To evaluate the shear bond strength (SBS) of orthodontic brackets bonded to enamel with four adhesive systems tested at three time periods.
MethodsA 180 cross sections of human premolars were randomly assigned into four groups according to the adhesive system used: Concise™ (G1), Transbond™ XT (G2), Transbond™ Plus Self-Etching Primer (TBS) (G3) and Heliosit®Orthodontic (G4). SBS was tested by producing bracket debonding after 15min of bonding, after 24h and after 24h plus 500 cycles of thermocycling (TC). Bond failure was determined with the modified adhesive remnant index (ARI) and composite resin cements, conditioned enamel surfaces and adhesive interfaces were observed by scanning electron microscopy (SEM).
ResultsTwo-way ANOVA determined no interaction between time or time and TC on the behavior of the adhesive systems (F=0.372, p=0.896). Post-bonding time induced a statistically significant increase in SBS (F=37.447, p<0.01), whereas thermocycling did not influence SBS (t=0.608, p=0.544). Adhesive systems were only different at 15min (F=4.75, p=0.005). ARI scores revealed differences between groups when the test was performed after 24h and after 24h+TC. Groups 1, 3 and 4 showed differences along testing periods. SEM observations revealed that TBS produced a more irregular, shallow structure with less defined indentations of enamel prisms than phosphoric acid.
ConclusionsRegardless of the adhesive system, SBS were significantly higher at 24h after bracket bonding procedure than after 15min. The self-etching primer tested can successfully be used for bracket bonding. The thermocycling protocol did not affect shear bond strengths.
Comparar as forças de adesão (FA) de quatro sistemas adesivos ortodônticos em três períodos de tempo.
MétodosCento e oitenta faces de pré-molares humanos foram distribuídas aleatoriamente em quatro grupos de acordo com os sistemas adesivos testados: Concise™ (G1), Transbond™ XT (G2), Transbond™ Plus Self-Etching Primer (TBS) (G3) and Heliosit®Orthodontic (G4). As FA foram determinadas em três períodos de tempo 15min; 24 horas e 24 horas seguida de termociclagem (TC). O tipo de fratura foi determinado com o índice de adesivo remanescente (IAR). As resinas compostas, os padrões de condicionamento e as interfaces adesivas foram observadas sob microscopia electrónica de varrimento (MEV).
ResultadosA ANOVA a 2 fatores não determinou interação entre o tempo ou o tempo e TC no comportamento dos sistemas adesivos (F=0.372, p=0.896). O tempo induziu um aumento estatisticamente significativo nas FA (F=37.447, p<0.01), enquanto que a termociclagem não influenciou as FA (t=0.608, p=0.544). Os sistemas adesivos apresentaram diferenças apenas no período de 15min (F=4,75; p=0,005). O IAR revelou diferenças significativas entre os grupos nos períodos de 24h e 24h+TC. Os grupos 1, 3 e 4 mostraram diferenças ao longo dos períodos de teste. As observações em MEV revelaram que o TBS produziu um padrão de condicionamento mais irregular e superficial relativamente ao ácido fosfórico.
ConclusõesIndependentemente do sistema adesivo, as FA foram significativamente superiores 24 horas após a colagem dos brackets relativamente aos 15min. O adesivo autocondicionante pode ser utilizado na colagem de brackets. A termociclagem não afetou as forças de adesão.
The introduction of the acid etch bonding technique by Buonocore in 19551 was particularly important for bracket bonding in bandless orthodontic treatments as it improved the micromechanical retention of the enamel surface needed to bond resins. Bracket bonding failure during orthodontic treatment is a relatively common problem. This feature may be related to various factors, including operator technique and skills, patient behavior, enamel morphology, and adhesive material properties.
Bond strength to enamel should withstand occlusion forces and stresses exerted by the archwires for tooth movement control in all three planes of space and, simultaneously, make possible the final bracket debonding without damaging the enamel surface.2,3 For orthodontic treatments, clinical bonding was considered to be successful when shear bond strengths vary between 5.9 and 7.8MPa.4 However, the maximum bond strength should be inferior to the tensile strength of enamel, which ranges between 11 and 25MPa, depending on the prismatic orientation.5In vivo bond strengths have been shown to be significantly lower than the in vitro ones, suggesting that the possibility of enamel damage might be lower under clinical conditions.2,6,7
Phosphoric acid solution remains the most widely used enamel conditioner among orthodontists as it promotes the most retentive etching pattern to enamel. Nevertheless, this routine etching technique has been described as a sensitive procedure due to the need of proper moisture control8 and to the potential mechanical damage to the enamel surface in the course of the debonding procedures.9–11 To simplify orthodontic bonding, self-etching primer adhesives (SEPs) were introduced, combining the etching and priming steps into one and eliminating the rinsing phase. Furthermore, it has been reported that, as SEPs produce more conservative etching patterns and reduce adhesive penetration, they potentially minimize the amount of enamel loss.11
Numerous in vitro studies were published revealing contradictory results concerning the effectiveness of the SEPs.3,12–21 In most cases, shear bond strengths are assessed exclusively at 24h after the bonding procedure, which does not reflect the most frequent daily clinical practice. On the one hand, the initial bond strength is of the utmost importance as archwires are inserted into the brackets slot 10–15min after the bonding procedure; on the other hand, routine exposure of the adhesive interfaces of brackets to chemical, mechanical and thermal changes occurring in the oral cavity induces stress capable of affecting the bond effectiveness.16,19,20,22
The aim of the present study was to evaluate shear bond strength of four orthodontic adhesive systems at three time point periods and examine the bracket/tooth failure interface. Following this, the null hypotheses formulated were:
- (1)
There is no difference in the behavior of the adhesive systems across the three testing setups.
- (2)
Within each setup there are no differences in shear bond strength between the four adhesive systems.
Ninety intact and caries free extracted human pre-molars were collected and stored in a solution of 0.5% chloramine T for up to 6 weeks after extraction. The crowns were split into two halves by cross-sectioning the tooth along the mesio-distal axis, using a Model 660 precision saw (South Bay Technology, Inc.; San Clemente, CA, USA), so that both lingual and buccal surfaces could be used, making 180 free surfaces for bracket bonding. The roots were partially cut-off and retentive notches were placed in the internal surface of the remaining structure. Each specimen was embedded in a self-curing acrylic resin (Orthocryl®, Dentaurum, Ispringen, Germany) using phenolic rings with the facial or lingual surface projecting above the rim of the ring. The teeth were cleaned and polished with nonfluoride oil-free pumice paste using a prophy-cup attached to a slow-speed hand piece for 10s. The teeth were rinsed and dried with oil-free compressed air.
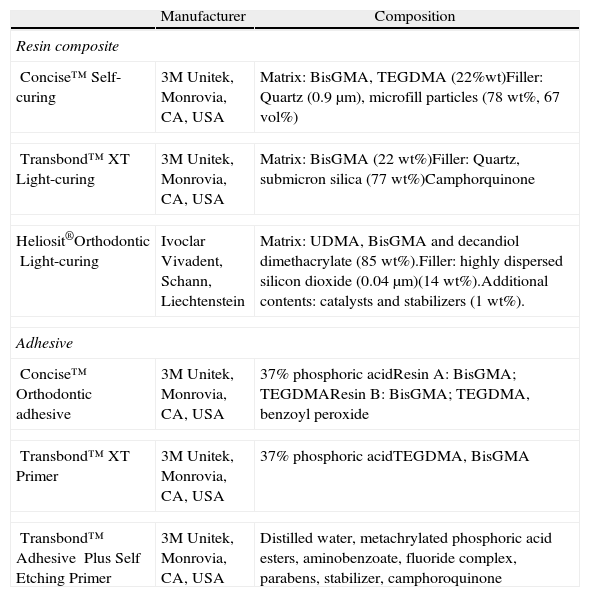
The 180 samples were randomly allocated into four groups and 12 subgroups according to the adhesive system to be tested and the time period/aging of testing (n=15) (Tables 1 and 2).
Materials studied.
| Manufacturer | Composition | |
| Resin composite | ||
| Concise™Self-curing | 3M Unitek, Monrovia, CA, USA | Matrix: BisGMA, TEGDMA (22%wt)Filler: Quartz (0.9μm), microfill particles (78wt%, 67vol%) |
| Transbond™ XTLight-curing | 3M Unitek, Monrovia, CA, USA | Matrix: BisGMA (22wt%)Filler: Quartz, submicron silica (77wt%)Camphorquinone |
| Heliosit®OrthodonticLight-curing | Ivoclar Vivadent, Schann, Liechtenstein | Matrix: UDMA, BisGMA and decandiol dimethacrylate (85wt%).Filler: highly dispersed silicon dioxide (0.04μm)(14wt%).Additional contents: catalysts and stabilizers (1wt%). |
| Adhesive | ||
| Concise™Orthodontic adhesive | 3M Unitek, Monrovia, CA, USA | 37% phosphoric acidResin A: BisGMA; TEGDMAResin B: BisGMA; TEGDMA, benzoyl peroxide |
| Transbond™XT Primer | 3M Unitek, Monrovia, CA, USA | 37% phosphoric acidTEGDMA, BisGMA |
| Transbond™ Adhesive Plus Self Etching Primer | 3M Unitek, Monrovia, CA, USA | Distilled water, metachrylated phosphoric acid esters, aminobenzoate, fluoride complex, parabens, stabilizer, camphoroquinone |
BisGMA: bisphenol A-glycidyl methacrylate; TEGDMA: triethylene glycol dimethacrylate; UDMA: urethane dimethacrylate.
Orthodontic pre-molar metal brackets (Victory Series, 3M Unitek Co., Monrovia, CA, USA) were used in this study. The average bracket base surface area was determined to be 12.2mm2. One experimented operator bonded all the brackets to the enamel surfaces along the axis of the crown according to the manufacturer instructions and following one of four adhesive protocols tested.
Group 1: Bonding with Concise™ (C): enamel was etched with 37% phosphoric acid gel for 30s. The surface was thoroughly washed and dried. One drop of resin A and B of Concise™ were then mixed and coated in a thin layer on the etched surface. Immediately, equal amounts of Concise™ paste A and B were mixed vigorously, applied over the bracket base, which was placed on the teeth.
Group 2: Bonding with Transbond™ XT (TXT): enamel was etched with 37% phosphoric acid gel for 30s. The teeth were thoroughly washed and dried. The TXT adhesive was applied to the etched surface and the composite Transbond™ XT was then applied into the bracket base, placed on the teeth and light cured for 20s.
Group 3: Bonding with Transbond™ Plus Self-Etching Primer (TBS): the self-etch primer was rubbed into enamel for 3s and dried with a gentle airburst. The composite Transbond™ XT was applied into the bracket base, placed on the teeth and light cured for 20s.
Group 4: Bonding with Heliosit®Orthodontic (HO): enamel was etched with 37% phosphoric acid gel for 30s. The teeth were thoroughly washed and dried. The composite Heliosit®Orthodontic was then applied into the bracket base, placed on the teeth and light cured for 40s.
In all groups excess composite material was removed with an explorer without disturbing bracket placement. Also, all light-curing procedures were performed with a halogen unit (Demetron Optilux 501, Kerr, Orange, CA, USA) operating in a continuous mode while emitting a light intensity around 800mW/cm2.
For each group, adhesive strength was assessed by the analysis of debonding in three different periods: 15min after bracket fixation; 24h after bracket fixation and storage in distilled water; and after 24h of storage in distilled water followed by a thermocycling (TC) regimen comprising 500 cycles in water with temperatures ranging from 5 to 55°C, according to the International Organization for Standardization (ISO) TR11450 standard (1994).
All samples were mounted in a universal testing machine (Model AG-I, Shimadzu Corporation, Kyoto, Japan) with the enamel surface parallel to the shearing rod. A shear force was applied to the bracket by lowering the shearing rod in an occlusogingival direction at a crosshead speed of 1mm/min. The shear bond strength was determined in Megapascal (MPa).
After debonding, brackets and enamel surfaces were examined under a stereomicroscope (Nikon® SMZ 1500, Tokyo, Japan) at 20× magnification to assess the fracture pattern. The enamel surfaces were scored from 1 to 5 according to the modified adhesive remnant index (ARI)23: Score 1 represented all adhesive left on the tooth surface, with a distinct impression of the bracket base; Score 2 represented more than 90% of the adhesive left on the tooth surface; Score 3 represented more than 10%, but less than 90% of adhesive left on the tooth; Score 4 represented less than 10% of the adhesive left on the tooth surface; Score 5 represented no adhesive left on the tooth surface.
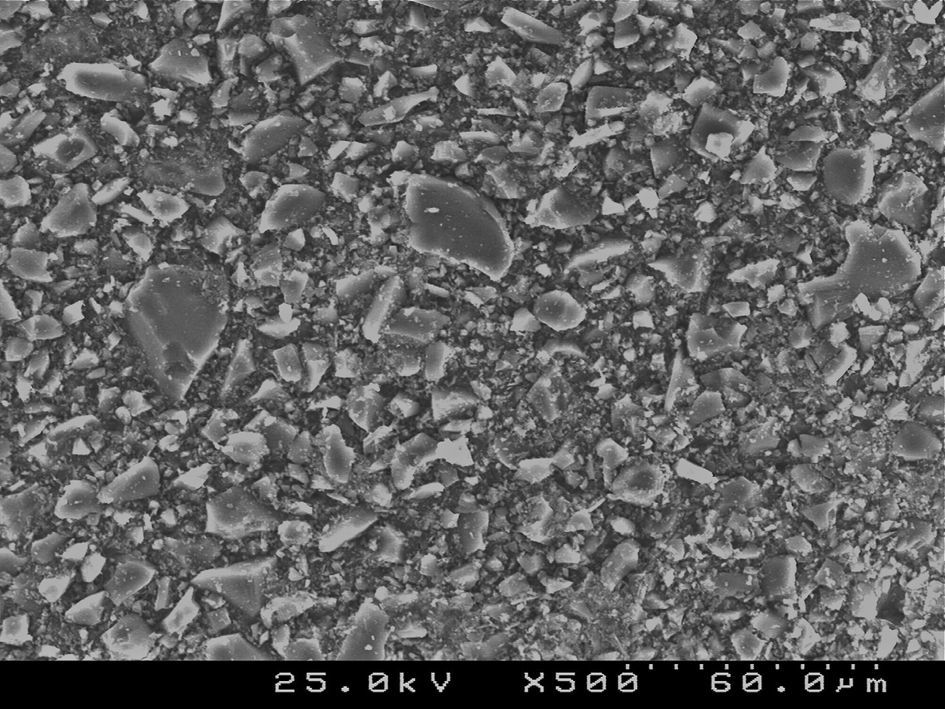
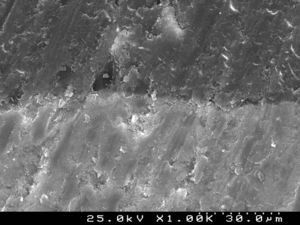
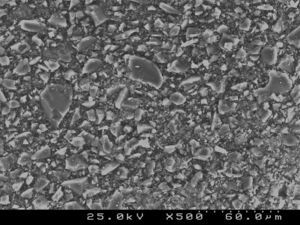
Samples of enamel/resin/bracket interfaces of each group and enamel etching patterns obtained by the self-etching primer versus 37% phosphoric acid were made for scanning electron microscope (SEM) evaluation. For bracket–resin interfaces evaluation, two samples of each group were made following the same protocol and cross-sectioned between the bracket wings in an occluso-cervical direction with a precision diamond saw (Exakt System®, Hamburg, Germany), polished and sonicated in absolute ethanol for 4min for dehydration. For etching pattern evaluation buccal enamel surfaces were conditioned with the self-etching primer (Transbond™Plus Self Etching Primer) according to manufacturer's instructions, but the resin was immediately rinsed off in order to examine the etching effect left in enamel. The etched enamel surface was rinsed with an ascending series of ethanol (30, 50, 70, 95%) for 1min each and further sonicated in absolute ethanol for 1min to dissolve the self-etching liquid, primer or adhesive, as well as dehydrating the specimens for SEM observation. For the 37% phosphoric acid samples, after conditioning buccal enamel surfaces for 30s, the acid was rinsed off with distilled water for 20s and then dehydrated in a similar manner. Additionally, some samples of the composite resin cements tested were prepared for SEM evaluation of their inorganic filler. All three sets of samples were sputter-coated with gold-palladium (Polaron E-5000 Sputter-Coater, Polaron Equipment Ltd, Watford, UK) before SEM analysis with a Hitachi S-4100 microscope (Hitachi, Tokyo, Japan). The samples were observed with an accelerating voltage of 20–25kV, at ×500, ×1500 and ×2000 magnifications for enamel conditioning patterns evaluation,24 at ×180 and ×1000 for adhesive interfaces and at ×500 and ×1500 for composite resin cements filler evaluation.
Statistical analysis was performed using SPSS® 12.0 (Statistical Package for the Social Sciences, SPSS Inc, Chicago, USA). Descriptive statistics of shear bond strengths were calculated for all groups. Two-way analysis of variance (ANOVA) was used to determine the effect of time or time + TC and the adhesive systems on shear bond strength, followed by one-way analysis of variance for each period and each adhesive system considering Tukey adjustment for post hoc multiple comparisons. Differences in ARI scores distribution between groups and along time were analyzed with Kruskal–Wallis non-parametric test, followed by Mann–Whitney test for pairwise comparisons. Significance level was set to α=0.05.
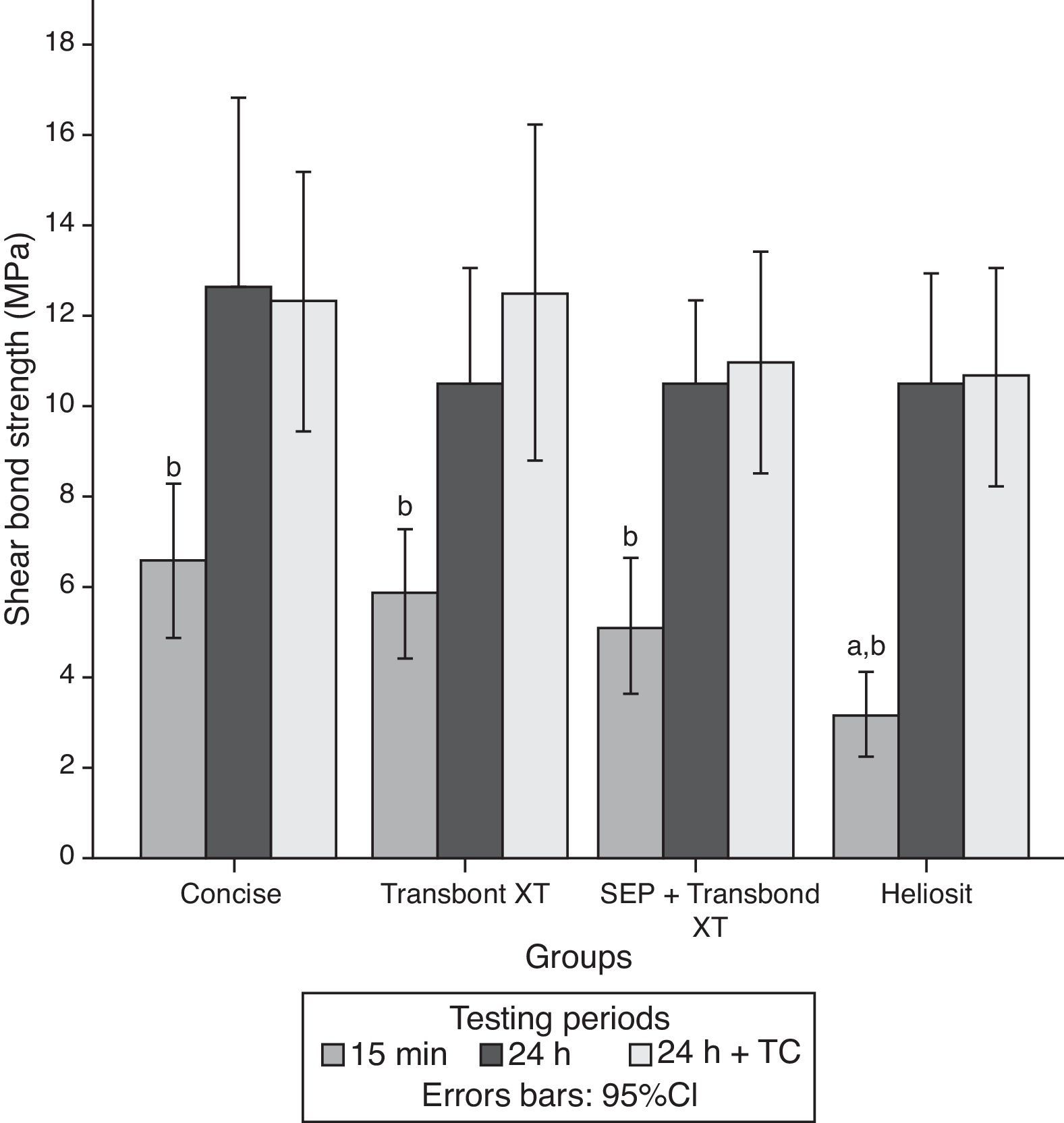
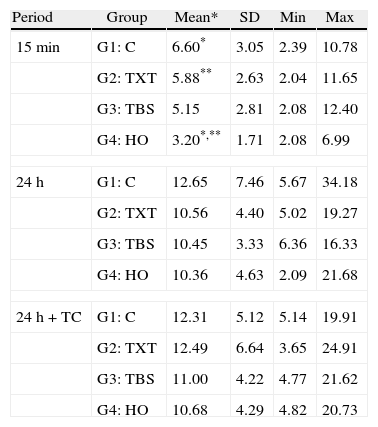
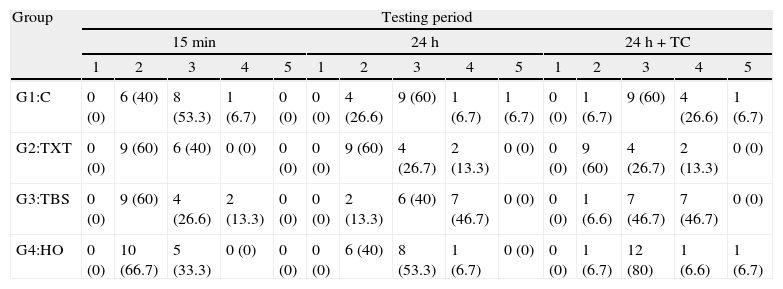
ResultsThe descriptive statistics for the shear bond strength are presented in Table 3 and Fig. 1. No interactions were detected between time or time+TC on the behavior of the adhesive systems, as stated by the two-way ANOVA: F(6, 168)=0.372, p=0.896. Thus, the first null hypothesis could not be rejected. Debonding time induced significant variations in SBS regardless of the adhesive system F(2, 168)=37.447, p<0.01. However, thermocycling was not responsible for a statistically significant variation between the samples with and without that procedure, inducing only a mean difference of 0.56MPa (95% CI, −1.27 to 2.40), t(118)=0.608, p=0.544 in SBS.
Descriptive statistics of SBS (in MPa) of the four groups according to the established testing periods.
| Period | Group | Mean* | SD | Min | Max |
| 15min | G1: C | 6.60* | 3.05 | 2.39 | 10.78 |
| G2: TXT | 5.88** | 2.63 | 2.04 | 11.65 | |
| G3: TBS | 5.15 | 2.81 | 2.08 | 12.40 | |
| G4: HO | 3.20*,** | 1.71 | 2.08 | 6.99 | |
| 24h | G1: C | 12.65 | 7.46 | 5.67 | 34.18 |
| G2: TXT | 10.56 | 4.40 | 5.02 | 19.27 | |
| G3: TBS | 10.45 | 3.33 | 6.36 | 16.33 | |
| G4: HO | 10.36 | 4.63 | 2.09 | 21.68 | |
| 24h+TC | G1: C | 12.31 | 5.12 | 5.14 | 19.91 |
| G2: TXT | 12.49 | 6.64 | 3.65 | 24.91 | |
| G3: TBS | 11.00 | 4.22 | 4.77 | 21.62 | |
| G4: HO | 10.68 | 4.29 | 4.82 | 20.73 | |
15min: F=4.75; p≤0.005; 24H: F=0.67; p≤0.57; 24h+TC: F=0.46; p≤0.71; *p=0.004; **p=0.033; ANOVA (one-way) followed by Tukey HSD post hoc test.
Within each adhesive, statistically significant differences were found between the three periods of debonding: G1 (F(2)=5.69, p=0.007); G2 (F=7.38, p=0.002); G3 (F=12.76, p<0.001); G4 (F=18.89, p<0.001). Post hoc analysis showed that for all groups, shear bond strengths were significantly lower when debonding was performed 15min after bracket bonding compared to 24h or 24h+TC periods (Fig. 1).
When comparing different adhesive systems at a given time, the results of the analysis of variance indicated significant differences only when debonding was performed within 15min after bonding the bracket to the tooth surface (F=4.75, p=0.005). At this time, HO (3.20±1.71MPa) showed statistically lower SBS than C (6.60±3.05MPa) and TXT (5.88±2.63MPa). Accordingly, the second null hypothesis only could be partially rejected.
Concerning ARI scores, no samples were assigned to score 1 and only three fell into the score 5 (Table 4). Statistically significant differences were found between the four groups when the test was performed after 24h (χ2(3)=9.92; p=0.019) and after 24h+TC (χ2(3)=13.22; p=0.004). Groups 1, 3 and 4 showed statistically significant differences in the ARI score distribution along the three testing periods (C: χ2(2)=6.50; p=0.039; TBS: χ2(2)=10.72; p=0.005 and HO: χ2(2)=11.84; p=0.003). At 15min all groups showed a similar distribution between scores 2 and 3. Twenty-four hour storage and thermocycling determined a more mixed failure mode among groups. Nevertheless, only the TBS group showed equal distribution of cohesive failures within the composite and adhesive failures at the enamel/composite interface at the 24h+TC period.
Absolute distribution frequency and percentages (between brackets) of the adhesive remnant index (ARI). Results for Kruskal–Wallis test for a significance level of 5%.
| Group | Testing period | ||||||||||||||
| 15min | 24h | 24h+TC | |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| G1:C | 0 (0) | 6 (40) | 8 (53.3) | 1 (6.7) | 0 (0) | 0 (0) | 4 (26.6) | 9 (60) | 1 (6.7) | 1 (6.7) | 0 (0) | 1 (6.7) | 9 (60) | 4 (26.6) | 1 (6.7) |
| G2:TXT | 0 (0) | 9 (60) | 6 (40) | 0 (0) | 0 (0) | 0 (0) | 9 (60) | 4 (26.7) | 2 (13.3) | 0 (0) | 0 (0) | 9 (60) | 4 (26.7) | 2 (13.3) | 0 (0) |
| G3:TBS | 0 (0) | 9 (60) | 4 (26.6) | 2 (13.3) | 0 (0) | 0 (0) | 2 (13.3) | 6 (40) | 7 (46.7) | 0 (0) | 0 (0) | 1 (6.6) | 7 (46.7) | 7 (46.7) | 0 (0) |
| G4:HO | 0 (0) | 10 (66.7) | 5 (33.3) | 0 (0) | 0 (0) | 0 (0) | 6 (40) | 8 (53.3) | 1 (6.7) | 0 (0) | 0 (0) | 1 (6.7) | 12 (80) | 1 (6.6) | 1 (6.7) |
15min: χ2(3)=2.60; p=0.466; 24H: χ2(3)=9.92; p=0.019; 24h+TC: χ2(3)=13.22; p=0.004; Concise: χ2(2)=6.50; p=0.039; TXT: χ2(2)=0.11; p=0.946; TBS: χ2(2)=10.72; p=0.005; HO: χ2(2)=11.84; p=0.003.
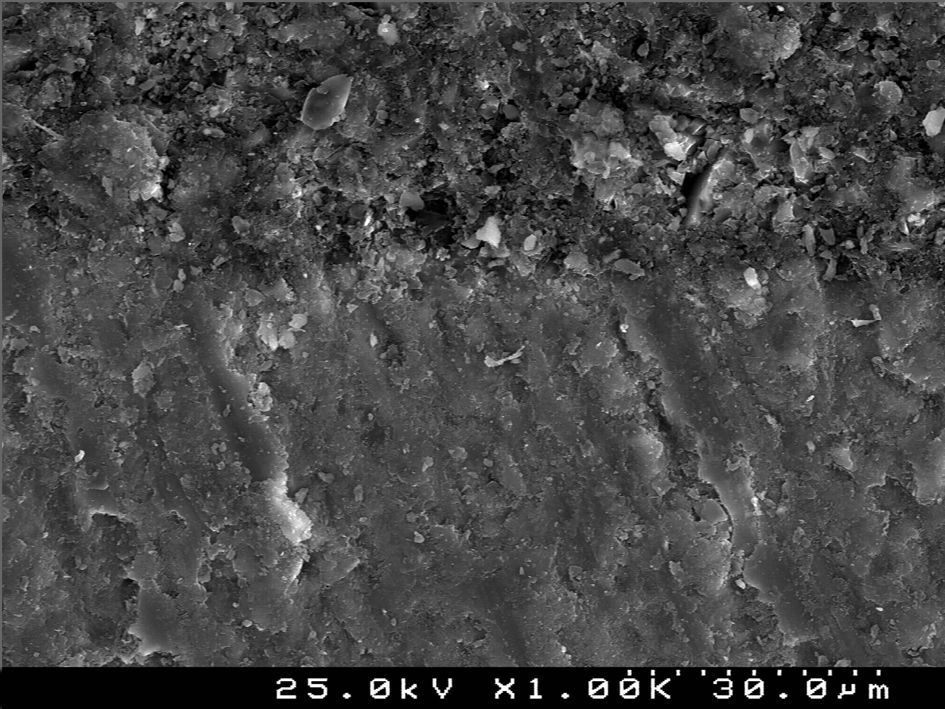
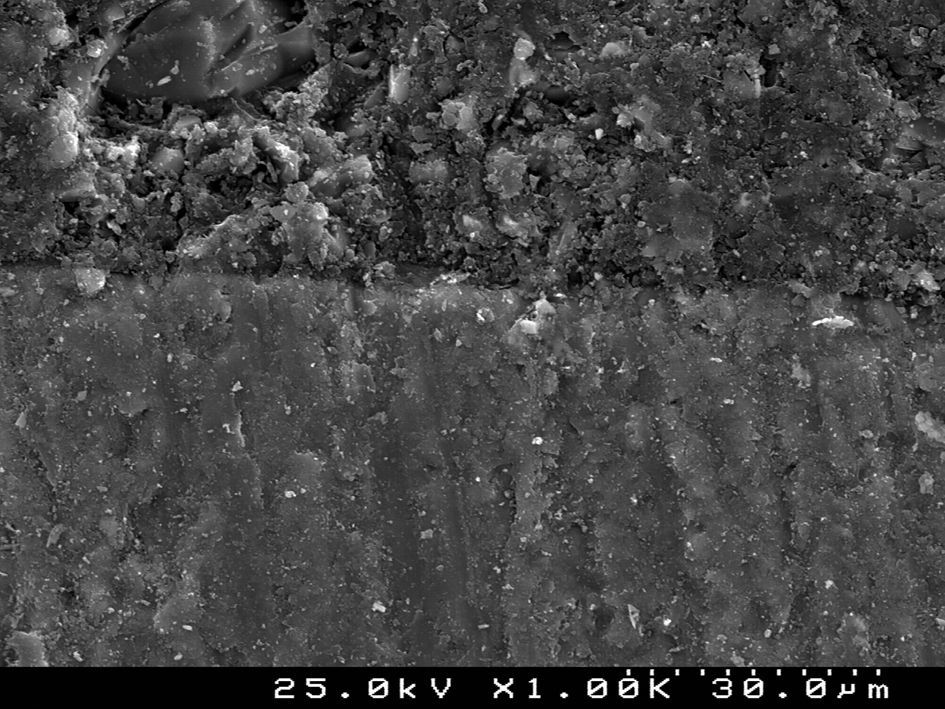
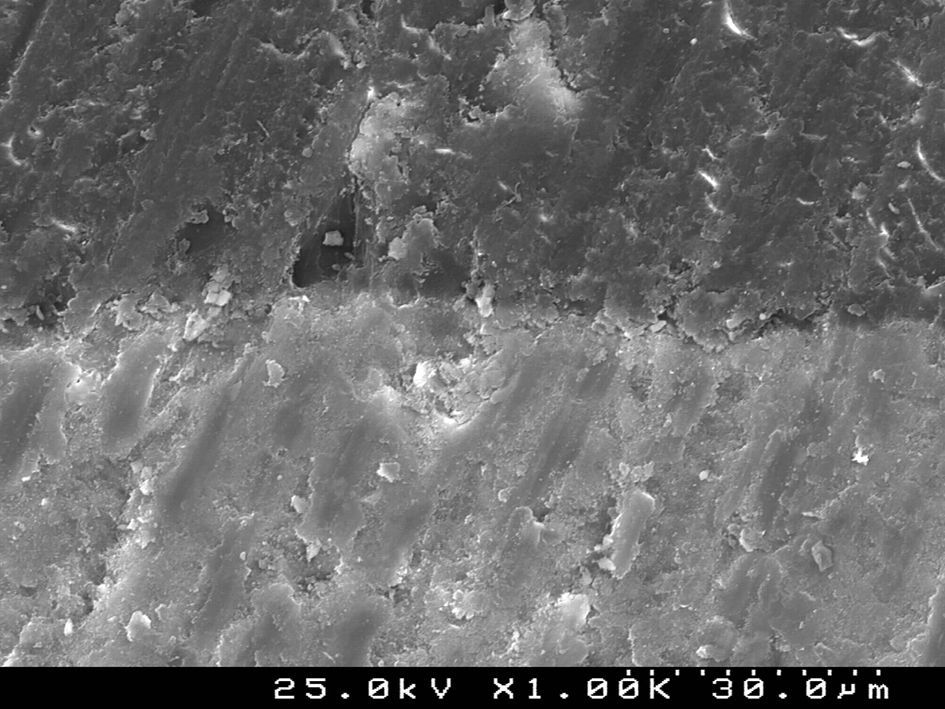
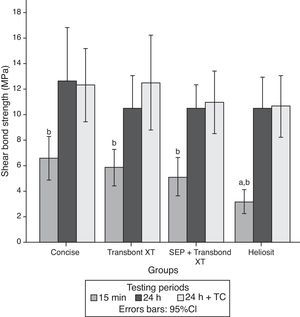
SEM examination revealed no marked distinct features in the enamel resin interface of the cross-sections samples for all the adhesive systems studied (Figs. 2–5). Concerning particle size, shape and distribution of inorganic filler, significant differences could be noticed between materials (Figs. 6–8). Concise™ and Transbond™ XT are well matched with macrofill composite resins, presenting a great variety of filler particle sizes, including large particles larger than 10μm. Contrariwise, HO resembles a microfill composite resin since particles are hardly noticed. Regarding enamel surface conditioning, SEM analysis revealed substantial differences between the etching pattern of the samples treated with 37% phosphoric acid, mainly characterized as type I (prismatic, “honeycomb/coral” structure) or type II (interprismatic) patterns, and the samples treated with TSE, which revealed a more irregular, shallow structure with less defined indentations of enamel prisms (Figs. 9 and 10).
Our study aimed at evaluating of shear bond strengths of four orthodontic adhesive systems applied over the enamel surfaces of premolars and tested at three different periods. Even though the adhesive systems used have been previously studied, the variations in bond strengths values found in the literature could be due to differences related to the operator or study methodology.25,26 In theory, the contour difference of lingual and buccal surfaces of premolars could affect bracket bond strength results. However, some studies report no significant differences in bond strength between surfaces, thus supporting the use of both surfaces for bracket bonding tests.27,28
The mean bond strengths of adhesive systems in the initial 15min were slightly below the limits suggested by Reynolds,4 yet in agreement with the findings of Bishara et al.29 Among all systems tested for this period, Concise™ exhibited the best result of shear bond strength. Although not significantly different from Transbond™ XT used with either one of the etching processes, the shear bond strength of the former was significantly higher than Heliosit® Orthodontic, which revealed the lowest mean bond strength value. These results could be explained by some differences in resin composition and filler content between the materials. Bis-GMA highly filled diacrylate resins have been reported as the strongest bonding adhesives for metal brackets.30,31 Besides, composite resins like Concise and Transbond™ XT contain large, coarse quartz or silica glass particles of highly variable size, ranging from 3 to 20μm, that improve some mechanical properties and light transmission. Conversely, Heliosit® Orthodontic contains a small fraction of submicron filler particles, highly dispersed silica, with an average size of only 0.04μm which may account for the inferior performance regarding some mechanical properties, high polymerization shrinkage and less degree of cure.31–33
Light curing under metallic brackets occurs by transillumination as the tooth structure transmits light.34,35 Still, light curing materials are unable to reach a complete degree of cure36 which can be potentially diminished by the presence of structures that reduces the intensity of the emission light source.37,38 Additionally, incomplete polymerization due to insufficient exposure time may result in reduced bond strengths.39 The dependence of several external factors on the polymerization kinetics of light-cured resin composites might affect their degree of cure, particularly in the early stages of bonding. Nevertheless, a high degree of monomer conversion is important to ensure maximum polymerization and adequate bond strengths to sustain early orthodontic forces, which might be better achieved within the first 15min of bonding by self-cured resins.40 Besides, depth of cure is directly related to filler particle size in dental composite resins.41,42 Light scattering within the composite is increased as the particle size of the fillers approaches the wavelength of the activating light and reduces the amount of light that is transmitted through the composite. Larger particle composites are less affected by light scattering thus presenting greater depths of cure.32 All those facts could explain the different mean bond strengths values within the first 15min between the high-filled self-cured and light-cured composite resins, where C, TXT and TBS gave best results than HO, even with a 40s light exposure for this last material. Furthermore, HO does not use a previous adhesive procedure before the composite resin application, which may also contributed for the lowest shear bond strength values obtained, as stated by Bishara.43
Some studies refer bond strengths of light-cured resins lower than those achieved by self-cured resins.44,45 Other studies have shown a reverse trend, with light activated materials giving stronger bond strengths.17,46–49 Most of those studies make specimen testing only after 24h storage. Effectively, at this period we found no differences between adhesive systems tested, which is in accordance with the results obtained by other authors.14,18,50,51 The 24h mean shear bond strengths values duplicated from those obtained at 15min for all adhesive systems, which is also in agreement with other studies.18,29,45 An increase of this magnitude could be explained by the continuous polymerization of the materials beyond the initial 15min irradiation period which is supported by Greenlaw et al.,46 who suggested that there is an initial production of free radicals at the periphery of the resin, where total light exposure is achieved, and internal diffusion of these free radicals along time. This allows the polymerization of the resin under the bracket base, which results in the increase of bond strengths.
In the present study thermocycling did not seem to negatively influence bond strength values, that were not significantly different from those obtained with debonding at 24h for all materials tested. Although the used thermocycling protocol is in accordance with ISO recommendations, some studies indicate that 500 cycles of thermocycling are probably insufficient to simulate the aging effect that occurs during long-term orthodontic treatment and might not affect bonding strengths.52,53 It is possible that some adhesive systems are sparsely affected by hydrolysis at the enamel interfaces. Some studies found statistically significant differences only for extended periods of thermocycling.16,50 However, Yuasa et al.20 report adequate shear bond strengths even after long term water storage and thermocycling.
In the present study SEM observations revealed more irregular and less noticeable enamel conditioning by TBS than with phosphoric acid. Though, Buyukyilmaz et al. examined by SEM the impressions of the enamel treated with both phosphoric acid and TBS and found higher bond strengths obtained with TBS, despite the lack of tag formation.21 Mechanical interlocking of the cured resin that is formed on the roughened enamel surface has been assumed as the main contributor to the enamel bond strength of orthodontic brackets bonded with composite resin adhesives.54 Nevertheless, Shinchi et al.55 were incapable to find a correlation between enamel bond strength and tag length and attributed the adhesive strength to the ability of the resin to penetrate between the enamel crystallites and rods. This could partially explain the similar bond strengths achieved by TBS when compared to the phosphoric acid groups.
Concerning failure patterns, all light-curing adhesives of the 15min testing groups had similar behavior at the site of bond failure, as the most frequent adhesive failures occurred at bracket/composite interface, which is in accordance with the results of Turk et al.18 The inability of the visible light to adequately cure the resin just behind the bracket mesh could account for these results.46 In opposition, the self-curing resin exhibited a mixed failure mode and cohesive failures within the composite resin occurred more often. One possible explanation for this finding is the degree of monomer conversion achieved by this resin within the first 15min, resulting in a more homogeneous polymerization and, consequently, initial stronger bond strengths.40
At the debonding period of 24h after water storage mixed failure patterns were observed for all materials, except for TXT in combination with conventional etching technique, which exhibited mainly the same pattern of adhesive failures at bracket/resin interface. Comparing to TBS, this aspect could be partially explained by the less dissolution of enamel surfaces found with self-etching primers.17,18 The greatest amount of organic matrix of HO is responsible for reduced resin cohesive strength, which could account for the mixed failure pattern registered.
At the last period, 24h plus thermocycling, a significant difference in score distribution was noticed between the adhesives. The TBS group showed an equal distribution of cohesive failures within the composite and adhesive failures at the enamel/composite interface. The more irregular, superficial and shallow etching patterns obtained by this adhesive could explain these findings. Clinically, this adhesive failure at the enamel resin interface is most desirable for final debonding and polishing at the end of the orthodontic treatment.20,23 Earlier studies have already demonstrated the potential of self-etching adhesives for bracket bonding to enamel while providing easy resin removal after treatment without damaging the enamel surface.12–15,17,18,20,21,50 More, non-rinsing conditioners reduce the number of steps during bonding procedures, minimizing the probability of contamination. This also corroborates clinical studies evaluating the retention of brackets to enamel bonded with either an etch and rinse or a self-etch system that did not shown higher retention rates for the former.56,57
Regardless the promising results presented in this study, care should be taken in the interpretation of the results, as well as applying into clinical situations as in vitro bond strengths are usually higher than those obtained in vivo.2,6,7
ConclusionsSelection of orthodontic adhesive system may play an important role, mainly in the initial period, at 15min after bonding, when flexible archwires are ligated. The present findings indicated that, regardless of the adhesive system, the shear bond strengths were significantly stronger at 24h after bracket bonding procedure.
The self-etching primer adhesive tested can successfully be used for bracket bonding with the potential advantage of minimizing enamel loss determined by a more limited etching depth.
The thermocycling protocol used did not affect shear bond strengths.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to express their gratitude to 3M Unitek and Ivoclar Vivadent for providing the materials used in this study.