The aim of this study was to evaluate the microtensile bond strength and the adhesive interface of four adhesive systems to primary dentin.
MethodsSixteen sound human primary molars were ground flat to expose dentin and randomly divided into four experimental groups according to the different adhesive material evaluated: three self-etching systems (Clearfil™ Protect Bond, Clearfil™ S3 Bond Plus and Futurabond® U) and one etch-and-rinse system (Prime&Bond® NT). The adhesives were applied under manufacturer's instructions and the crowns “restored” with a composite resin (Synergy® D6). The “restored” teeth were then cross-sectioned to obtain sticks. Each stick was evaluated using a microtensile test in a universal testing machine. Additionally, eighteen dentin samples from four temporary molars were prepared for dentin conditioning and interface morphology evaluation using scanning electron microscopy. The bond strength results were analyzed using one-way ANOVA and a Tukey HSD test (confidence level of 95%).
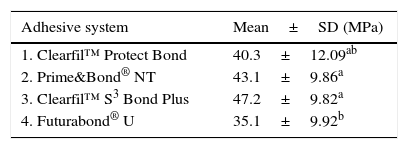
ResultsClearfilTMS3 Bond Plus (47,28MPa), Prime&Bond® NT (43.11MPa) and Clearfil™ Protect Bond (40.33MPa), presented the highest adhesion values without statistical differences. Futurabond® U bond strengths (35.16MPa) was statistically similar to Clearfil™ Protect Bond (p=0.271) but significantly lower from Prime&Bond® NT (p=0.022) and Clearfil™ S3 Bond Plus (p<0.001). An ultra-morphological evaluation showed marked differences in smear layer dissolution, depth of dentin demineralization and thickness of the hybrid layer promoted by the different adhesive strategies evaluated.
ConclusionsConsidering the limitations of this in vitro study, some self-etch adhesives may be capable of producing high bond strengths to primary dentin, similar to the etch-and-rinse adhesive evaluated.
Avaliar forças de adesão e interfaces adesivas obtidas por quatro sistemas adesivos em dentina decídua.
MétodosSeccionaram-se dezasseis molares decíduos humanos expondo uma superfície de dentina plana, dividindo-os aleatoriamente em quatro grupos experimentais de acordo com o adesivo avaliado: três autocondicionantes (Clearfil™ Protect Bond, Clearfil™ S3 Bond Plus e Futurabond®U) e um de “condicionar e lavar” (Prime&Bond® NT). Após aplicação de acordo com as instruções dos fabricantes seguiu-se a “restauração” com uma resina composta microhíbrida (Synergy® D6). Os dentes foram posteriormente seccionados obtendo-se bastonetes de secção quadrangular subsequentemente sujeitos a um teste de adesão por microtração. Os valores obtidos (MPa) analisaram-se pelos testes paramétricos ANOVA e de comparação múltipla de Tukey HSD (p≤0,05). Adicionalmente preparam-se dezoito amostras de dentina provenientes de quatro molares temporários tratadas com os mesmos adesivos objetivando estudar o condicionamento dentinário obtido e a ultramorfologia da interface por microscopia electrónica de varrimento.
ResultadosOs adesivos Clearfil™ S3 Bond Plus (47,28MPa), Prime&Bond®NT (43,11MPa) e Clearfil™ Protect Bond (39,38MPa), registaram valores de adesão mais elevados sem diferenças estatisticamente significativas entre si. Os valores obtidos com o Futurabond®U (35,16MPa) foram estatisticamente semelhantes ao Clearfil™ Protect Bond (p=0.271), mas estatisticamente inferiores aos do Prime&Bond®NT (p=0.022) e Clearfil™ S3 Bond Plus (p<0.001). Relativamente ao padrão de condicionamento dentinário e interfaces adesivas foram encontrados resultados substancialmente diferentes entre os adesivos.
ConclusõesDentro das limitações inerentes pode ser concluído que alguns adesivos autocondicionantes proporcionam valores de adesão em dentina temporária elevados, similares ao adesivo do tipo “condicionar e lavar” avaliado.
Modern restorative dentistry focuses on conserving tooth structure using adhesives and restorative materials. The clinical success of such restorations depends on the adhesive system, which provides a durable bond between the composite and the dentin and enamel.1–3
Presently, there are two main different approaches where adhesive systems can be used: etch-and-rinse and self-etching modes.4,5 Current self-etch systems may represent an attractive addition to the day-to-day dental practice due to their shortened application protocol, a particularly significant advantage in pediatric dentistry.6–10
Despite extensive research in dental adhesion, it has been common practice that knowledge acquired by in vivo or in vitro studies using permanent teeth has been extrapolated to primary teeth. Regardless of eventual chemical and morphological peculiarities, the same protocols have been recommended for bonding to primary and permanent teeth.11 Evidence regarding morphological differences suggests that the density and diameter of dentinal tubules is higher in primary than in permanent dentin, resulting in a reduced area of intertubular dentin available for bonding.12 Also, the higher prevalence of microchannels in primary teeth would further reduce bond strength.13 Chemically, the concentration of calcium and phosphate in peritubular and intertubular dentin is lower in primary teeth than in permanent teeth,12,14 which increases the reactivity of primary dentin to acidic solutions, resulting in the formation of thicker hybrid layers compared with permanent teeth.11,12,14–16 The differences between permanent and primary dentin may influence adhesive performance, leading to lower bond strength for primary dentin.13,17
The aim of this study was to evaluate the dentin conditioning, adhesive interface and microtensile bond strength (μTBS) of four adhesives to primary dentin. The null hypothesis was that “there are no significant differences in the bond strength between the different adhesive systems evaluated”.
Materials and methodsSixteen sound human primary molars were used in the microtensile study. The pulp tissue of each tooth was gently removed with an excavator and the pulp chamber was adhesively filled with a dual-cure composite resin (ParaBond® adhesive system and ParaCore® white, Coltène/Whaledent AG, Switzerland).
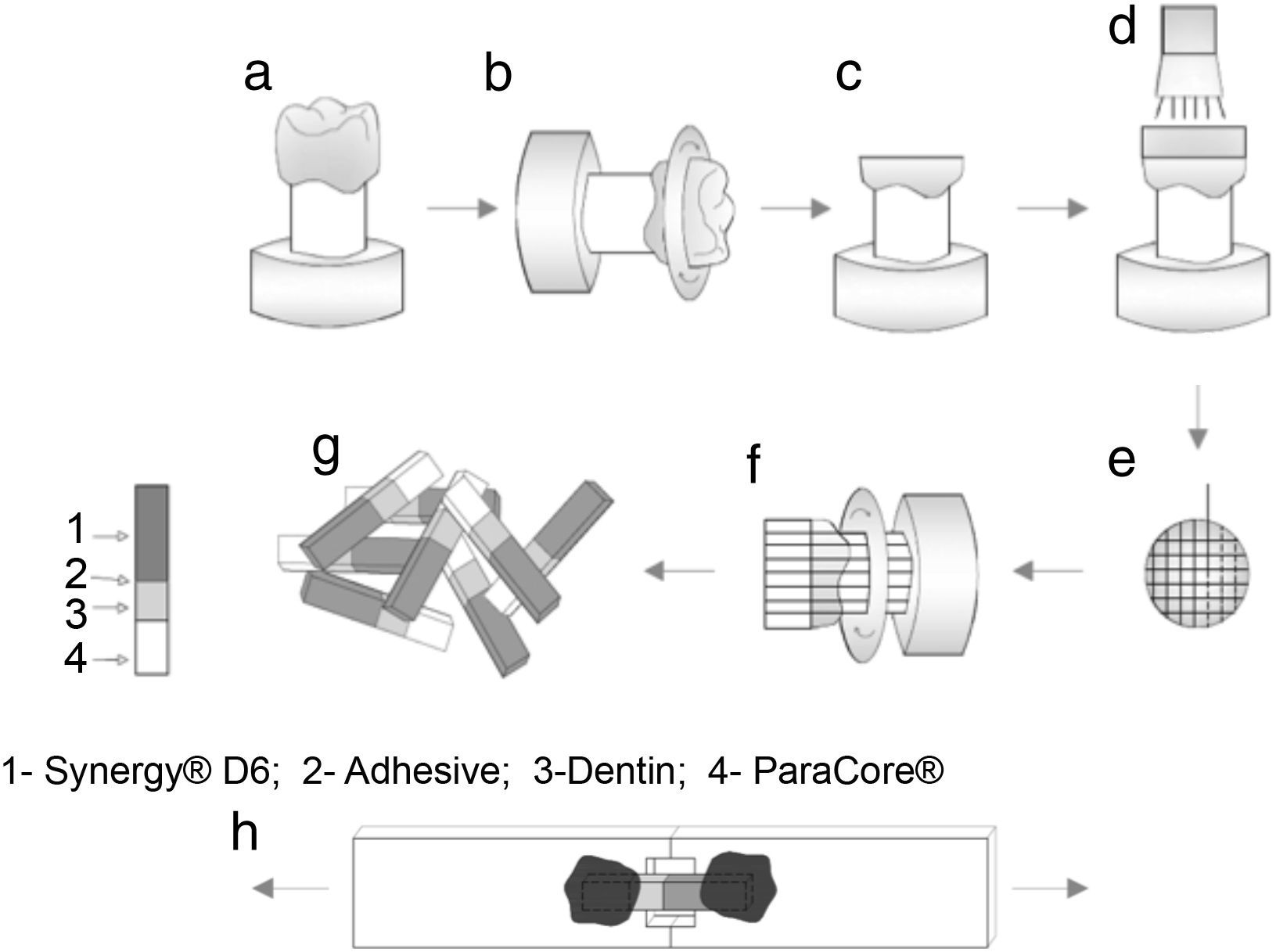
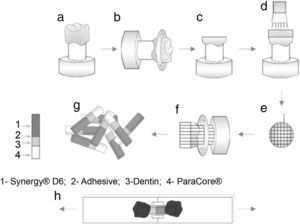
The occlusal surfaces of the teeth were cut just below the dentino-enamel junction to expose a flat area of dentin (Accutom 50, Struers, Denmark) under water refrigeration (Figure 1a–c). The exposed dentin surfaces were further wet polished with 240-, 400- and 600-grit silicon-carbide sandpaper in a circular motion, 60s each, to create a uniform smear layer. Dentin prepared surfaces was observed under an optical microscope (Leica, Switzerland) to ensure the absence of residual enamel.
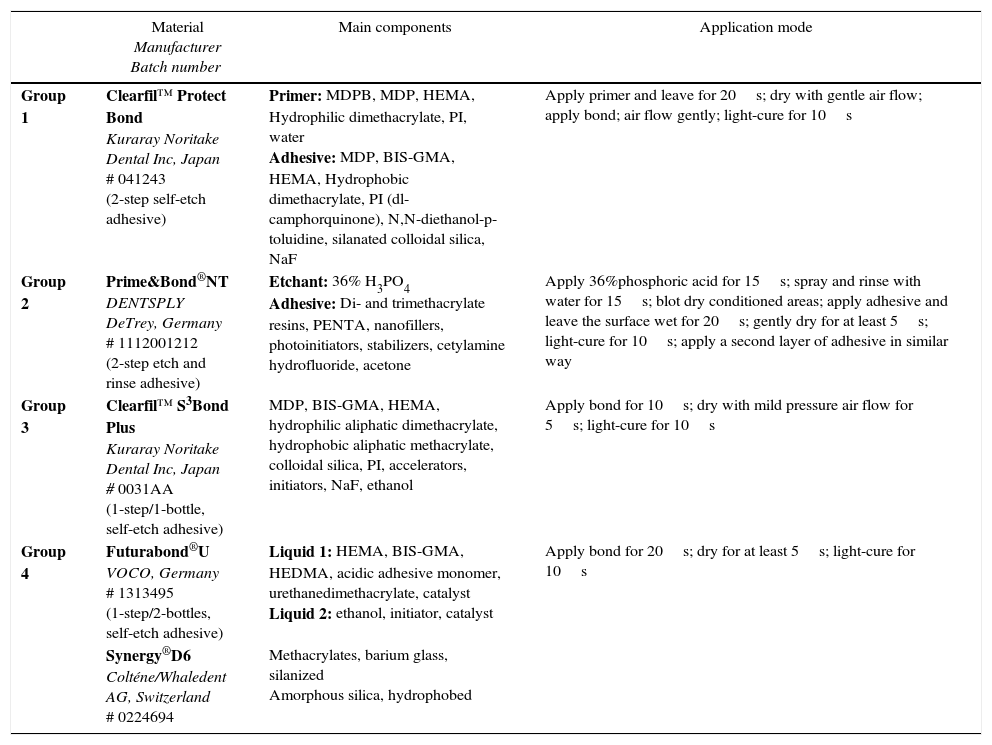
Teeth were randomly assigned into four groups (n=4), according to the adhesive system: (1) Clearfil™ Protect Bond (CPB) (Kuraray Noritake Dental Inc., Japan); (2) Prime&Bond® NT (PBNT) (DENTSPLY DeTrey, Germany); (3) Clearfil™ S3 Bond Plus (CSBP) (Kuraray Noritake Dental Inc., Japan); and (4) Futurabond® U (FBU) (VOCO, Germany) (Table 1). The adhesives were applied according to the manufacturer's instructions and light-curing performed using an LED device (Bluephase®, Ivoclar Vivadent, Liechtenstein) used in the “High Power” mode with a light intensity of 1200mW/cm2). After applying the adhesive system, a composite resin – Synergy® D6, A1/B1 (Coltène/Whaledent AG, Switzerland) – was built up using increments approximately 1.5mm thick; the first increment was light-activated for 10s with the same light-unit and the next increments for 20s, complemented by a final polymerization time of 40s. Figure 1 shows a schematic diagram of tooth preparation, restoration, specimen sectioning and subsequent testing. A single operator carried out all the bonding procedures.
Materials’ composition, characteristics and application mode.
| Material Manufacturer Batch number | Main components | Application mode | |
|---|---|---|---|
| Group 1 | Clearfil™ Protect Bond Kuraray Noritake Dental Inc, Japan # 041243 (2-step self-etch adhesive) | Primer: MDPB, MDP, HEMA, Hydrophilic dimethacrylate, PI, water Adhesive: MDP, BIS-GMA, HEMA, Hydrophobic dimethacrylate, PI (dl-camphorquinone), N,N-diethanol-p-toluidine, silanated colloidal silica, NaF | Apply primer and leave for 20s; dry with gentle air flow; apply bond; air flow gently; light-cure for 10s |
| Group 2 | Prime&Bond®NT DENTSPLY DeTrey, Germany # 1112001212 (2-step etch and rinse adhesive) | Etchant: 36% H3PO4 Adhesive: Di- and trimethacrylate resins, PENTA, nanofillers, photoinitiators, stabilizers, cetylamine hydrofluoride, acetone | Apply 36%phosphoric acid for 15s; spray and rinse with water for 15s; blot dry conditioned areas; apply adhesive and leave the surface wet for 20s; gently dry for at least 5s; light-cure for 10s; apply a second layer of adhesive in similar way |
| Group 3 | Clearfil™ S3Bond Plus Kuraray Noritake Dental Inc, Japan # 0031AA (1-step/1-bottle, self-etch adhesive) | MDP, BIS-GMA, HEMA, hydrophilic aliphatic dimethacrylate, hydrophobic aliphatic methacrylate, colloidal silica, PI, accelerators, initiators, NaF, ethanol | Apply bond for 10s; dry with mild pressure air flow for 5s; light-cure for 10s |
| Group 4 | Futurabond®U VOCO, Germany # 1313495 (1-step/2-bottles, self-etch adhesive) | Liquid 1: HEMA, BIS-GMA, HEDMA, acidic adhesive monomer, urethanedimethacrylate, catalyst Liquid 2: ethanol, initiator, catalyst | Apply bond for 20s; dry for at least 5s; light-cure for 10s |
| Synergy®D6 Colténe/Whaledent AG, Switzerland # 0224694 | Methacrylates, barium glass, silanized Amorphous silica, hydrophobed |
MDPB, 12-methacryloyloxydodecylpyridinium bromide; MDP, 10-methacryloxydecyl dihydrogenphosphate; HEMA, 2-hydroxyethyl methacrylate; MFM, multifunctional methacrylate; PI, photoinitiator; NaF, sodium fluoride; PENTA, dipenta-erythritol penta acrylate monophosphate; BIS-GMA, Bisphenol A-diglycidyl methacrylate; H3PO4, phosphoric acid.
After storage in distilled water at 37°C for one week the bonded samples were cross-sectioned perpendicular to the adhesive interface into quadrangular bonded sticks (Accutom 50 machine, Struers, Denmark) under water refrigeration at 300rpm (Figure 1e–f). The sticks were measured with a digital caliper rule (1.2mm×1.2mm of square section) (Figure 1g) and examined with an optical microscope at 40× magnification.
The sticks were individually attached to the microtensile testing jig with a cyanoacrylate adhesive (Permabond® 735, Permabond International Co., Englewood, NJ) (Figure 1h) and the bond strength was evaluated using a universal testing machine (Model AG-I, Shimadzu Corporation, Kyoto, Japan) with a crosshead speed of 0.5mm/min until failure occurred. Bond strength values were registered in MegaPascal (MPa). A total of 123 sticks were evaluated (31 for CPB; 38 for PBNT; 30 for CSBP; and 24 for FBU).
Fractured surfaces were inspected with an optical microscope (Leica, Switzerland) at a magnification of 40× to characterize the failure modes, which were classified as: (a) adhesive; (b) cohesive in dentin; (c) cohesive in composite; or (d) mixed (failure partial at the resin/dentin interface including some cohesive pattern on the neighboring substrates). Two examiners crosschecked this observation and confirmed the different findings.
Dentin conditioning and ultra-morphological evaluation of the adhesive interface was tested using 18 dentin samples, obtained from 4 split dentin disks from 4 primary molars. All of the disks were fixed in 2.5% glutaraldehyde for 24h. Two disks were split in 5 sections and only subjected to some dentin pre-treatment procedures in order to observe the chemical interaction of the adhesives with the smear-layer; the other 2 disks were split in 4 sections, with each prepared using the complete application procedure for the 4 adhesive systems evaluated. After longitudinal section these last specimens were soaked in 6Mol/L HCL for 30s and then immersed in 5% sodium hypochlorite for 10min. All the specimens were sequentially dehydrated in increasing concentrations of ethanol (50% – 75% – 95% – 100%), immersed in hexamethylisilazane, and completely air-dried. Finally, the specimens were mounted in aluminum stubs, sputter-coated with a gold-palladium layer and observed using scanning electron microscopy (SEM) (Hitachi S-4100, Japan).
Stick-based bond strength data were found to be normally distributed (Shapiro–Wilk test) and homogeneous in variances (Levene's test). One-way ANOVA test was performed to examine the effect of different adhesive systems with post hoc multiple comparisons using the Tukey HSD test. Failure modes were analyzed using chi-square test of independence. All statistical tests were applied with a confidence level of 95%.
ResultsThe μTBS means and standard deviations for all experimental groups are represented in Table 2. ANOVA reported statistically significant differences in μTBS values among the groups [F(3.119)=6.355, p<0.01]. Further statistical analysis using Tukey's HSD showed the highest mean μTBS value associated to CSBP, which was not significantly different from PBNT (p=0.364) and CPB (p=0.052). Conversely, FBU obtained the lowest μTBS, but statistically significant differences were only found between FBU and CSBP (p<0.01) and between FBU and PBNT (p=0.022).
Distribution of the failure/fracture mode is summarized in Table 3. Adhesive failures occurred mostly with PBNT than with CSBP. CSBP and CPB showed higher percentage of cohesive failures in composite resin than FBU. Regarding dentin cohesive fractures there were no differences between the groups.
Distribution of the failure mode for each group: N (% within failure mode).
| Clearfill™ Protect Bond | Prime&Bond® NT | Clearfil™ S3 Bond Plus | Futurabond® U | ||
|---|---|---|---|---|---|
| Failure mode | AD | 8 (23.5) | 18 (52.9) | 3 (8.8) | 5 (14.7) |
| CC | 13 (37.1) | 7 (20) | 15 (42.9) | 0 (0) | |
| M | 5 (14.7) | 9 (26.5) | 8 (23.5) | 12 (35.3) | |
| CD | 5 (25) | 4 (20) | 4 (20) | 7 (35) |
AD, adhesive failure; CD, cohesive failure in dentin; CR, cohesive failure in resin; M, mixed failure.
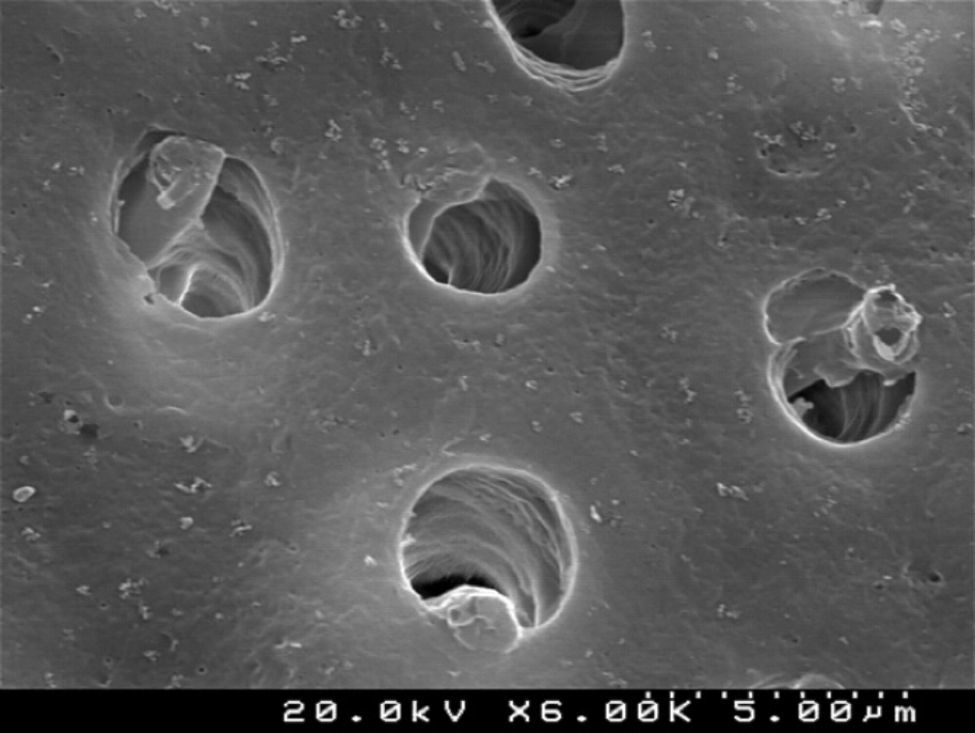
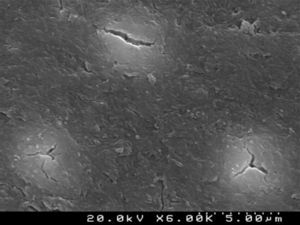
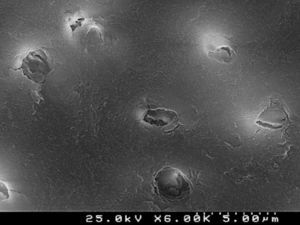
Figures 2 and 3 are representative SEM images of the smear layer adhered to dentin surface and almost occluding the dentinal tubules before any adhesive procedure.
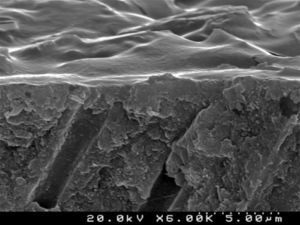
When the dentin surface was treated with 36% phosphoric acid (Figures 4 and 5), the smear layer and plugs were removed and dentinal tubules were totally opened and enlarged.
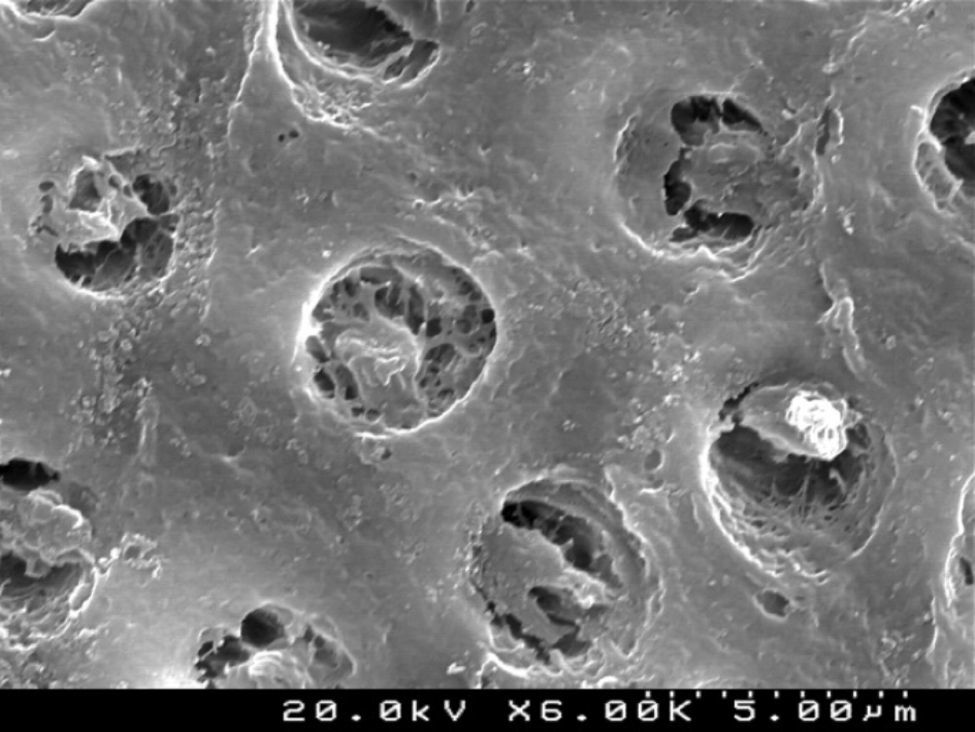
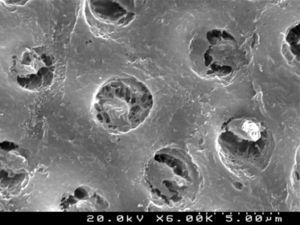
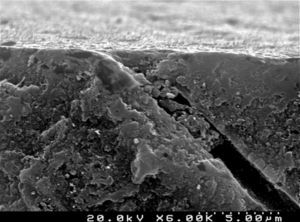
The self-etch primer of the CPB (Figures 6 and 7) removed various smear plugs, opening the dentinal tubules and partially demineralizing the peritubular dentin collar.
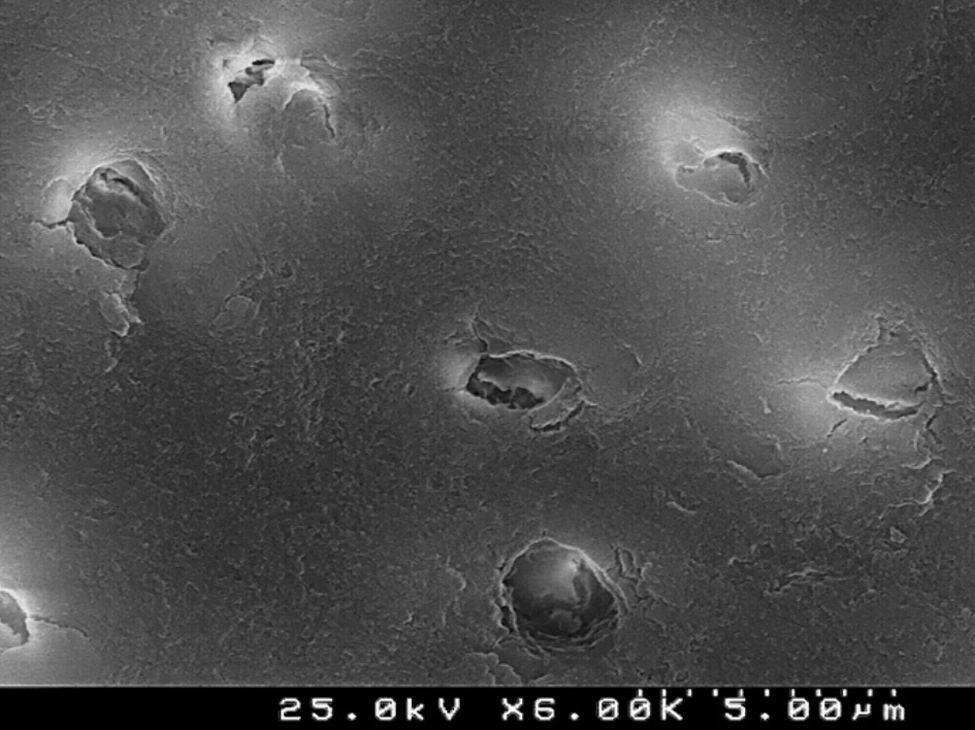
CSBP (Figures 8 and 9) and FBU (Figures 10 and 11) did not remove the smear layer or smear plugs, so that the dentinal tubules are not left open. The demineralization was superficial and did not show a noticeable difference between the intertubular or peritubular dentin collar around the lumen of the tubules.
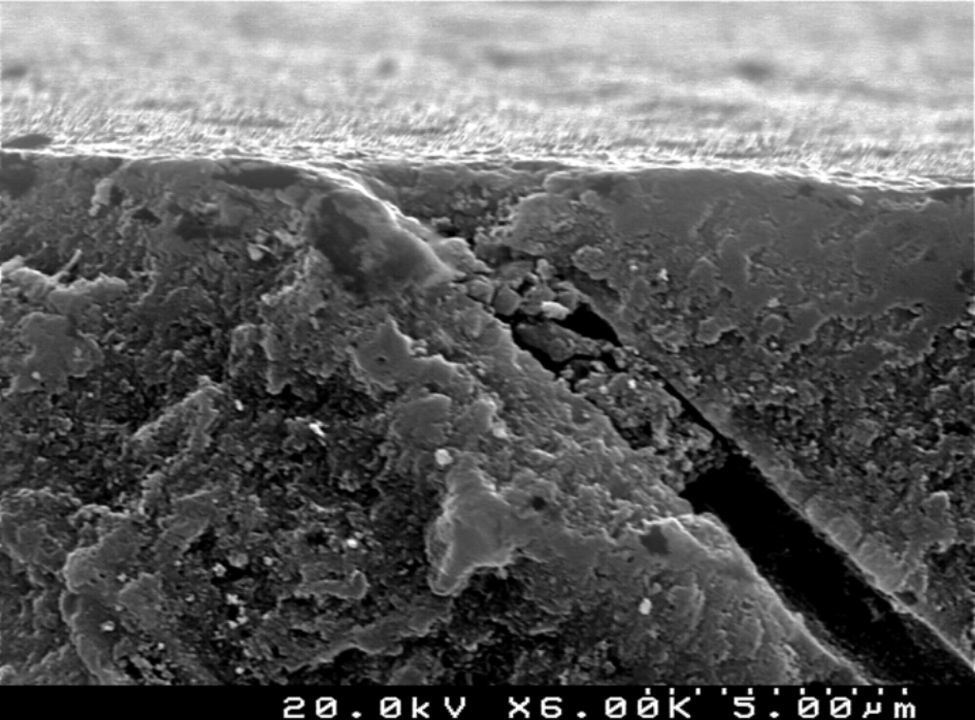
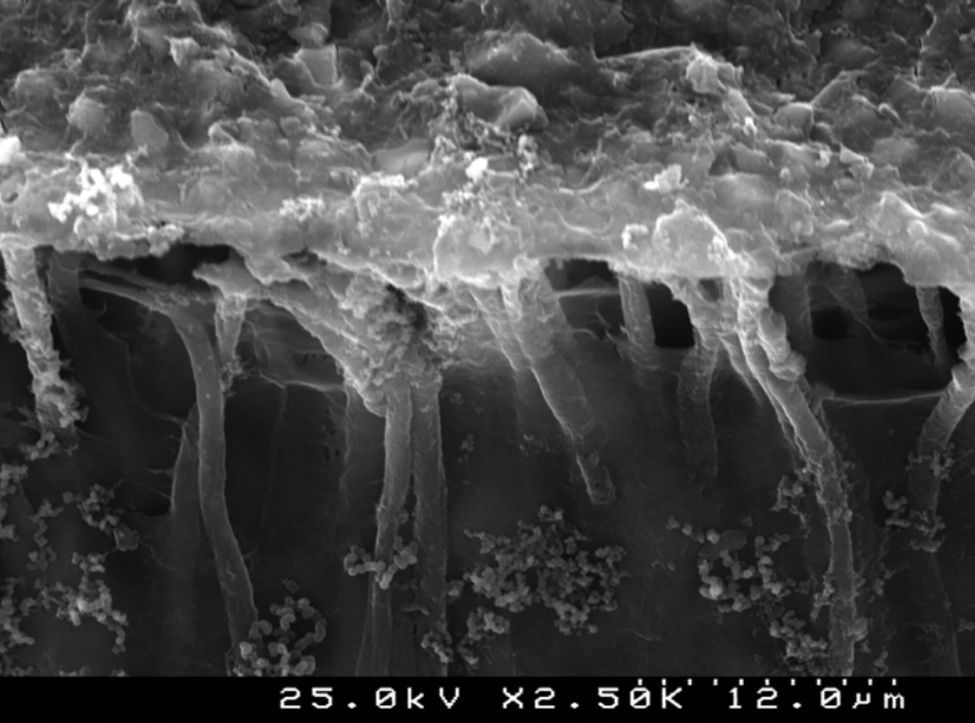
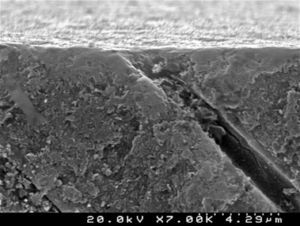
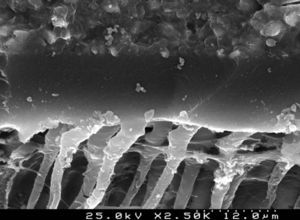
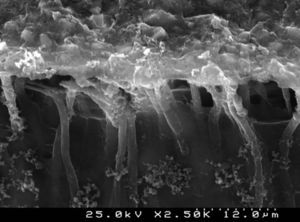
Analysis of the specimens in longitudinal sections revealed that the thickness of the hybrid layer produced by CPB and PBNT is thicker than that produced by CSBP and FBU. CSBP and FBU exhibited cylindrical resin tags with scarce lateral branches. The funnel-shaped configuration of the resin tags is evident mainly in PBNT (with numerous lateral branches) and partially in CPB (Figures 12–15).
Clinical demand has driven the development of simplified versions of adhesive systems, such as self-etch, particularly in pediatric restorative dentistry. However, their efficacy is material dependent and most of them still need to be effectively evaluated in temporary teeth.3,6,10,14,18–24
The mean adhesion values in this study showed that the self-etch all-in-one CSBP system exhibited the highest bond strength values, but statistically similar to PBNT (two-step, etch-and-rinse) and CPB (two-step, self-etch). Thus, the null hypothesis was rejected. One probable factor contributing for the high values obtained by CSBP could be the acidity of the adhesive that determines the depth to which resin monomers can penetrate into dentin.25 In 2001, Tay subdivided self-etching primers into mild, moderate and aggressive, according to their etching aggressiveness.26 Under this classification, CSBP (pH 2.3) should be considered a mild self-etching adhesive as it only causes dentin demineralization to a depth of 1μm.26 Moreover, only partially demineralization occurs, leaving a substantial amount of residual hydroxyapatite still attached to the collagen.5 The preservation of hydroxyapatite within the submicron hybrid layer may serve as a receptor for additional chemical bonding and hybrid layer stabilization.27 Along with the pH, bond durability is significantly influenced by the kind of adhesive monomers within bonding agents.5,27 Researchers have pointed out that some functional monomers in self-etch adhesives, such as 10-MDP present in CSBP, have the potential to chemical bond to the calcium in the residual hydroxyapatite.27,28 The combination of micromechanical and chemical adhesion is probably responsible for the high bond strengths obtained with CSBP. Another feasible reason for the high values obtained could be the application time of this adhesive system. Osorio et al. evaluate the effect of shortening the application time of a one-step self-etch adhesive (One-Up® Bond) compared with the time recommended by the manufacturer. They concluded that halving the application time of One-Up® Bond improved bond strength to primary dentin.29 Similarly, CSBP has a recommended application time of 10s, which is significantly less than most of the self-etch adhesives.
Previous studies would lead us to expect that PBNT would produce better results than the self-etch adhesives tested.8,18 However, the present study found that CSBP exhibited higher μTBS than PBNT, although not statistically significant. The etching time can influence the values obtained by PBNT. Several studies compared the bond strengths in primary teeth depending on the etching time.15,29–31 All of them concluded that a reduction in etching time might produce an increase in microtensile bond strength. According to the manufacturer's instructions, 36% phosphoric acid should be applied at for least 15s, before PBNT application; although this is the recommended etching time for permanent teeth, the more reactive characteristic of primary dentin to acidic conditioners means that an eventual reduction in the etching time can prevent the formation of a non-impregnated demineralized dentin, which compromises the bonding efficacy.11 Osorio et al. (2010) evaluated the effect of shortening the etching time on the bond strength of Single Bond (etch-and-rinse) and concluded that halving the phosphoric acid etching time compared to the manufacturer's recommendations (from 15 to 7s), promoted a significant increase in microtensile bond strength (29.38–42MPa).29 Thus, we can speculate that shortening the phosphoric acid etching time for PBNT could be advantageous in primary dentin and deserves specific research.
CPB (two-step self-etch) exhibited similar bond strength to CSBP (one-step self-etch). This result is contrary to other studies, which report that two-step self-etch adhesive systems exhibit superior in vitro performance in comparison to one-step self-etch systems.25,32,33 The effect of introducing an antibacterial monomer (MDPB) in CPB is controversial. One study reported that it causes a decrease in bond strength to primary teeth dentin, whereas other studies state that introducing MDPB has no influence on the bond strength of adhesive systems.34–36 In theory, the introduction of MDPB can influence bond strength in water-based adhesives.27
Another important factor to be considered is the HEMA concentration in CPB (25–45%). High amounts of HEMA in the adhesive composition result in flexible polymers with inferior qualities, and a potential reduction in bond strength due to the attraction of water.27,37
The lowest microtensile bond strength was obtained with FBU, which was statistically different to CSBP and PBNT. FBU is characterized by a relatively mild pH (2.3) and high HEMA concentrations. The lack of scientific data for this adhesive system, tested in primary dentin, makes it difficult to discuss the results.
SEM evaluation of the hybrid layer showed that the adhesive with the best bond strength results (CSBP) produced a thinner hybrid layer compared to PBNT and CPB. Effectively, literature has not yet established a positive correlation between the thickness of the resin infiltrated layer and bond strength in primary dentin. The quality, rather than the thickness of the resin-infiltrated layer, can assume the fundamental role.
ConclusionsWithin the limitations of this in vitro study the results can lead us to draw the following conclusions:
- 1.
Out of the adhesives tested, some self-etching systems can achieve high bond strength in primary dentin, comparable with the etch-and-rinse adhesive evaluated. Clearfil™ S3 Bond Plus and Prime&Bond® NT showed the highest microtensile bond strength values.
- 2.
There are marked differences in smear layer dissolution, depth of primary dentin demineralization and thickness of the hybrid layer resulting from the different adhesive strategies evaluated. More aggressive and deeper dentin demineralization was obtained with the phosphoric acid, which promoted the complete removal of the smear layer. The self-etching 2-step adhesive Clearfil™ Protect Bond provided greater dissolution of the smear layer and depth of demineralization than the self- etching 1-step systems Clearfil™ S3 Bond Plus and Futurabond® U.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors gratefully acknowledge to Dr. Fernando Marques for his precious support with all technical procedures, to Dra. Vânia Sobral for her collaboration and also to the manufacturers for the availability of the materials under study.