To evaluate the effect of acetylcholinesterase on the cytotoxicity of three autopolymerizing acrylic reline resins through the effect of the materials’ eluates, liquids and respective pure compounds on the cellular viability of primary dermal fibroblasts cultures.
MethodsDisk shaped specimens of two direct Acrylic Reline Reins (ARR), Kooliner and Ufi Gel Hard, and one indirect ARR, Probase Cold, were studied. Cytotoxicity was studied through spectrophotometric determination of tetrazolium reduction (MTT assay) and lactate dehydrogenase activity (LDH assay). Moreover, at least 7 concentrations of each liquid and compound were prepared to determine the IC50 parameter. All data were evaluated using Kruskall–Wallis or Mann–Whitney test, after verification with Kolmogorov–Smirnov test.
ResultsThe fibroblasts exposed to the direct ARR eluates resulted in inhibition of the mitochondrial activity. Probase Cold eluates did not diminish cellular viability. LDH remained unaltered when fibroblasts were exposed to the eluates. Acetylcholinesterase groups of direct reline resins showed to be less cytotoxic when compared with control groups without changing their cytotoxic potential. The non-cytotoxic effect of Probase Cold did not change. The cytotoxicity of the pure compounds increased in the following order: Methacrylic Acid (MA), Isobutyl Methacrylate (IBMA) and Hexanediol Dimethacrylate (HDMA). Methyl Methacrylate (MMA) showed no cytotoxicity at the concentrations used. The direct reline resins liquids and respective pure compounds exhibited similar behavior.
ConclusionsAcetylcholinesterase did not change the cytotoxic potential of the reline resins studied. HDMA and IBMA revealed higher levels of cytotoxicity than MA, and their behavior was similar to the respective liquids.
Avaliar o efeito da acetilcolinesterase na citotoxicidade de três resinas acrílicas de rebasamento autopolimerizáveis (RRA), através do efeito dos extractos totais dos materiais, dos líquidos e dos respetivos monómeros puros na viabilidade de culturas primárias de fibroblastos.
MétodosForam avaliadas duas RRA diretas, Kooliner e Ufi Gel Hard, e uma resina de rebasamento indirecto, Probase Cold. A citotoxicidade foi determinada através de ensaios espectrofotométricos da redução do brometo de tetrazólio (MTT) e da atividade da enzima lactato desidrogenase (LDH), em culturas primárias de fibroblastos. Adicionalmente, foram preparadas, pelo menos, 7 concentrações de cada monómero e líquido, para determinar o parâmetro IC50. Os dados foram analisados por meio do teste Kruskall–Wallis ou Mann–Whitney, após verificação com teste de Kolmogorov–Smirnov.
ResultadosA exposição dos fibroblastos aos extratos das RRA diretas resultou na inibição da atividade mitocondrial, enquanto o Probase Cold não provocou diminuição da viabilidade celular. A atividade da LDH não sofreu alterações quando exposta aos extratos. Os grupos com acetilcolinesterase das RRA directas revelaram-se menos tóxicos, quando comparados com os grupos controlo, sem alterar o seu potencial citotóxico. A citotoxicidade dos monómeros puros aumentou na seguinte ordem: ácido metacrílico (MA), isobutilmetacrilato (IBMA) e hexanodioldimetacrilato (HDMA). Os líquidos das resinas de rebasamento direto demonstraram uma curva de citotoxicidade semelhante aos respetivos monómeros.
ConclusõesA enzima acetilcolinesterase não alterou o potencial citotóxico dos materiais estudados. O HDMA e IBMA demonstraram maiores níveis de citotoxicidade que o ácido Metacrílico, e o seu comportamento foi semelhante ao líquido das respetivas resinas.
The use of autopolymerizing acrylic reline resins (ARR) has recently gained popularity in dentures readjustment to the continuous reabsorbed underlying tissues, providing better retention and stability for complete removable prostheses.1,2
However, these materials have been associated with in vitro toxicity and also, in vivo manifestations such as chemical irritation, allergic reactions,1,3 erythema, erosion of oral mucosa and burning mouth sensation.4 These adverse reactions caused by denture base polymers have been attributed to substances leached from these materials, especially unreacted residual monomers (RM), that remained in the resin net after polymerization.5–7
Given the generally reliable manufacturers intended lifetime of polymeric devices,8 several studies have shown that polymers may be subject to numerous biodegradation processes in the oral cavity,9 due to the important role that esterases plays in the enzymatic activity. Acetylcholinesterase (AChE) catalytic activity has recently been shown to be detectable in saliva where its catalytic activity is stable.10 However, other study findings indicated that the intra-individual coefficient of variance of saliva AChE was 35%,11 showing that levels of this enzyme are highly variable.
Although well demonstrated in composite resins,12–14 the role of esterases on the biodegradation of ARR needs further investigation.
The main purpose of the present study was to investigate the influence of acetylcholinesterase on the level of cytotoxicity of three widely used autopolymerizing ARR. In addition, the purpose was to assess the level of cytotoxicity of three specific pure compounds, that are known to be present in the eluates, and the cytotoxicity of the resin liquids through the determination of the half maximal inhibitory concentration (IC50).
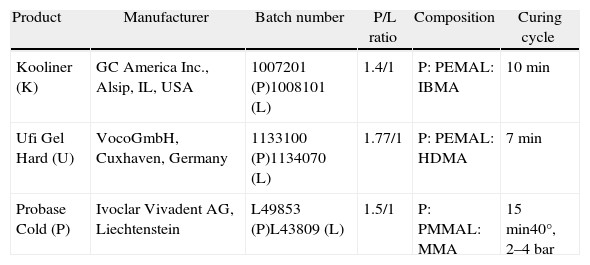
Materials and methodsThis study enrolled two direct ARR, Kooliner (GC America Inc., Alsio, IL, USA), Ufi Gel Hard (VocoGmbH, Cuxhaven, Germany), and one indirect ARR, Probase Cold (Ivoclar Vivadent AG, Schaan, Liechtenstein), in powder liquid form (Table 1). Disk shaped specimens were prepared from three separate mixtures in stainless steel molds, with an average diameter of 50±0.1mm and an average thickness of 2±0.01mm, according to ISO recommendation for biological evaluation of biomaterials.15,16
Materials under evaluation in the study.
| Product | Manufacturer | Batch number | P/L ratio | Composition | Curing cycle |
| Kooliner (K) | GC America Inc., Alsip, IL, USA | 1007201 (P)1008101 (L) | 1.4/1 | P: PEMAL: IBMA | 10min |
| Ufi Gel Hard (U) | VocoGmbH, Cuxhaven, Germany | 1133100 (P)1134070 (L) | 1.77/1 | P: PEMAL: HDMA | 7min |
| Probase Cold (P) | Ivoclar Vivadent AG, Liechtenstein | L49853 (P)L43809 (L) | 1.5/1 | P: PMMAL: MMA | 15min40°, 2–4bar |
Direct ARR were set at 37±2°C for the recommended polymerization time (Table 1) in order to simulate the intra-oral polymerization of the material. Polymerization of indirect ARR was carried out in a Ivomat pressure device (Ivoclar Vivadent, Lichenstein) for the recommended time, temperature and pressure (Table 1).
After UV sterilization,17–19 specimens of each material (n=6) were randomly divided into two groups: experimental, immersed in 5mL of serum-free DMEM with 5U/mL of AChE and control, immersed only in 5mL of serum-free DMEM. The volume of the medium was selected in order to cover the entire surface of each specimen.15
Specimens were incubated for 72h at 37°C under constant agitation to allow the soluble components to leach into the medium.20 Every 24h, 5U/mL of AChE (Sigma–Aldrich Co., St. Louis, MO, USA) was added to experimental specimens in order to maintain the enzyme activity and DMEM was added to control specimens. The medium without specimens was also incubated as above to serve as the negative control and medium with enzyme without specimens to serve as the enzyme control.
All specimens’ eluates were then diluted in fresh supplemented DMEM as follows: no dilution (100%), 3:4 dilution (75%) and 1:2 dilution (50%), to check the dose-dependent response of the cultured cells, following ISO instructions to measure accuracy.21
The cytotoxic evaluation of the eluates was carried out through the reduction of 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium bromide by mitochondrial dehydrogenases (MTT) and by the release of a soluble cytosolic enzyme, lactate dehydrohenase (LDH), into the cell culture medium as the marker for membrane damage.22,23
Cell culture procedure was adapted from a method previously described.24 Human Adult Dermal Fibroblast Cells (Zen-Bio Inc., Chapel Hill, USA) were routinely cultured in DMEM (Sigma–Aldrich Co., St. Louis, MO, USA) with 3.15g/L of d-glucose (Sigma–Aldrich Co., St. Louis, MO, USA), 11.4% FBS (Sigma–Aldrich Co., St. Louis, MO, USA), and 1% penicillin–streptomycin solution (Sigma–Aldrich Co., St. Louis, MO, USA). The cells were grown under an atmosphere containing 5% of CO2.
Cells were then inoculated into 96-well culture plates at a density of 3.2×103 cells/well and incubated at 37°C under a 5% CO2 atmosphere. After 24h, the supernatant was removed and cells were then treated for a further 24h period with 200μL per well of serial dilutions of the eluates (n=8) per combination. Previously explained enzyme and negative control as well as positive control (incubation with DMSO 20%) were used in each assay.25
After the 24h incubation, the medium was carefully removed from each plate and pipetted to a new vial, to be used later in the LDH assay (Sigma–Aldrich Co., St. Louis, MO, USA).
The remaining cells were incubated with 200μL of MTT solution (0.5mg/mL, Sigma–Aldrich Co., St. Louis, MO, USA) for a further period of 2.5h at 37°C. After this period, the MTT solution was discharged and a soluble solvent, DMSO, was added to each well to dissolve the formazan crystals. Absorbance was read at a wavelength of 595nm (Spectrostar Omega, BMG LABTECH, Ortenberg, Germany).
The medium previously removed from the 96 well plates was then centrifuged at 10,000×g for 10min. The supernatant (25μL) was moved to a new 96-well plate along with a mixture of PBS and reconstituted substrate mix already prepared from the LDH Kit (tox-7, Sigma–Aldrich, St. Louis, USA). Plates were then kept for 24min in the dark at room temperature. Absorbance was recorded both at 490 and 690nm on a spectrophotometer (Spectrostar Omega, BMG LABTECH, Germany).
Three independent experiments were performed with eight replicate cultures used for each test solution and controls in each independent experiment. The mean and standard error of the mean absorbance for each test solution were calculated from the triplicate samples. Results of the colorimetric assays were expressed as percentage of viable cells yielded by the test solutions compared to negative controls.
The Kolmogorov–Smirnov test was used to assess the normality of cell viability variable. Mann–Whitney tests were used to compare cell viability between control and AChE groups. To compare materials, test compounds and dilutions, Kruskall–Wallis was used, followed by post testing Tukey multiple comparison. p-Values≤0.05 were considered significant.
In order to get clear insight on the role of monomers on the cytotoxicity of these materials, the cellular viability was also assessed after exposure to Isobutyl Methacrylate (IBMA), Hexanediol Dimethacrylate (HDMA), Methyl Methacrylate (MMA) and the common hydrolysis by-product Methacrylic Acid (MA), taking into account the IC50 (half maximal inhibitory concentration of a substance). The IC50 of the ARR liquids was also studied. All the above mentioned compounds were purchased from Sigma–Aldrich Co. (St. Louis, MO, USA) with the exception for MMA which was obtained from Merck, KgaA (Schuchardt, Germany).
At least seven concentrations of each liquid and compound were diluted in DMEM supplemented with ethanol at a final concentration of ≤0.3%, in order to obtain the IC50, through the MTT assay.26,27
IC50 was determined using a non-linear regression of dose–response – inhibition type [log(inhibitor) vs. normalized response−variable slope].
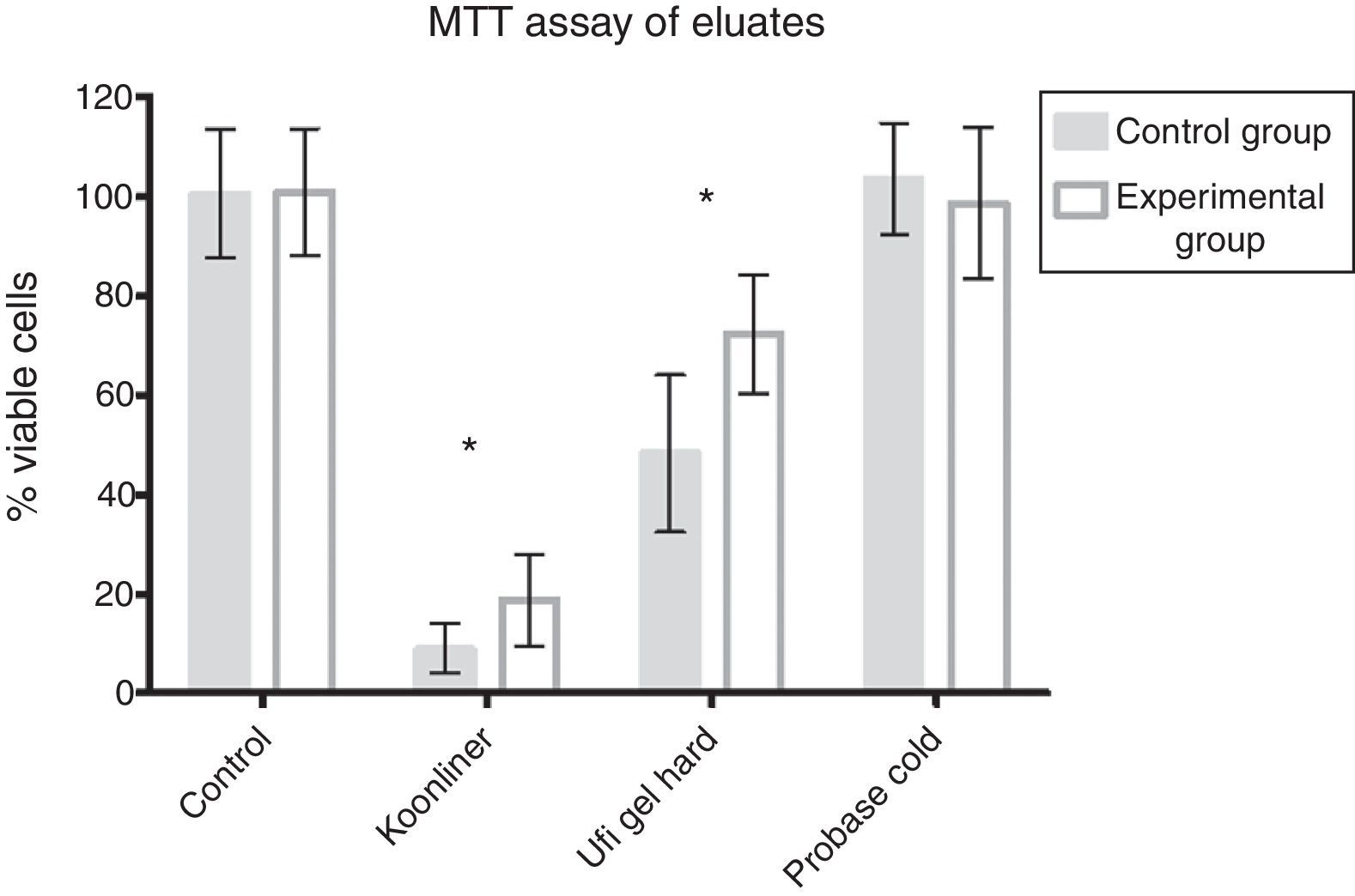
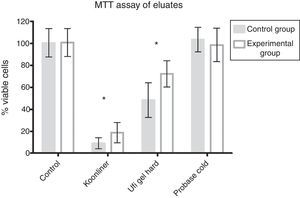
ResultsFig. 1 shows no cytotoxicity for Probase Cold control specimens, ∼90% decrease in cell viability for Kooliner control specimens and ∼51% decrease in cell viability for Ufigel Hard control specimens. Differences between the three materials were statistically significant (p<0.001).
Kooliner specimens submitted to treatment with AChE showed a slight increase of cell viability (18.8±9.2%) compared with the control specimens (9.0±4.9%, p<0.001). For Ufi Gel Hard specimens, the cell viability of the experimental group submitted to AChE (72.5±12%) also showed an increase compared with the specimens incubated only in the culture medium (48.3±15.8%, p<0.001) as recorded in Fig. 1. AChE did not change the non-cytotoxic effect of Probase Cold specimens.
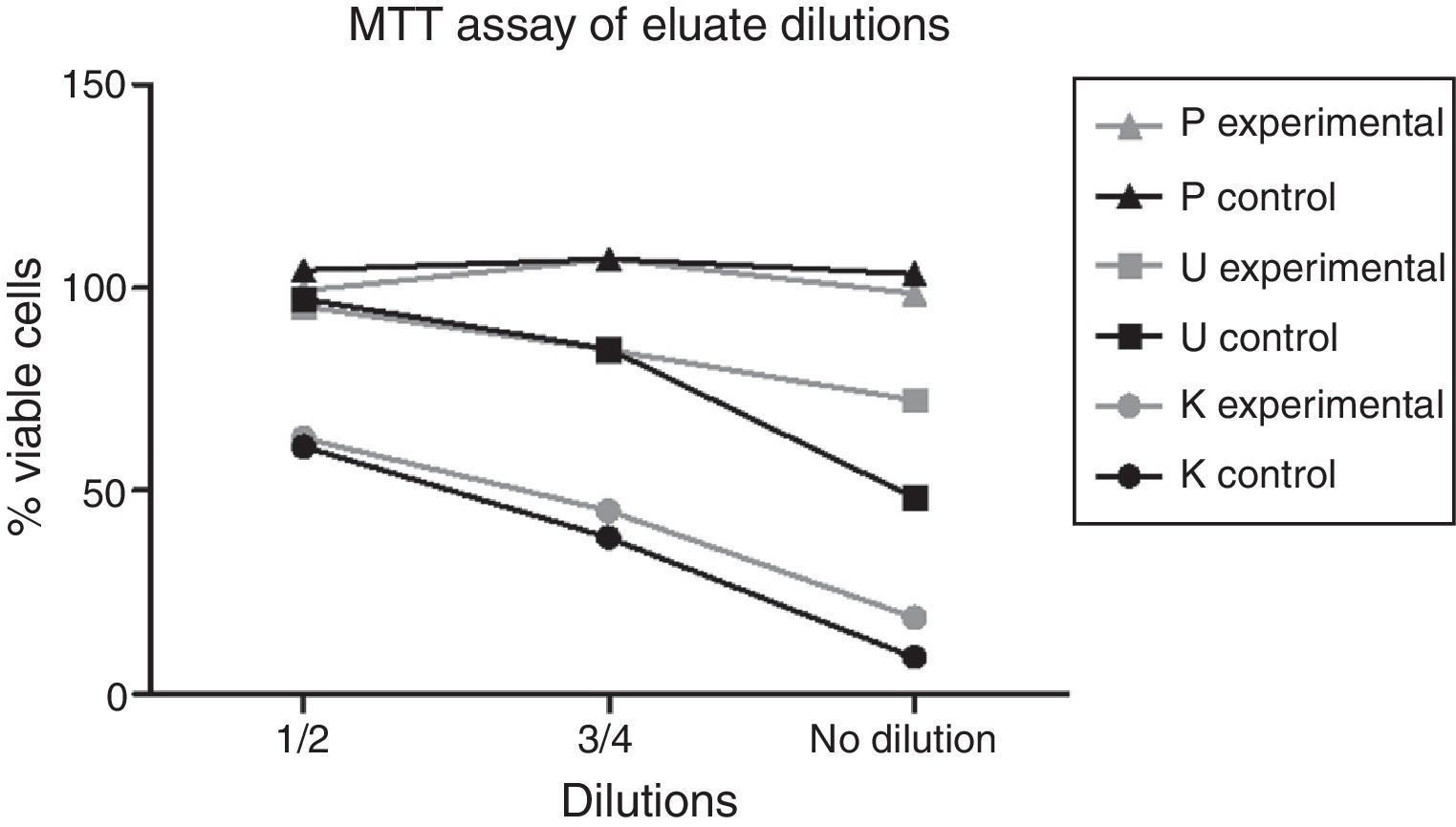
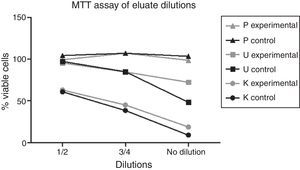
The data indicated a dose-dependent effect on cytotoxicity for the different dilutions of Kooliner and Ufi Gel Hard eluates, as shown in Fig. 2.
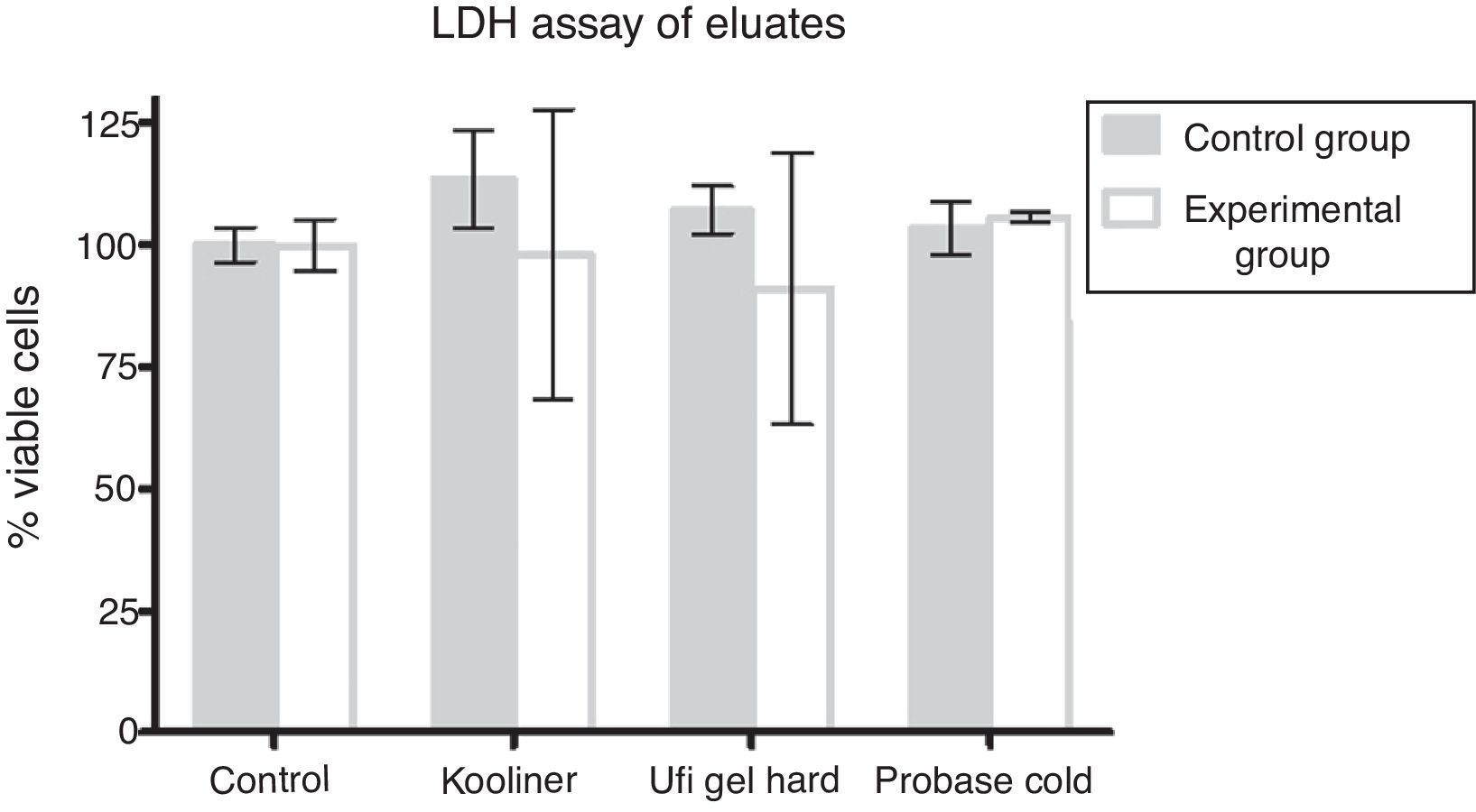
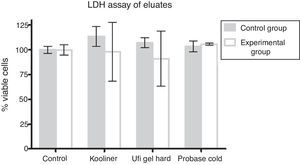
At the LDH assay, neither control nor AChE groups demonstrated differences when compared with negative control groups (Fig. 3).
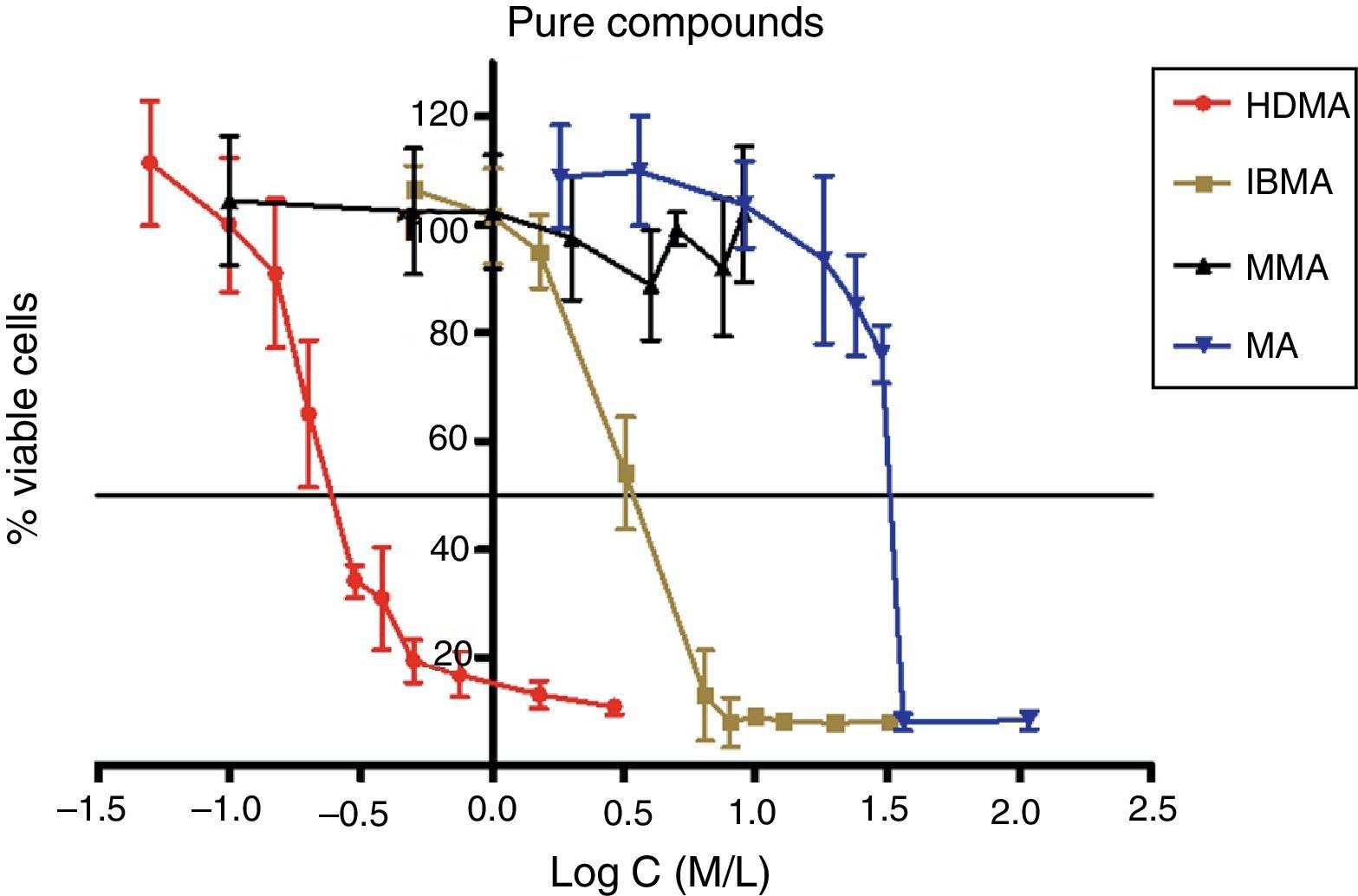
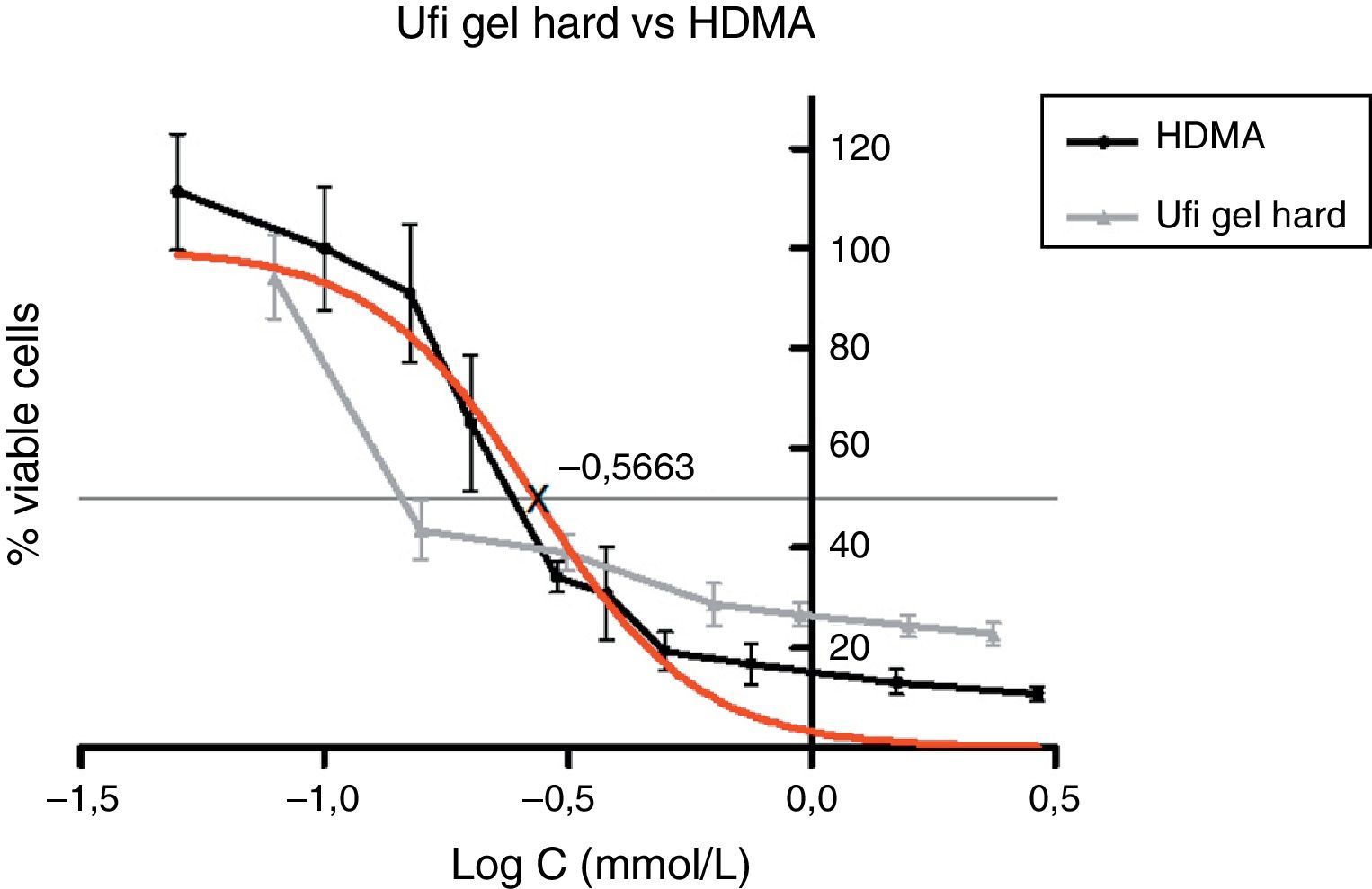
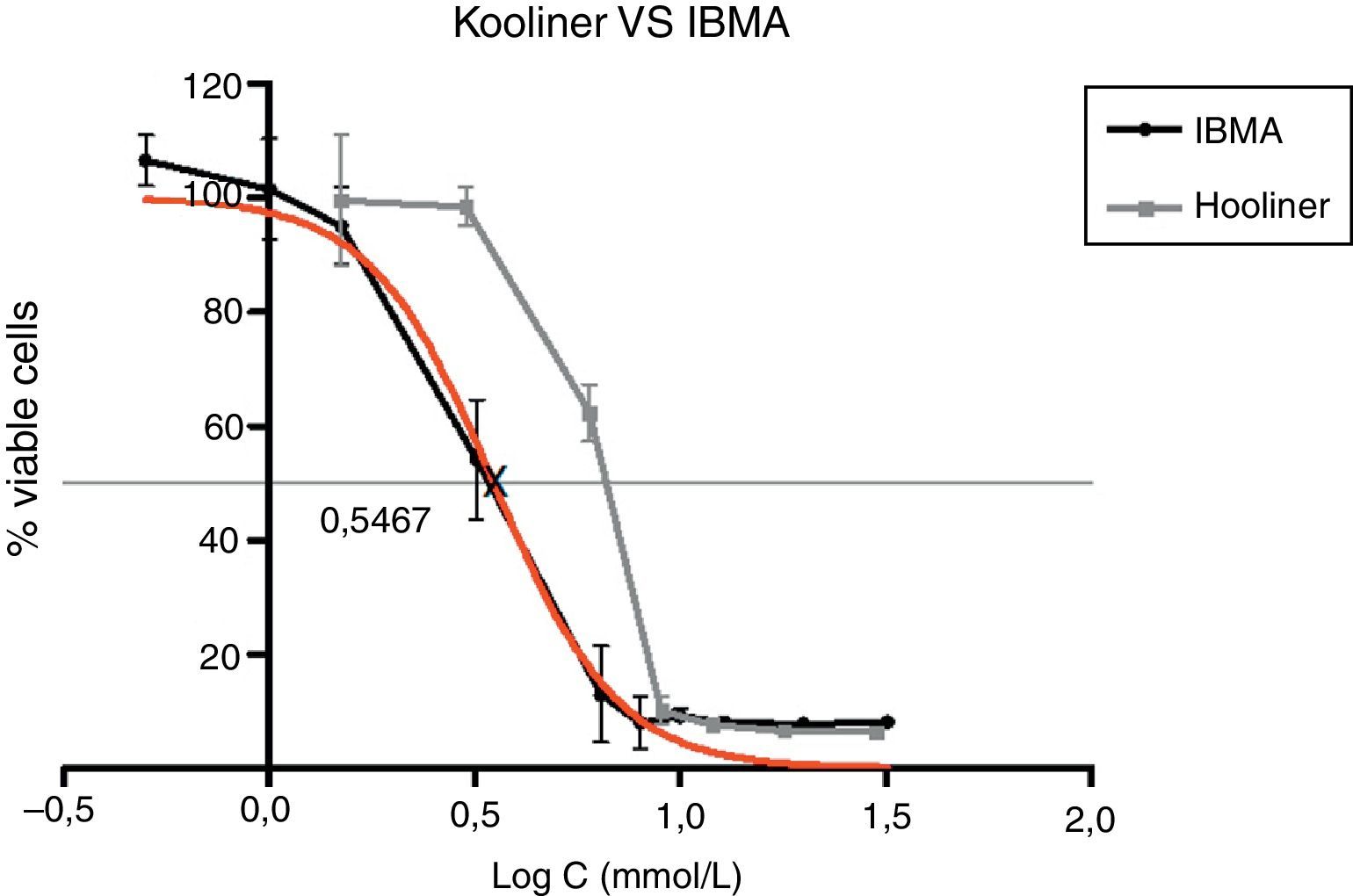
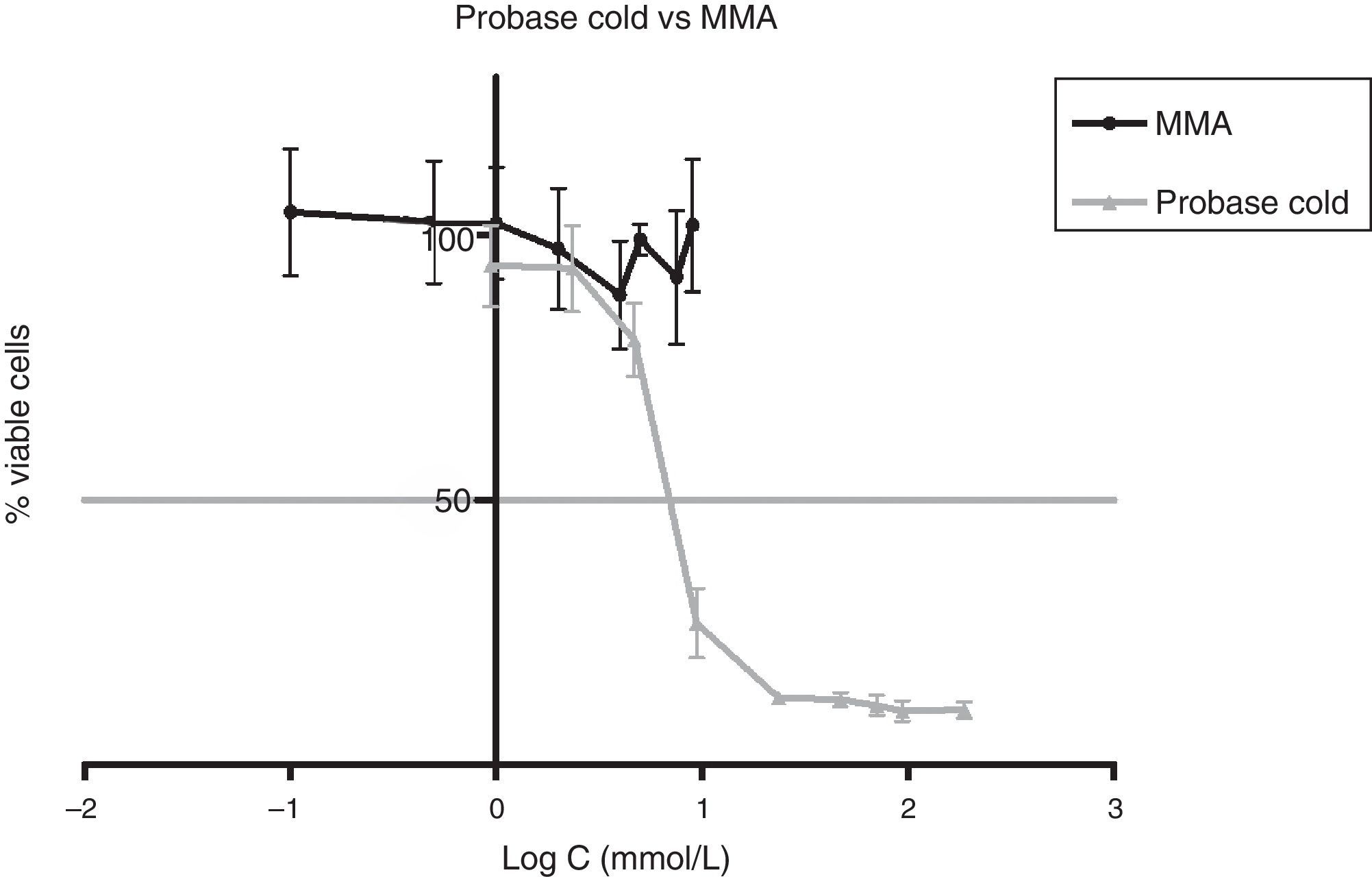
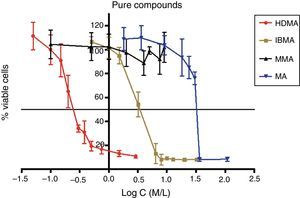
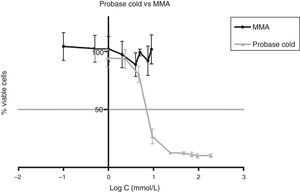
Approximately 50% of the cellular viability of the compound groups were affected when 0.2715mmol/L of HDMA, 3.521mmol/L of IBMA, 31.88mmol/L of MA were used. MMA showed no cytotoxicity at the concentrations used, and hence it was not possible to determine IC50 (Figs. 4–7).
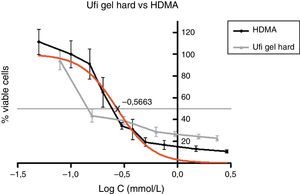
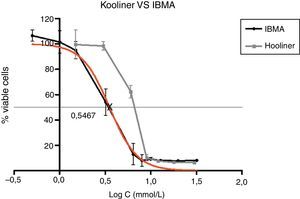
Figs. 5–7 exhibit point-to-point curves of resins liquids and respective monomers. The IC50 of the ARR liquids is obtained with, respectively, 0.2587mmol/L of Ufi Gel Hard, 6.496mmol/L of Kooliner and 7.124mmol/L of Probase Cold.
The curve shape of the monomers in Figs. 5 and 6, matched with the resins liquids, exhibiting similar behavior.
DiscussionThe exposure of fibroblasts to direct ARR eluates resulted in a significant suppression of the mitochondrial activity. This result is in accordance with a previous study that also used human fibroblasts.24 Nevertheless, in the other study,19 direct ARR eluates did not show any toxic effects on the L929 mouse lung fibroblasts cell line. These results could be explained by the distinct type of cells used in the studies. Several authors reported that primary cells have greater sensitivity than transformed lines when testing various biomaterials used in dentistry.3,28 Primary cultures correlate to an in vivo response more accurately, so they can be considered to be more appropriate for testing toxicity of materials for human use.24
Even though several previous studies have shown that indirect autopolymerized eluates were cytotoxic to fibroblasts,29–31,17 the present study did not find cytotoxicity on eluates of Probase Cold. This can be explained by the recommended pressure and temperature treatment during the polymerization, in which the indirect ARR used in our study was exposed, in opposition to the polymerization at room temperature used in the mentioned studies.3,32
Kooliner showed a higher cytotoxic effect than Ufi Gel Hard in control specimens. The fact that Kooliner showed a higher percentage of RM content than Ufi Gel Hard24,33,34 could explain this difference. A previous study35 found that the more monomer added to the mixture the greater the amount of RM and therefore the higher the potential for cytotoxicity. The greater RM available in the Kooliner can be explained by the lower powder/liquid ratio. Previous studies have already shown that post-polymerization treatment whether with immersion in hot water or ethanol aqueous solutions at 55°C can reduce this RM content; the latter being more effective in decreasing the cytotoxicity of both materials.36
In this study, Ufi Gel Hard eluates suppressed around 51% cell viability. In spite of the severe cytotoxicity potential of HDMA, defended by some authors,24,26 the low levels of RM content of this resin promoted only moderately cytotoxic effects over the fibroblast cells.
In contrast, the highly toxic effect of Kooliner eluates (∼10% of viable cells) cannot be explained solely by a higher percentage of residual IBMA content of specimens. This may also be due to differences in quantity and quality of other potentially toxic compounds32,37,38 that may be released from the resins as cross linking agents, initiator, plasticizers like ethylene glycol dimethacrylate (EGDMA) or tetramethylene dimethacrylate (TMDMA),39 pigments, degradation by-products like MA and newly formed formaldehyde.40 In addition, potential synergetic effects of the leachable chemicals should also be considered.24
The experimental specimens from direct ARR revealed an increase of cell viability when compared to respective control groups. The increase of cell viability of experimental Kooliner specimens can be explained by the hydrolysis of IBMA promoted by the enzymatic reaction. MA was found to be a product of this reaction, but the lower cytotoxic potential of MA comparing to IBMA demonstrated by this study and before by several groups24,26 can explain the reduction of the cytotoxicity. In addition, MA proved to be a very unstable compound in aqueous solutions.41
In contrast, the slender increase of cell viability of experimental Ufi Gel Hard specimens could not be related to the enzymatic reaction since HDMA was found to be resistant to AChE. However, levels of MA obtained in other studies reveal that AChE promoted production of MA by hydrolysis of monomers others than HDMA that can be present in Ufi Gel Hard specimens.24
Within the present study, results of the dilutions of eluates showed an increase of cell viability in a dose dependent manner.
Among the tested materials, the Ufi Gel Hard liquid and its monomer, 1,6-HDMA, showed the greatest toxic effects, whereas MMA had the smallest effect. Both Kooliner liquid and IBMA showed moderate cytotoxicity.
The presence of IBMA and 1,6-HDMA explains the cytotoxic effects observed for Kooliner liquid and Ufi Gel Hard liquid, respectively. However, even in higher concentrations, MMA showed no cytotoxic effect on fibroblasts. MMA alone cannot completely explain the effects of Probase Cold liquid on the viability of cells. The effects of Probase Cold liquid in the cellular viability can be explained by the hydrolysis of the monomer MMA in MA, or by its composition which besides MMA contains a plasticizer, tetramethylene dimethacrylate. The effect of this compound on the fibroblasts viability is still unknown.
ConclusionsWithin the limitations of this study, the main conclusions are:
- •
Incubation with acetylcholinesterase did not change the non-cytotoxic effect of Probase Cold.
- •
Incubation with AChE caused a slight increase in cell viability of both direct ARR (Kooliner and Ufi Gel Hard), without changing their cytotoxic potential.
- •
The cytotoxicity of the pure compounds increased in order: MA<IBMA<HDMA. MMA showed no cytotoxicity at the concentrations used.
- •
The direct ARR liquids and respective pure compounds exhibited similar behaviors.
The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Voco GmbH (Cuxhaven, Germany) for the donation of the Ufi Gel Hard material evaluated in this study and Fundação para a Ciência e Tecnologia (Portugal) (PEst-OE/SAU/UI4062/2011; PEst-OE/SAU/UI4013/2011; Ciência 2008 for J. P. M. and EXCL/CTMNAN/0166/2012) for providing financial support to this project.