The main aim of this study was to assess the clinic atmosphere quality regarding the Index of Air Microbial contamination (IMA), according to dental aerosols bacterial counts, when different dentistry procedures are performed.
Material and methodsThe aerosols generated by dentistry and endodontic procedures were analyzed in 26 dental units of the dental clinic. Blood agar plates (n=244) were incubated at 37°C/48h and the colony forming units (CFU) were calculated.
All statistical analysis methods were conducted using the SPSS® vs.17.0 software (SPSS Inc., IL, Chicago, USA), using α=0.05 for the comparison tests used (non parametric tests of Mann–Whitney and Wilcoxon).
ResultsThe IMA median value in the dental clinic was of 10.4CFU/dm2/h. Aerosols’ CFU counts were significantly higher at 0.5m and during endodontic treatments. Longer treatment times were associated with higher CFU counts both in dentistry and endodontic procedures. The use and time of turbine use did not significantly affect the CFU counts. Micrococcus sp., Staphylococcus sp. and Streptococcus sp. were identified with presumptive tests after isolation of representative colonies.
ConclusionConsidering the IMA, the dental clinic atmosphere quality was found to be good.
O objetivo principal deste trabalho foi determinar a qualidade do ar da clínica utilizando o Index of Air Microbial contamination (IMA), mediante utilização de contagens bacterianas em aerossóis dentários produzidos durante a realização de diferentes procedimentos dentários.
Material e métodosForam analisados aerossóis gerados por procedimentos de dentística e endodontia em 26 unidades dentárias distribuídas pela clínica de medicina dentária. As placas de agar de sangue (n=244) foram incubadas a 37°C/48h e foram calculadas a unidades formadoras de colónias (UFC).
A análise estatística foi realizada recorrendo ao software SPSS® vs. 17.0 software, utilizando α=0.05 em todos os testes de comparação estatística realizados (testes não paramétricos de Mann–Whitney e de Wilcoxon).
ResultadosA mediana do IMA na clínica de medicina dentária foi 10,4UFC/dm2/h. As contagens de UFC nos aerossóis foram significativamente superiores na distância 0,5m e durante tratamentos endodônticos. Tratamentos mais longos estão associados a contagens mais elevadas de UFC, tanto para procedimentos de dentística como de endodontia. A utilização e tempo de utilização de turbina não afetou significativamente as contagens de UFC. Após isolamento de colónias representativas e utilizando de testes presumptivos, foi possível identificar Micrococcus sp., Staphylococcus sp. e Streptococcus sp.
ConclusãoA qualidade do ar na clínica de medicina dentária, utilizando o IMA, foi considerada como boa.
In dental clinic environment dentistry professionals and patients are daily exposed to a great variety of infectious agents and toxic substances transported by aerosols and droplets, produced during dental operative procedures,1 promoting an increased risk of cross infection.2,3 Aerosols are particles small enough to stay airborne for an extended period before they settle on environmental surfaces.4,5 As 75% of these particles drop on a desktop with a diameter of 2m (meters) from the patient position,6 the environment (water, air and surfaces) play an important role in this context.2 Mouth fluids are grossly contaminated with bacteria, mostly aerobic bacteria (streptococci and staphylococci) and viruses.2,7 Most dental procedures that use handpieces, turbines, ultrasonic scalers, air polishers and abrasion units removes material from the operative site, that becomes aerosolized3,7,8 by the instrument rotary action or the water sprays and compressed air combined actions1; So, there is a strong possibility that aerosols, besides the presence of bacteria,9 will include viruses, blood, and supra- and sub-gingival plaque organisms.10,11
Several studies12–16 have been conducted to determine which dental procedure produces the highest airborne bacterial contamination using nonselective bacterial growth media (blood agar). When an aerobic bacterium settles on the plate and grows as a colony, it is counted as a colony-forming unit, or CFU.1 Although this method does not provide any differentiation between whether the bacteria are relatively benign or a pathogenic species, it is a sterile, easy to implement and low cost, reproducing the most common conditions and gives a good perspective regarding total airborne bacterial CFUs from a particular procedure.17
Pasquarella et al.17 described the Index of Air Microbial contamination (IMA) based on the count of the microbial fallout on to Petri dishes left open to the air according to the 1/1/1 scheme (for 1h, 1m from the floor, at least 1m away from walls or any obstacle). Classes of contamination and maximum acceptable levels have been established, and a threshold of 25 was considered adequate.2,3 IMA has been tested in many different places and proved to be a reliable and useful tool for monitoring the microbial surface contamination settling from the air in any environment2,17,18 although its use is not consensual for critical environments such as operating theatres.2 A few studies on air quality microbial counts in dental clinics were presented in literature.2,8,19
The aim of this study was to assess the aerosols contamination, by quantifying the aerobic bacteria CFUs when different dental treatments were performed and to analyze the effect of type and time of treatment and time of turbine use on the quantitative bacterial variation of aerosols produced during restorative dentistry and endodontic treatments, and to identify the representative aerobic bacterial colonies. Another purpose of this study was to check the dental clinic atmosphere quality comparing with the IMA values.
Material and methodsThis study was conducted in the teaching Dental Clinical of the Faculty of Health Sciences, University Fernando Pessoa (FHS-UFP). This clinic has a total area of 390m2, divided into 37 dental units spaces (DUs), each one with 5m2. The ventilation system was checked periodically and was not altered during the study period.
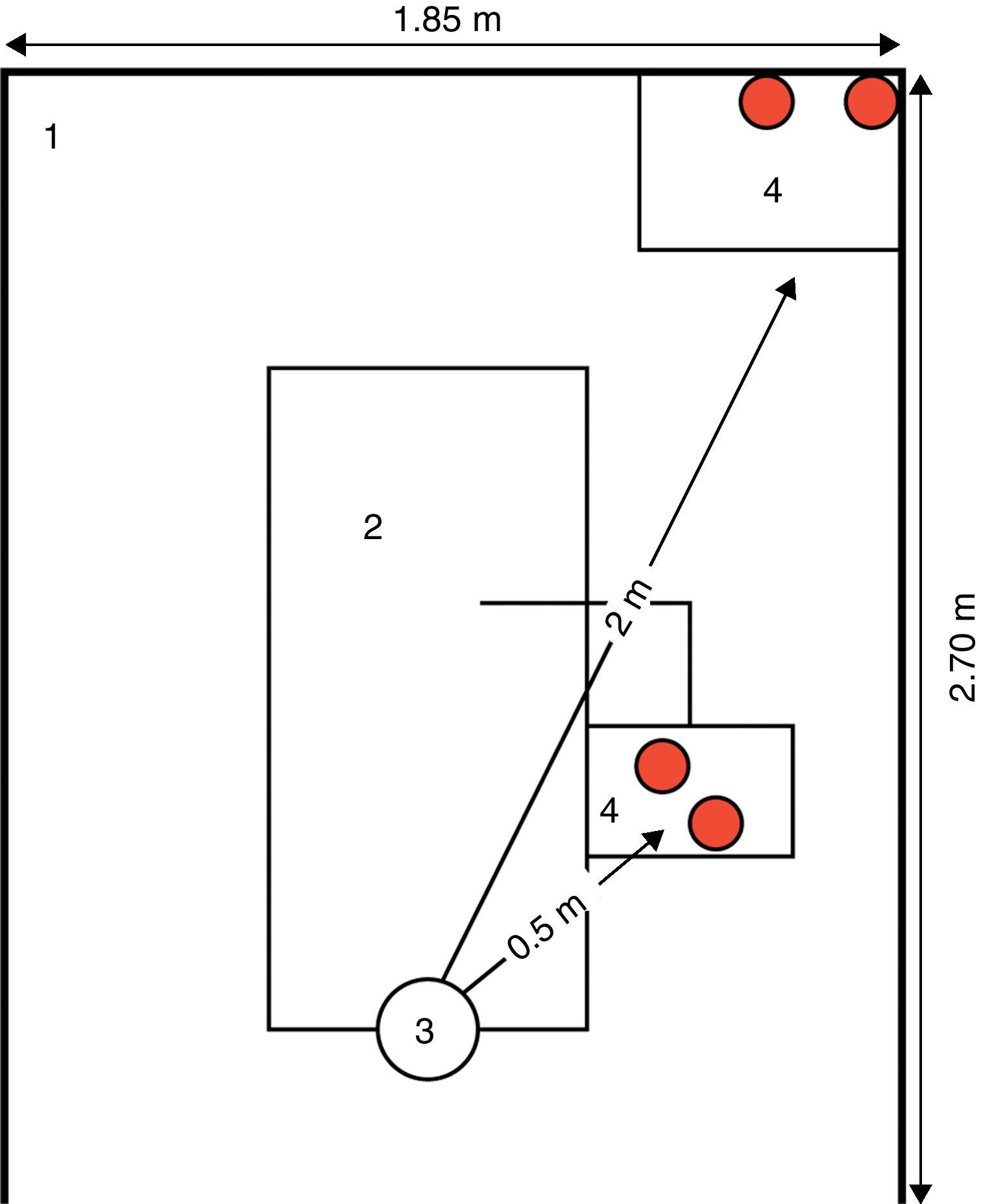
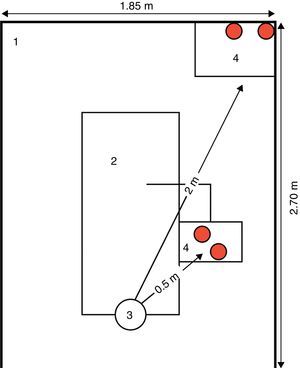
A baseline register of CFU counts was conducted before any appointment/dental procedure performed in the dental clinic. This control group was constituted by 12 opened plaques placed in three randomly selected DUs. IMA was assessed by passive sampling, using blood agar plates (bioMerieux Ref. 43041, Linda-a-Velha, Portugal) 9cm (centimetre) in diameter that were exposed to air during dental treatments (two at 0.5m and two at 2m from the patient head position) (Fig. 1). The plates were opened at the beginning of each dental procedure and remained so for at least 1h to a maximum of 4h (duration of the dental clinical appointments). Overall, 244 air samples in blood agar plates were collected from 26 DUs of the dental clinic.
The restorative dentistry procedures included cavities preparation of dental hard tissues disorders that were directly restored with adhesive or non-adhesive dental materials. All biomechanical preparation was done with manual instruments and high-speed handpieces (turbine) with water-cooling. The restored surfaces were polished by rotary action, with water-cooling; Relative operatory field was done with cotton rolls and surgical aspirator.
All endodontic treatments, performed in mono- and multi-canal teeth, were non-surgical and included biomechanical preparation with turbine (to prepare the access cavity) with water-cooling, manual instrumentation, disinfection with antimicrobial rinses and the root canal filling; Rubber dam was used for operatory field isolation.
To perform the microbial quantitative analysis of air all blood agar plates were incubated at 37°C for 48h. Colonies were counted by CFU/plate. The CFU/dm2/h was calculated to determine the IMA. Bacterial levels <39CFU/dm2/h with passive sampling were considered as acceptable values for the air microbial contamination.17,20 The threshold value of 25CFU/dm2/h for IMA was considered as a good air performance.2,17
The methodology employed for the qualitative analysis of microbial air followed the classical microbiologic identification. The isolation of representative colonies predominantly present in the blood agar plates was based on macroscopic colony morphology: colour, shape, size, texture and hemolytic characteristics. Further identification was determined using the Gram staining and cell morphology21,22; and the enzymatic tests: catalase, oxidase, coagulase and DNAse. Based on these information it was possible to select appropriate procedures for identification of isolates by the usual commercial biochemical tests, using the API Staph (bioMérieux, Linda-a-Velha, Portugal) and API 20 Strep (bioMérieux, Linda-a-Velha, Portugal).
All statistical analysis methods were conducted using the SPSS® vs. 17.0 software (SPSS Inc., IL. Chicago, USA), using α=0.05. The comparison of the median CFU value in dentistry procedures performed (restorative dentistry/endodontic), time of treatment (≤2h/>2h), use of turbine (yes/no) and time of turbine use (≤30min/>30min) was performed using the Mann–Whitney test (as the assumption of normality of the observations does neither hold nor the symmetry of the distributions). The Wilcoxon test was used for comparing median CFU values projected at two different distances (paired measurements, at 0.5m and 2m).
ResultsThe IMA value in the dental clinic was significantly (Mann–Whitney test, p<0.001) lower before the opening and patient attendance (Mean (±SD); Median: 3.7 (±1.0); 3.4CFU/dm2/h) than during the clinical attendance (11.9 (±6.7); 10.4CFU/dm2/h). There were significant differences (Mann–Whitney test, p<0.001) in the CFU/plate counts between the control group (7.0 (±1.9); 6.4CFU/dm2/h) and the experimental (restorative dentistry and endodontic treatments) group (17.3 (±10.1); 15.0CFU/dm2/h). The CFU/plate counts mean value was significantly (Mann–Whitney test, p<0.001) higher for endodontic treatments (19.7 (±10.8); 17.1CFU/dm2/h) than for restorative dentistry (15.1 (±8.9); 13.9CFU/dm2/h) procedures.
During dentistry and endodontic treatments aero-transported CFU counts (Table 1) were significantly (Wilcoxon test, p<0.001) higher at 0.5m (19.0 (±11.5); 16.0) than at 2m (15.6 (±8.2); 13.9) and significantly higher for endodontic treatment for both distances (Mann–Whitney test, p=0.001, at 0.5m and p=0.001 at 2m). During restorative dentistry procedures the CFU counts were significantly higher (Wilcoxon test, p<0.001) at 0.5m (16.6 (±10.4); 15.0) than at 2m (13.6 (±6.9); 12.7).
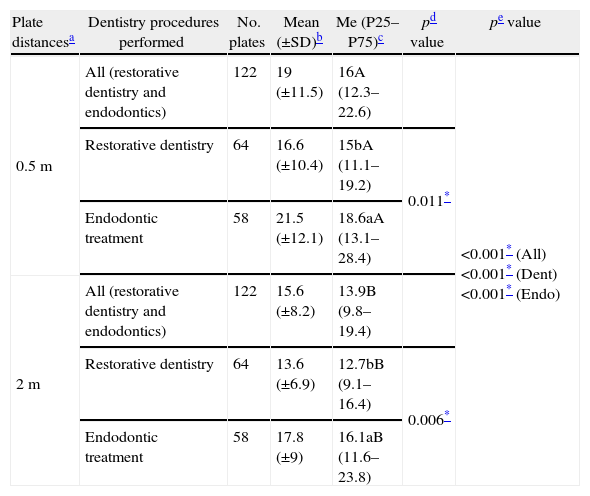
Descriptive statistics of CFU/plate count during different dentistry procedures (restorative dentistry and endodontic treatments) projected at two distances (0.5 and 2m).
| Plate distancesa | Dentistry procedures performed | No. plates | Mean (±SD)b | Me (P25–P75)c | pd value | pe value |
| 0.5m | All (restorative dentistry and endodontics) | 122 | 19 (±11.5) | 16A (12.3–22.6) | <0.001* (All)<0.001* (Dent)<0.001* (Endo) | |
| Restorative dentistry | 64 | 16.6 (±10.4) | 15bA (11.1–19.2) | 0.011* | ||
| Endodontic treatment | 58 | 21.5 (±12.1) | 18.6aA (13.1–28.4) | |||
| 2m | All (restorative dentistry and endodontics) | 122 | 15.6 (±8.2) | 13.9B (9.8–19.4) | ||
| Restorative dentistry | 64 | 13.6 (±6.9) | 12.7bB (9.1–16.4) | 0.006* | ||
| Endodontic treatment | 58 | 17.8 (±9) | 16.1aB (11.6–23.8) |
Blood agar plates placement regarding the patient head position, on the dental unit space (5m2 area); distance in meters (m).
Mean (±SD): mean and standard deviation of CFU/plate counts according to the dentistry procedures performed.
Me (P25–P75): median, 25 and 75 percentiles of CFU/plate counts according to the dentistry procedures performed.
Mann–Whitney test; different letters (a and b) after the median value stand for significant differences with treatment.
Longer treatment times (≥2h) were associated with higher CFU count (Mann–Whitney test, p<0.005) in restorative dentistry (Mann–Whitney test, p<0.004) and in endodontic treatments (Mann–Whitney test, p<0.002) procedures, for both growth plate distances, at 0.5m and at 2m (Table 2). No significant differences were found in CFU/plate counts formed during the restorative dentistry or the endodontic treatments procedures, considering the use or no turbine use and the short time (≤30min) or the longer time (>30min) of turbine use (Table 2).
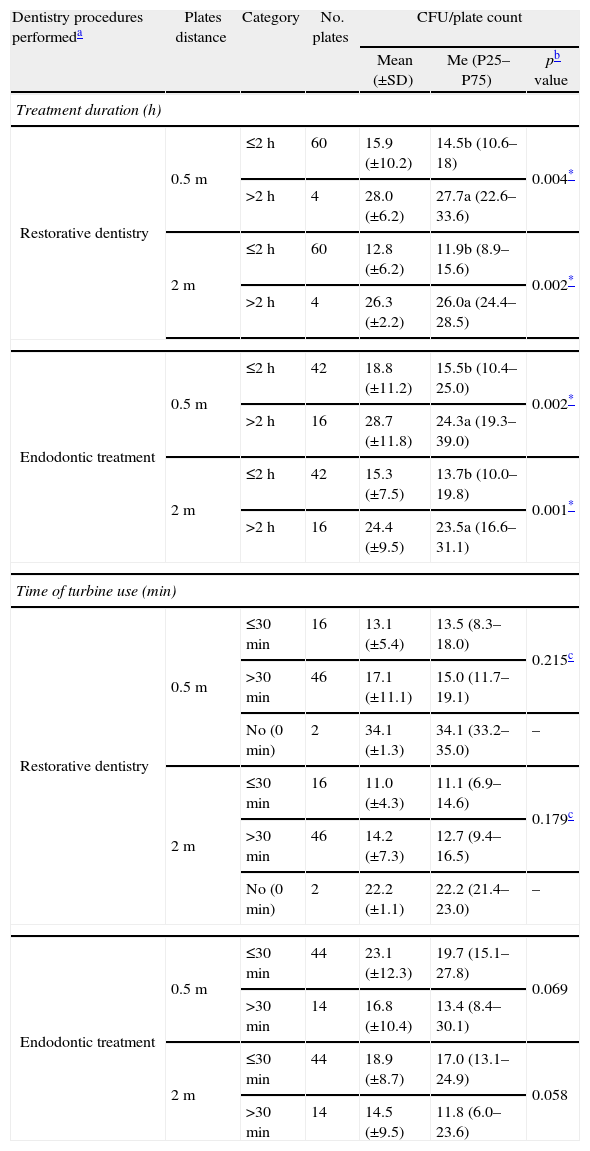
Descriptive statistics of CFU/plate count formed during different dentistry procedures (restorative dentistry and endodontic treatments) projected at two distances (0.5 and 2m) according to treatment duration, in hours (≤2h and >2h), and to length of turbine use, in minutes (0minutes (no), ≤30min and >30min).
| Dentistry procedures performeda | Plates distance | Category | No. plates | CFU/plate count | ||
| Mean (±SD) | Me (P25–P75) | pb value | ||||
| Treatment duration (h) | ||||||
| Restorative dentistry | 0.5m | ≤2h | 60 | 15.9 (±10.2) | 14.5b (10.6–18) | 0.004* |
| >2h | 4 | 28.0 (±6.2) | 27.7a (22.6–33.6) | |||
| 2m | ≤2h | 60 | 12.8 (±6.2) | 11.9b (8.9–15.6) | 0.002* | |
| >2h | 4 | 26.3 (±2.2) | 26.0a (24.4–28.5) | |||
| Endodontic treatment | 0.5m | ≤2h | 42 | 18.8 (±11.2) | 15.5b (10.4–25.0) | 0.002* |
| >2h | 16 | 28.7 (±11.8) | 24.3a (19.3–39.0) | |||
| 2m | ≤2h | 42 | 15.3 (±7.5) | 13.7b (10.0–19.8) | 0.001* | |
| >2h | 16 | 24.4 (±9.5) | 23.5a (16.6–31.1) | |||
| Time of turbine use (min) | ||||||
| Restorative dentistry | 0.5m | ≤30min | 16 | 13.1 (±5.4) | 13.5 (8.3–18.0) | 0.215c |
| >30min | 46 | 17.1 (±11.1) | 15.0 (11.7–19.1) | |||
| No (0min) | 2 | 34.1 (±1.3) | 34.1 (33.2–35.0) | – | ||
| 2m | ≤30min | 16 | 11.0 (±4.3) | 11.1 (6.9–14.6) | 0.179c | |
| >30min | 46 | 14.2 (±7.3) | 12.7 (9.4–16.5) | |||
| No (0min) | 2 | 22.2 (±1.1) | 22.2 (21.4–23.0) | – | ||
| Endodontic treatment | 0.5m | ≤30min | 44 | 23.1 (±12.3) | 19.7 (15.1–27.8) | 0.069 |
| >30min | 14 | 16.8 (±10.4) | 13.4 (8.4–30.1) | |||
| 2m | ≤30min | 44 | 18.9 (±8.7) | 17.0 (13.1–24.9) | 0.058 | |
| >30min | 14 | 14.5 (±9.5) | 11.8 (6.0–23.6) | |||
Different letters (a and b) after the median value stand for significant differences with duration of treatment according to the Mann–Whitney test.
Gram-positive cocci were predominant in the samples: Micrococcus sp. (99.9%), Staphylococcus capitis (99.8%), Streptococcus sp. (99.9%), Staphylococcus epidermidis (84.8%) and Staphylococcus sp. (99.9%) colonies were identified (% of identification reliability given by the API system).
DiscussionIn the present study the air quality of the dental clinic was found to be good, with acceptable values for the air microbial counts17,20 since the bacteria levels (11.9 (±6.7)CFU/dm2/h) registered were lower than 39CFU/dm2/h.2,17 Passive air sampling is one effective way of quantifying airborne bacteria once it measures the live microorganisms that can settle, growth and multiply, as used in the present study, reflects the extent of aerobic bacteria contamination on the surfaces, and highlights clinical areas of primary importance for cross infection prevention.2,8,17
The results (Table 1) showed higher number of CFU counts during the endodontic treatments in which the rubber dam was used. One likely explanation is that during the restorative dentistry procedures, the operatory field was relatively isolated with cotton rolls and splatter aspiration. Although the use of rubber dam has been shown to be highly significant in reducing contamination of the atmosphere,8,23 another data reported suggest that the use of rubber dam can concentrate and spread24 the aerosols produced when high-speed rotating instruments are used or when endodontic treatment is performed, and momentarily contaminates the air; but as the bacteria settle on the surfaces, then the air quality increases. How far the aerosols spread and what level of contamination they cause in the dental surgery is of concern8 and should be analyzed.
During both dentistry and endodontic treatments CFU counts (Table 1) were significantly higher at 0.5m than at 2m and significantly higher in endodontic treatment for both distances, results corroborated by Timmerman et al.,25 but not by Rautemaa et al.,8 that registered higher CFU/m2/h counts at distances >1.5m than at distances <1m, explaining that may be related to the increased speed of instruments rotation, that gives a greater speed and higher angular trajectory of bacteria.
Longer treatment times (≥2h) were associated with higher CFU count in dentistry and endodontic procedures, at both distances (0.5m and 2m) (Table 2), but the time of turbine use did not affect the aerosol CFU counts. These results are partially in agreement with Grenier (1995) study, regarding the level of air bacterial contamination that was higher at the end of dental treatments with about 6h time duration,24 but considering the use of turbine, are not in agreement with the Rautemaa et al.8 findings, where the CFU counts were less intense during dental treatments where high-speed and ultrasonic instruments were not used.
In the present study the most common microorganisms isolated were included in Micrococcus sp., Streptococcus sp., and Staphylococcus sp. genus, with a good identification by using the commercial biochemical test system, in spite of their own limits. Rautemaa et al.8 evaluated samples taken from personnel facial masks and surfaces in dental rooms where treatments were performed using high-speed rotating instruments, and registered Gram positive-cocci namely viridans streptococci (Streptococcus genus) and Staphylococcus, as the most common bacterial findings. Streptococcus is the main cause of bacterial endocarditis in compromised patients, and the normal habitat is the human upper respiratory tract and skin.7 Similarly, Staphylococcus is a normal commensal on the skin surface and anterior nares which has the characteristic of an opportunist pathogen and may cause catheter related sepsis and infection of artificial joints. Micrococcus are frequently present on normal skin.7,26 Despite the importance of air microbial level evaluation as a step toward cross infection prevention, the design studies variability limits the conclusions and difficult the comparison between published results regarding the quantity (acceptability levels of CFU) and quality (microbial identification) assessment of microbial contamination in dental aerosols. In the present study given the reduced dimension of the sample size, the presented statistical values are merely indicative, even though several variables were analyzed and the records obtained tend to suggest that dentistry procedures promote a risk of infection if the extent and nature of microbial aerosols created by high-speed handpieces are underestimated.27 A huge number of bacteria are frequently isolated from air in dental offices as well as from dental unit waterlines.9 Further studies with simultaneous qualitative and quantitative counts related with usual dental treatments would be of the utmost importance.
The air quality of the UFP pedagogical dental clinic regarding bacterial counts was found to be good, but in dental clinic environments microbial transmission can occur by air, so preventive measures should be promoted to avoid pathogenic microorganisms spread.
ConclusionsCFU counts in aerosols are influenced by the dentistry procedures (restorative dentistry and endodontic), the operative site distance and the treatment times performed. Qualitative and quantitative composition of dental aerosols probably varies with each patient and operative site. Dental aerosols produced during dentistry procedures should be controlled, to the greatest extent possible, to a health reassurance of patients and dental team in clinical work environments.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Author M.C. Manso acknowledge Fundação para a Ciência e a Tecnologia through grant PEst-C/EQB/LA0006/2011.