
Soares et al. summarized the results of published studies on BRAF. Comparing the results in PTMC with those observed in PTC, no substantial differences were found in BRAF mutation prevalence.33
Others studies tried to establish a correlation between histology of PTMC and BRAF status.
It is important to notice the disparate frequency of the BRAF mutation in the two major subtypes of PTMC. Through the analysis of Table 1 it is clear that BRAF mutation is much more common in the classical than in the follicular variant.
The prevalence of the BRAFV600E mutation is reported to be as high as 80–100% in tall cell variants, but what we know about this PTMC subtype is not much.16 In a study from 2013, it was demonstrated for the first time that, despite the small size, these microcarcinomas are significantly associated with ETE and more advanced stage at presentation, and they are more frequently associated with multifocality, lymphovascular invasion, LNM and BRAFV600E mutation when compared to classic PTMC.16
Virk et al. concluded that PTMC has an idiosyncratic morphology in relation with BRAF mutation status.11 Mutated microcarcinomas presented significantly higher prevalence of infiltrative tumor borders, presence of stromal desmoplasia, fibrosis and/or sclerosis, classic nuclear characteristics of papillary carcinoma and cystic change. Tumors harboring the mutation were also significantly associated with LNM particularly lateral cervical LNM, and ETE.
Niemeier et al. projected a risk-stratifying model for patients with PTMC.34 A combined molecular-pathology (MP) score was proposed based on BRAF mutational status of the tumor more three histopathological features – multifocality/intraglandular tumor spread, tumor fibrosis and superficial tumor location in the gland. They offer a very simple score that can be helpful in the evaluation of cancer aggressiveness. The combined MP score allows to stratify tumors in one of three categories of probability of LNM or tumor recurrence (low risk: 0%, moderate-risk: 20%, and high-risk: 60%). This score proved to be better than either molecular or histopathologic evaluation alone.
The recent study done by Biase et al. tried to clarify the distribution of BRAFV600E and, by using a sample of 85 BRAF positives PTCs, it was able to identify three groups of tumors by the distribution of mutated cells: greater than 80% in 43.5%, 30–80% in 45.9%, and less than 30% in 10.6%.35 The statistical association between PTMC and the tumor group featuring less than 30% BRAFV600E mutated cells highlights the possibility of considering PTMCs as an early stage of PTCs. There was a trend for larger tumors to have a higher proportion of BRAF mutated cells supporting the hypothesis that BRAF mutation promotes tumor growth and, in the cases with a higher proportion of mutated cells, BRAFV600E may be the founding genetic alteration.
Based on previous reports that BRAF mutation could be detected peripherally in the serum or blood of PTCs patients, Kwak et al. tried to establish if it is worth to detect the peripheral BRAFV600E mutation in order to use it as serum tumor marker.12 For that, they obtained 94 serum samples from patients with PTC harboring BRAFV600E mutation in the tumor itself, of which 67 patients (71.3%) had PTMC. Unfortunately, they were incapable to identify peripheral BRAFV600E mutations with real time PCR. So, until now, even if we consider to use BRAF mutation to help on the management of PTMCs, we only have an invasive procedure such as FNAB to identify this genetic alteration.
We must emphasize a recently published meta-analysis by Li et al. performed in order to ascertain the relationship between BRAFV600E mutation and the clinicopathological features of PTMC.36 Nineteen studies comprising 3437 patients were included and 47.48% of these patients had BRAFV600E mutation, with no significant difference according to patient gender or age. In comparison with the wild-type, BRAFV600E mutation was associated with tumor multifocality, ETE, LNM, and advanced stage of PTMC. So, the findings from this meta-analysis clearly demonstrate that PTMCs harboring BRAFV600E mutation have a greater tendency for increased aggressiveness. However, a subgroup analyses done according to the country of the study revealed that BRAFV600E mutation is not significantly correlated with aggressive clinicopathological behaviors of PTMC in patients from Korea, where the mutation is highly prevalent. Therefore, BRAF mutation may have relatively restricted prognostic value in areas where BRAFV600E mutation has an extremely high prevalence.
RET/PTC rearrangementThe RET/PTC rearrangement, the first genetic alteration assigned to PTC, also triggers MAPK pathway and was considered to have some prognostic value.33 However, it has been reported that RET/PTC rearrangement, present in PTMC in up to 52% of the cases, is a finding that does not seem to be related to cancer aggressiveness.18 Nevertheless, the great prevalence of RET/PTC translocations reported in PTMCs suggests that the activation of this oncogene has a responsibility in a premature stage of PTMC.8
The results of the published studies on RET/PTC were summarized in a recent report.33 Comparing the results in PTMC with those observed in PTC, no substantial differences were found in RET/PTC rearrangement prevalence. On the same report they concluded that this rearrangement, as we said, highly prevalent in PTMC, is rare in less-differentiated thyroid cancers. This is consistent with previous reports confirming the weakness of RET/PTC in contrast with other oncogenes, and could explain why most PTMCs do not grow, or even if they do, they do not evolve to poorly differentiated or undifferentiated carcinomas with a more hostile behavior, despite their ability of regional metastization. Tumors with RET/PTC rearrangement are more frequently, small, slow growing and predominantly with papillary architecture.
NAD(P)H and NRH polymorphismsNAD(P)H Quinone Oxidoreductase 1 (NQO1) and NRH Quinone Oxidoreductase 2 (NQO2) seem to protect against oxidative stress and its carcinogenic effect. Polymorphisms on these enzymes have been suggested as predictive factors for cancer susceptibility and development.37
Lee et al. investigated the link between NQO1/NQO2 genotype and clinicopathological characteristics of PTMC, concluding that NQO1 and NQO2 polymorphisms are associated with aggressive phenotypes.37 The homozygous genotype of the polymorphic variant of NQO2 could be a prognostic marker because of the significant relationship with an increased risk of LNM. Also, PTMC with polymorphic NQO1 frequently exhibited ETE when compared to PTMC NQO1 wild-type. It was also investigated the response of Nrf2, a marker against oxidative stress. PTMC harboring the polymorphic variants showed higher Nrf2 expression, signifying that the lack of normal NQO1 and NQO2 might cause strong oxidative reaction.
TERT mutationMutations in the promoter region of telomerase reverse transcriptase (TERT), which can lead to persistent telomere lengthening, are indicators of thyroid tumors aggressiveness being intensely associated with increased risk of recurrence and mortality.6
In a retrospective study, TERT mutations were associated with a high risk of disease specific mortality in patients with PTC compared with those without the mutation. In what concerns to PTMCs, no TERT mutations were described in tumors smaller than 1cm.38
In another study, with a very small series to allow a grounded conclusion, TERT mutations were observed in 8% of PTC and in 0% of PTMC cases.33,39
Other molecular pathways in PTMCP53The p53 gene, which encodes a nuclear tumor suppressor protein, is involved in the cell cycle being capable of inhibiting cell proliferation and transformation.9,40 It has been described that p53 gene expression is an indicator of a worse prognosis raising the question of its importance in PTMC.40
Recent findings demonstrated no association between p53 expression and poor prognostic in PTMC.9 Probably because they are an early cancer, whereas p53 is usually a late genetic event usually associated with aggressive tumors and particularly to less differentiated/undifferentiated thyroid tumors.
In 2006, Corapcioglu et al. studied a total of 44 patients with PTMCs.40 Of them, 29 had preparations viable to stain for p53 expression. They found p53 positivity in 34.5% and this was not significantly correlated with gender, age, LNM, multifocality, capsular invasion, histological type, tumor location or size.
In 2007, Lim et al. evaluated the histopathological and clinical characteristics of 217 Korean patients with PTMC and P53 immunohistochemical staining was performed for 87 specimens.9 The p53 expression was not associated with any known prognostic factors.
Also, in the three cases with fatal outcome, mentioned above, there were no evidences of p53 nuclear accumulation.31
A recent study tried to find out whether the expression of some molecules associated with the biological behavior of PTMC differed between patients with ETE and no ETE.41 In that context, immunohistochemical staining of p53 did not differ between either groups.
P27Another tumor suppressor gene is p27, important in cell cycle regulation as an anti-cell cycle cyclin.8,32
In 2008, Min et al. analyzed the p27 expression in relation to LNM.4 Loss of p27, associated with thyroid cancer and previously reported as a predictive factor for recurrence and LNM, was observed in 125 of 198 PTMCs (63%) without any association with LNM.4,32
Other study, comparing PTC and PTMC, studied the expression of some molecular markers.32 The immunohistochemical analysis revealed no different expression pattern of p27 in relation to the tumor size.
COX-2, EGFR and ki-67The enzyme cyclooxygenase-2 (COX-2), which is responsible for the formation of prostaglandins from arachidonic acid, is induced by several growth factors, cytokines and oncogenes.9 It is frequently expressed in PTC and in some reports is more frequent in PTMC than in PTC. It has been suggested that it might serve as a useful diagnostic as well as a prognostic molecular marker for PTC.
Epidermal growth factor receptor (EGFR) has been reported as an independent prognostic factor for thyroid cancer.9 Also, its overexpression has been related to cancer recurrence. However, this has been shown mainly for PTC larger than 1cm.9,41
The ki-67 antigen is a marker of proliferative activity and seems to relate with worse clinical outcome.9 It was also reported that its expression increases significantly with age explaining the poorer prognosis in older patients.
In the same 2007 report from Lim, mentioned above, immunohistochemical staining of COX-2, EGFR and ki-67 for 87 specimens was performed.9
After evaluating the expression of those molecular markers they proved that the absence of EGFR expression was associated with ETE and LNM while the absence of COX-2 expression was correlated with bilaterality and multifocality. Moreover, ETE showed a trend of positive relation to the absence of EGFR expression, although not statistically significant. The ki-67 expression was very low, suggesting a slowly progressive disease and was not associated with recognized prognostic factors.
EGFR may still control the growth in small tumors explaining why higher EGFR expression was inversely correlated with ETE and LNM.9 Other study revealed no difference in the expression of EGFR pattern in relation to ETE.41
In a recent study, the expression of ki-67 was assessed in PTMCs tumors and LNMs.42 The ki-67 index was generally low in both primary PTMC and in the metastasis and was not associated significantly with clinicopathological characteristics. No difference was found in ki-67 index at the invasive front compared to the center of the tumor.
S1004S100A4 is a member of the S100 family of calcium-binding proteins involved in tumor progression, metastasis and angiogenesis promotion.8 It has been shown to be a prognostic marker in various human cancers.4,8
In a series of 198 PTMCs, S100A4, expressed in 69% of the cases predicted LNM.4 Moreover, expression of S100A4, particularly its strong expression in the invasive front of the tumors, was significantly and independently associated with lateral node metastasis and macrometastasis, suggesting that its overexpression is associated with invasion and nodal metastasization of PTMCs. Expression of S100A4 may be useful for prediction of metastatic potential of PTMCs.
Cyclin D1Cyclin D1, which activates cyclin-dependent kinases, may participate in cancer progression, but we still are in face of inconclusive results.32 Overexpression of cyclin D1 on immunohistochemistry is strongly associated with LNM in PTMC.43 However, similar results were observed in patients without LNM.18,43
Through the immunohistochemical analysis of 35 cases of PTMC, Lantsov et al. found a significant association between cyclin D1 expression and tumor size and LNM. The level of cyclin D1 was significantly lower in PTMC<5mm than in PTMC>5mm or PTC. All PTMC cases with LNM were>5mm and 80% showed a high level of cyclin D1 overexpression. Thus, cyclin D1 may be up-regulated early in thyroid carcinogenesis promoting tumor growth and metastatic process.44
Antonaci et al. evaluated cyclin D1 expression in 31 PTCs and 36 PTMCs.45 Staining for cyclin D1 was observed in all PTCs and in 61.1% of PTMCs. Moreover, they showed that cyclin D1 overexpression is correlated with the expression of survivin, an anti-apoptotic protein that also intervenes in cell proliferation. Cyclin D1 and survivin over-expression are presumably early events, since a high percentage of PTMCs showed the same profile as PTCs. They also found cyclin D1 is over-expressed in LNM and emphasize that the higher expression of both cyclin D1 and survivin in tumor tissues than in normal tissues could be useful to detect single cell transformation in FNAB samples facilitating early diagnosis.
Also in the study by Min et al., in a cohort of 198 PTMCs, the expression of cyclin D1, with intense nuclear staining in 60%, predicted LNM.4 Cyclin D1 expression was significantly higher in the older age group and larger tumors and lower in follicular variants.
Londero et al. studied 131 PTMCs in order to evaluate if the expression of cyclin D1 could predict metastization.46 Forty-three of PTMCs (33%) had regional or distant metastases. Cyclin D1 median expression was significantly higher in patients with metastases in comparison to those without, indicating a correlation with tumor aggressiveness. Nonetheless, both groups showed wide variation in expression, which disqualify the marker as a discriminator for metastasis detection.
Park et al. found no differences between PTC larger than 1cm and PTMC in cyclin D1 expression.32
Immunohistochemical analysis for cyclin D1 was also performed in the three cases mentioned previously.31 Cyclin D1 was expressed in both primary PTMC and metastatic cells. Findings in these three cases suggests that cell cycle deregulation is relevant in the progression of PTMC and supports its potential as a marker to predict LNM.31,47
Galectin-3Overexpression of galectin-3 revealed to be a reliable marker for thyroid cancer. This molecule is involved in interactions between cells and between them and the extracellular matrix. Galectin-3 also controls cell growth, malignant transformation and metastatic process, allowing resistance to apoptosis.32
Cvejic et al., in 2005, analyzed galectin-3 expression in 63 cases of PTMC, concluding that its presence in incidentally found microcarcinomas has no relation with tumor growth or aggressiveness.48 Immunohistochemical reactivity was evident in 51 cases (80.9%). Only three cases involved LNM, and they were galectin-3 positive. The other 48 cases expressed galectin-3, without LNM, suggesting that galectin-3 expression, itself, has not a metastatic potential.
Other studies evaluated whether galectin-3 expression in PTMC could be a marker of LNM but the results showed no significant relation.18
In Londeros's study, it was also evaluated if galectin-3 could predict metastization but its expression showed no significant correlation with PTMC metastases.46
A recent report from Kim41 showed no difference in the expression of galectin-3 between patients with and without ETE.
HMWK, CK-19 and HBME-1High molecular weight keratin (HMWK) and cytokeratin-19 (CK-19) are useful markers for differentiating papillary carcinomas from benign lesions and are sensitive markers for PTCs.32 Hector Battifora mesothelial-1 (HBME-1) is helpful in the diagnosis of follicular cell-derived thyroid tumors.32 In 2010, Park et al. in a comparison between PTC and PTMC explored the expression of these three molecular markers.32 The immunohistochemical analysis exhibited no different expression pattern according to the tumor size suggesting that larger PTCs and PTMCs have similar molecular characteristics.
HGF and c-METThe hepatocyte growth factor (HGF)/c-Met pathway has been related with tumor invasion in various cancers and HGF and c-Met expression are low and rare in benign lesions when compared with their high levels in thyroid cancer.49 Vascular endothelial growth factor (VEGF) overexpression is involved in cell proliferation, migration and survival and was found to be increased in thyroid metastasis when compared with the expression in primary lesions.
A recent report, from Koo et al., was the first that tried to evaluate the significance of HGF and c-Met as a predictive marker for subclinical LNM (SLNM) in PTMC.49 They analyzed the association between HGF and c-Met expression and SLNM in 113 patients with clinically identified PTMCs. Positive immunohistochemical HGF and c-Met staining was found in 107 (95%) and 103 (91%) cases, respectively, and it was strongly correlated with SLNM. The in vitro studies that they performed demonstrated that HGF stimulation and constitutive c-Met activation increases the migration and invasiveness of cancer cells by rising VEGF-A expression.
Membrane mucinsMembrane mucins include a heterogeneous family of glycoproteins that are characteristic of epithelial cells and have a protective function, behaving like a barrier against infection and being responsible for lubricating epithelial cell surfaces. However, their protective function can be harnessed by neoplastic cells to defend them against immunological mechanisms. They may serve, as well, as cell surface receptors directing signals, conducting to responses such as differentiation, proliferation or apoptosis and, once again, cancer cells might use mucins to protect themselves from hostile environment and to adapt the local conditions during invasion.50
MUC1 expression was also analyzed in Min's study and was detected in 96 cases (49%) and did not predict LNM, but was associated with larger tumor size.4
In 2011, Nam and colleagues evaluated the expression levels of MUC4 and MUC15 and their prognostic meaning in PTC.50 They not only concluded that those mucins were overexpressed in PTC, but also observed that high MUC4 expression was significantly correlated with small tumors and PTMC subtype, so MUC4 may have a relevant intervention in early oncogenesis.
Cell adhesion moleculesKim et al. performed an oligonucleotide microarray analysis of PTMCs to disclose their gene expression profiles and to compare the results with those of PTCs.3 Most of the commonly up-regulated and down-regulated genes in PTMCs were functionally associated cell adhesion and cell-mediated immunity. In the comparative analysis of gene expression profiles of PTMCs and PTCs, no significant difference was found in a way that they cannot be distinguished by gene expression profiles.
Three others studies focused on the relationship of specific adhesion molecules, such as epithelial cell adhesion molecule (EpCAM) and E-cadherin, and clinicopathological factors of PTMC.42,51,52
Epithelial cell adhesion molecule (EpCAM) has an intracellular domain (Ep-ICD), a transmembrane domain and an extracellular domain (EpEx). EpCAM intervenes in a variety of cell processes including proliferation, adhesion, differentiation, cell cycle regulation and is involved in cancer signaling.
Kunavizarut et al., hypothesized that accumulation of Ep-ICD and loss of EpEx could be useful for distinguishing metastatic PTMC, since it was previously reported that Ep-ICD accumulation and loss of EpEx correlated with a worse prognosis in thyroid malignancy.52 Tissue samples from 36 patients were stained for Ep-ICD and EpEx by immunohistochemistry. Cytoplasmic and nuclear Ep-ICD expression and loss of membranous EpEx showed to be positively correlated with metastasis in PTMC patients. An index of aggressiveness, Ep-ICD subcellular localization index (ESLI), was defined as the sum of the immunohistochemistry scores for accumulation of Ep-ICD and loss of EpEx. ESLI was significantly associated with LNM in PTMC and therefore may be useful in identifying metastatic potential of these tumors.
The loss of E-cadherin occurs in the process of cancer cell transformation when they change their characteristics from an epithelial to a mesenchymal-like type.42 E-cadherin is frequently expressed in differentiated thyroid cancer and loss of its expression was reported to be an independent prognostic factor for these cancers.
Nakamura et al. evaluated 93 PTMCs patients and 57 LNM for E-cadherin expression.42 From these, 73 tumors (78.5%) and 49 LNM (86%) were immunohistochemically positive. In comparison to the center of the tumor, E-cadherin expression was significantly less common at the invasive front. Tumors that had lost E-cadherin expression at the invasive front frequently presented with LNM. Small tumors (≤5mm) expressed E-cadherin significantly more frequently when compared with the larger ones. Observing that the tumors which lost E-cadherin expression at the invasive front, commonly presented with LNM suggests that, even in small PTMCs, the process of cancer cell dissemination has already begun.
Batistatou et al. examined the expression of dysadherin, an anti-cell–cell adhesion glycoprotein, in PTMC, to relate it with E-cadherin expression and find out the differences with PTC.51 A statistically significant difference in dysadherin and E-cadherin expression between PTC and PTMC and a negative correlation between E-cadherin and dysadherin expression were observed. E-cadherin expression was retained in about half of the PTMCs and was low in only 10% of them, whereas reduction of E-cadherin expression was significantly higher in PTCs. The indolent course of PTMC may be due, at least in part, to the absence of high dysadherin expression in consequence of the maintenance of the E-cadherin, which prevents tumor cells from separating easily from each other and metastasize. Increased dysadherin expression is, maybe, one of the mechanisms responsible for E-cadherin downregulation in thyroid papillary cancer.
ConclusionThe approach of PTMCs remains controversial due to discrepant natural history of these apparently benevolent small tumors. Hereupon, it is of great importance to distinguish between nonincidental PTMC – clinically recognized PTMC – and incidental PTMC – asymptomatic PTMC found after surgery or during US performed for other motives. These two groups appear to be biologically distinct. From one side we have indolent tumors with nearly no potential for progression and, in the other side, tumors with the predisposition for a more aggressive course with clinical features comparable to those of conventional PTC.
In addition to clinical and histopathological factors, biomarkers are urgently required to assist in identification of the minority of patients that belong to the aggressive group. Unfortunately, until now, there is no biological marker that defines prognosis with certainty.
Despite the results not being entirely consistent, BRAFV600E is associated, in most reports, with aggressive clinicopathological characteristics such as tumor size, male gender, LNM, ETE, advanced TNM stages, multifocality and bilaterality, being highly prevalent in the tall cell variant.
Nevertheless, one should look critically to those associations because, ultimately, we cannot forget how prevalent this mutation is in PTMCs and, by contrast, how low is the mortality associated to this malignancy.
It is not wrong if we say that BRAF status analysis can improve the diagnostic accuracy of preoperative thyroid lesions. As suggested by Xing, considering the prevalence of BRAF mutation in PTMC around 30%, it appears practicable to treat more aggressively (ex. total thyroidectomy vs lobectomy) the one-third of PTMC patients that are positive for BRAF mutation on a FNAB sample and might have a worse prognosis.17 The remaining cases, the ones negative for BRAF mutation, could be managed more conservatively, if no other factors indicated other approach.
In a recent review, the utility of BRAF testing in the management of PTC was equated; unfortunately, with regard to PTMCs, no conclusion was reached.53 On the other side, the MP score proposed by Niemeier seems an interesting tool for patients with PTMC, although it is useless if we want to stratify risk before the surgical approach.
Singly, all genetic alterations, even BRAFV600E mutation, and biomarkers have, yet, little potential to overcome the barrier between the laboratory and the clinical practice. The RET/PTC rearrangement does not have value in predicting a poor outcome; on the contrary, NQO1 and NQO2 polymorphisms could be used as prognostic markers because of their relation with invasiveness. TERT mutation was not found in PTMCs. The tumor suppressor genes p53 and p27 are not helpful. The expression of COX-2 and EGFR may play a role in prognosis by their association with ETE, LNM, multifocality and bilaterality. S100A4 immunohistochemistry seems to be valuable for predicting metastatic potential. Cyclin D1 may predict LNM, but results are inconclusive. Galectin-3, HMWK, CK-19 and HBME-1 are not of great utility since their expression is similar in PTMCs and PTCs. HGF and c-MET expression were identified as significant factors for SLNM. From the existing data about membrane mucins we cannot achieve many conclusions. Cell adhesion molecules, especially EpCAM and E-cadherin, need to be studied in more detail in order to clarify their possible contribution in the metastatic process.
If a variety of molecular markers were evaluated many patients could be accordingly stratified for management. Thus, further studies are needed in order to try a combination of several markers for the purpose of increasing the probability of identifying the cases with more aggressive behavior and thus allow better and targeted treatment. Long-term randomized prospective studies are required as well as more information in what concerns to molecular findings.
In regards to clinicopathological features with prognostic value, we should remember the dichotomy inherent to the age at diagnosis. Although older age at diagnosis has been recognized as an element suggesting worse prognostic, it has been shown by Ito et al. that young age is an independent predictor of PTMC progression.59 Therefore, old patients with subclinical low-risk PTMC may be the best candidates for observation. Although PTMC in young patients may be more progressive than in older ones, it appears that surgery remains a viable option even after progression of subclinical PTMC to clinical disease, without compromising the outcome.
Several questions about the genetics events associated to PTMC remain unanswered. The main interrogations are the correlation between pathogenesis and clinical outcome as well as the best way to stratify clinically relevant subtypes of PTMC.
Determining a biological signature able to predict tumor aggressiveness would be a major discovery with enormous clinical relevance that, ultimately, could prevent unnecessary and aggressive treatment because of such a small tumor as a PTMC.
Conflicts of interestThe authors declare no conflicts of interest.