The importance of neuropsychological functioning in First-Episode Psychosis (FEP) has led to the publication of a growing number of studies in this area of research. The present study pursued three goals: first, to examine verbal and visual memory in a sample of Child and Adolescent FEP, second, to evaluate the effect of other cognitive domains on verbal and visual memory, and finally, to examine the relationship between performance in this cognitive dimension and the use of cannabis at this age.
MethodA sample of 41 FEPs and 39 healthy subjects were evaluated. The variables assessed were verbal and visual memory, attention, working memory, processing speed, mental flexibility, verbal fluency, motor coordination, planning ability and intelligence.
ResultsOur results found impairment of short and long-term recall of verbal memory, and short-term visual memory in early psychosis. They also found relationships between cognitive dimensions, such as visual memory and intelligence and motor coordination. Finally, a «paradoxical» effect was found in patients who used cannabis, as the FEP consumers performed the visual memory test better than those who had not used it.
ConclusionsPatients showed impairment of short and long-term recall of verbal information and short-term visual reproduction. In the second place, motor coordination and intelligence influenced short-term visual memory in patients in the early stages of the illness. Third, use of cannabis in patients with FEP was associated with better performance in the test that evaluated the short-term visual memory, as measured by task completion time, that is, efficiency in performing the test. However, when measured by task execution accuracy, their visual memory was no better than the controls.
La importancia que se otorga al funcionamiento neuropsicológico durante el primer episodio de psicosis (PEP) ha dado lugar a la publicación de un número creciente de artículos de investigación sobre este aspecto en poblaciones de pacientes. El objetivo del presente estudio fue triple: por un lado, examinar la memoria verbal y visual de una muestra de niños y adolescentes con un PEP; en segundo lugar, evaluar el efecto de otros dominios cognitivos sobre la memoria verbal y visual y, en tercer lugar, analizar la relación entre el rendimiento cognitivo en memoria y el consumo de Cannabis.
MétodoLa muestra incluyó a 41 pacientes con PEP y a 39 individuos de control sanos. Las variables analizadas fueron la memoria verbal y visual, atención, memoria de trabajo, velocidad de procesamiento, flexibilidad mental, fluidez verbal, coordinación motora, capacidad de planificación e inteligencia.
ResultadosEn pacientes con un PEP, comparado con el grupo de individuos sanos que sirvieron como control, se encontraron alteraciones en los dominios de memoria verbal a corto y largo plazo y de la memoria visual a corto plazo; además, también identificamos una influencia de la inteligencia y la coordinación motora en la memoria visual. Por último, entre los pacientes consumidores de Cannabis, detectamos un «efecto paradójico» del consumo, puesto que ejecutaron mejor el test que mide la memoria visual en comparación con los que no consumieron dicha sustancia.
ConclusionesEn primer lugar, en pacientes con un PEP se identifican alteraciones en la memoria verbal a corto y largo plazo y en la reproducción visual a corto plazo. En segundo lugar, la coordinación motora y la inteligencia son 2 dimensiones cognitivas que influyen en la memoria visual a corto plazo de los pacientes. Y, por último, en la muestra del presente estudio de pacientes con PEP, el consumo de Cannabis se asoció a un mejor rendimiento en el test que evalúa la memoria visual a corto plazo, determinado mediante el tiempo requerido para la ejecución del test, es decir, la eficiencia en su ejecución. No obstante, la precisión en la ejecución de la tarea de memoria visual no fue mejor que la observada entre individuos sanos del grupo de control.
Cognitive performance is one of the main factors associated with problems in the personal lives and on-the-job performance of schizophrenic patients.1 It is considered one of the most stable conditions of this pathology, because, unlike other characteristics such as symptoms, it has been demonstrated that cognitive performance does not change with the evolution of the illness.2
The importance of neuropsychological functioning in these patients has led to the publication of a growing number of related studies.3–8 Although studies on cognitive performance in this illness are of special interest in its early stages in children and adolescents,9,10 as well as in adults, there are much fewer studies concentrating on the cognitive profile in Child and Adolescent First-Episode Psychosis11 (FEP).
Verbal memory has been shown to be one of the cognitive domains most affected in early psychosis in childhood and adolescence.10 Authors, such as Kravariti et al.12 and Fagerlund et al.,13 found that their samples of subjects with first episodes showed significantly poorer verbal memory than healthy controls (HC). However, while some authors have found that visual memory in FEP is noticeably impaired,14,15 others have not found any difference between HC and patients with schizophrenia beginning in adolescence.16
In addition to verbal and visual memory, available data show that these patients have impaired working memory, attention, executive functioning,3,10,14 processing speed,17 verbal fluency,18 motor skills19 and intelligence. Nevertheless, we have not found any studies showing whether impaired memory in FEP could be due to any of these other impairments.
Moreover, data collected reflect the existence of a problem with illegal drug use, especially cannabis, among young people in the population. The latest report of the European Observatory for Drugs and Drug Addiction20 showed a considerable increase in use of cannabis by teenagers (14–18 years) from 18.2% in 1994 to 36% in 2004, and placed the mean age when use is begun at 14.8 years.21 These results are especially alarming in view of recent research finding the use of cannabis to be a risk factor for psychosis,22–24 and a catalyst of disease.25 There are no conclusive neuropsychological data at this time on the influence of cannabis in patients with FEP, because whereas some researchers have found that cognitive performance is poorer in substance-use patients26 others have noted an improvement in cognitive functioning of schizophrenic patients who used cannabis before they were 17,27 and whose first episode had taken place in childhood and adolescence.4 These unexpected results have been called a “paradox effect” of cannabis in psychotic patients.28 Fewer minor neurological signs have also been found in first-episode patients who use cannabis.23
This study pursued three goals. The first was to find out whether there are differences in verbal and visual memory between FEP in childhood and adolescence and a group of HC. The second goal attempts to demonstrate whether other cognitive domains influence verbal and visual memory in these patients. And finally, we investigated whether the use of cannabis before the start of the first episode modifies verbal and visual memory performance in a sample of children and teenagers with FEP.
Materials and methodsParticipantsThe forty-one teenagers who had had a FEP included in the study (schizophrenia n=21, bipolar disorder n=12, and “other psychoses” n=8) were recruited from the Children and Youth Mental Health Units at five hospitals in Andalusia located in Jaen, Granada, Almeria, Cordoba and Seville, and were evaluated from October 2007 to June 2010. These diagnoses were confirmed after 12 months from the first assessment. The criteria for inclusion in the group of patients were that they had experienced the FEP from 12 to 18 years of age, had had it for less than five years (M=10.42; SD=20.74 months) and completed the verbal and visual memory test. Exclusion criteria for patients were symptoms not having stabilized (based on administration of the Positive and Negative Syndrome Scale, PANSS,29 and the Children’ Global Assessment Scale, CGAS),30 having an IQ below 65 (based on administration of the WISC-IV and WAIS-III), and having had an organic central nervous system disease or a cranioencephalic traumatism with loss of consciousness for over an hour.
The 39 HC recruited were all evaluated at the same time as the patients and were recruited at several primary attention outpatient clinics in Jaen Province. In the waiting rooms at the paediatric care units, they were asked if they wanted to participate, and those with characteristics similar to the group of patients were selected. They were given a first clinical assessment to discard psychotic symptoms. None of them met DSM-IV diagnostic criteria. The criteria for inclusion in the control group were age and sex similar to the group of patients, for which controls were grouped by age (12–14, 15–17 and 18), and each control was matched to a patient so the two groups would have the same sex distribution. Other inclusion criteria for this group were absence of psychotic symptoms and completion of the verbal and visual memory test.
Exclusion criteria for controls were psychotic symptoms, an IQ below 65 (based on the WISC-IV and WAIS-III), an organic central nervous system disease or cranioencephalic traumatism with loss of consciousness for over an hour.
When the participants had been informed about the study, their parent, family member or legal guardian signed an informed consent form and they proceeded to the first assessment. When it was confirmed that the subjects met the criteria for inclusion in the sample, the complete assessment was done. This study was approved by the Research Ethics Committees at the hospitals of Jaen, Granada, Almeria, Cordoba and Seville.
Clinical assessmentWhen the subjects had given their consent to participate in the study, children and their parents were individually assessed by administering the semi-structured interview, Kiddie Schedule of Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SAD S-PL).31,32 This initial assessment was completed with an interview in which they were asked their age, education of the subject, education of subject's parents,33 subject's pharmacological treatment, substance use and neuropsychological assessment.
Patients and controls were assessed by an experienced clinical researcher using the validated Spanish adaptation of the Kiddie Schedule of Affective Disorders and Schizophrenia, Present and Lifetime Version31,32 semi-structured interview following the criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV-TR).34 Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS)29 and the Child Global Assessment Scale (CGAS).30 Patients were assessed while hospitalized for the first time.
The Positive and Negative Syndrome Scale (PANSS)29 consists of 30 items (symptoms) scored from 1 (absent) to 7 (extreme). It is made up of three subscales: Positive (delusions, conceptual disorganization, hallucinatory behaviour, excitement, grandiosity, suspiciousness/persecution and hostility), Negative (blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, difficulty in abstract thinking, lack of spontaneity and conversation flow, stereotyped thinking), and general psychopathology (somatic concerns, anxiety, guilt feelings, tension, mannerisms/posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgement and insight, disturbance of volition, poor impulse control, preoccupation, active social avoidance).
Functioning was assessed using the Child Global Assessment Scale (CGAS).30 This is a numerical scale (from 1 to 100) used by mental health specialists to evaluate the overall functioning of children under 18 years of age.
Neuropsychological assessmentCognitive assessment was done by psychologists trained in the standardized tests when the patients’ symptoms had stabilized according to clinical criteria.
Immediate, short and long-term verbal memories were measured based on the scores on Word List I (which includes the total score on Immediate and Short Term Memory) and Word List II (which reports on Long-Term Memory and Recognition) of the Spanish version of the Weschler Memory Scale-Third Edition.35
Visual and short-term memoryThe Spanish version of the Rey Complex Figure Test36 was using a previously copied figure, after 3min. The variables were the time it took the subject to reproduce the figure (RCF-1) and the direct scores (RCF II) showing accuracy and detail of figure reproduction.
Attention was measured using the digit span test on the Spanish version of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III)37 and the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV)38 in direct order.
Working memoryThe digit span test included in the WAIS-III37 and WISC-IV38 Weschler Intelligence Manuals in reverse order.
Processing speedThis was measured based on the number code subtest in the Weschler Intelligence Scale Manual.37,38
Mental flexibilityThe interference scale in the Spanish adaptation of the Stroop Test was used.39
Motor coordinationEvaluated by the time it took the subjects to take the Grooved Pegboard test with the dominant hand.40
Planning abilityIt is found based on the time after planning that it took to perform the battery of tests in Version 1 of the Zoo Map in the Behavioural Assessment of Dysexecutive Syndrome for Children (BADS-C)41 and the Behavioural Assessment of Dysexecutive Syndrome for Adults (BADS).42
IQ was measured using the Weschler Intelligence Scale for subjects under 16 years of age (WISC-IV),38 which includes Word Reasoning, Similarities, Block Design, Digit Span, Matrix Reasoning, Picture Completion, Coding, Information, Symbol Search and Letter Number Sequencing subtests. The IQ of subjects over 16 years old was found using the Axelrod and Ryan estimation43 with the Vocabulary, Similarities, Block Design, Digit Span, Matrix Reasoning, Figure Completion, Coding and Information subtests in the Weschler Intelligence Scale for Adults (WAIS-III).37
Substance-use assessmentTo find the cannabis use pattern, both patients and controls were assessed using Section L of the Spanish version of the CIDI semi-structured interview.44 The interview criteria allowed us to divide the sample into “Cannabis users” (those who had used it more than five times in their lives), and “Nonusers” (those who had not used it or had done so fewer than five times in their lives), and distribute the users by frequency of use into “almost every day”, “1–2 days a week”, “1–3 times a month” or “less than once a month”. It also includes information on the use of other substances (cocaine, MDMA, ketamine, hallucinogens, solvents and speed); however, their use was not within the scope of this study, and was therefore not evaluated.
Statistical analysisBefore analysis, the data were tested for normality (Kolmogorov–Smirnov) and homoscedasticity (Levene's Test), and as the sample was found to meet both criteria, the pertinent statistical analyses were performed.
The categorical sociodemographic variables (sex, parents’ education and use of cannabis and other substances) were analyzed using the Chi Square test, while the continuous variables were compared with the Student t-test for independent samples.
An analysis of covariance (ANCOVA) was done to find the differences between patients and controls in the memory domains (verbal and visual), that is, to test for the first goal of this study. The differences found between the two groups in the age, education and IQ variables were controlled by ANCOVA with these variables as covariates.
For the second goal of this study, in which it was intended to analyze the effect that the rest of the neuropsychological variables might have on verbal and visual memory in patients with FEP, a linear regression model was used to explore the relationship among the various cognitive domains and verbal and visual memory.
Thirdly, to find out how the use of cannabis influences verbal and visual memory, an ANCOVA was done using “use of cannabis” as the fixed factor.
Finally, a post hoc analysis was done to analyze the effect that use of cannabis had on the sample by subdividing it into four groups (patients who use it vs those who do not, and controls who use it vs those who do not).
All the analyses were done using the SPSS 15.0 statistical package with a significance level of p<0.05 as a reference.
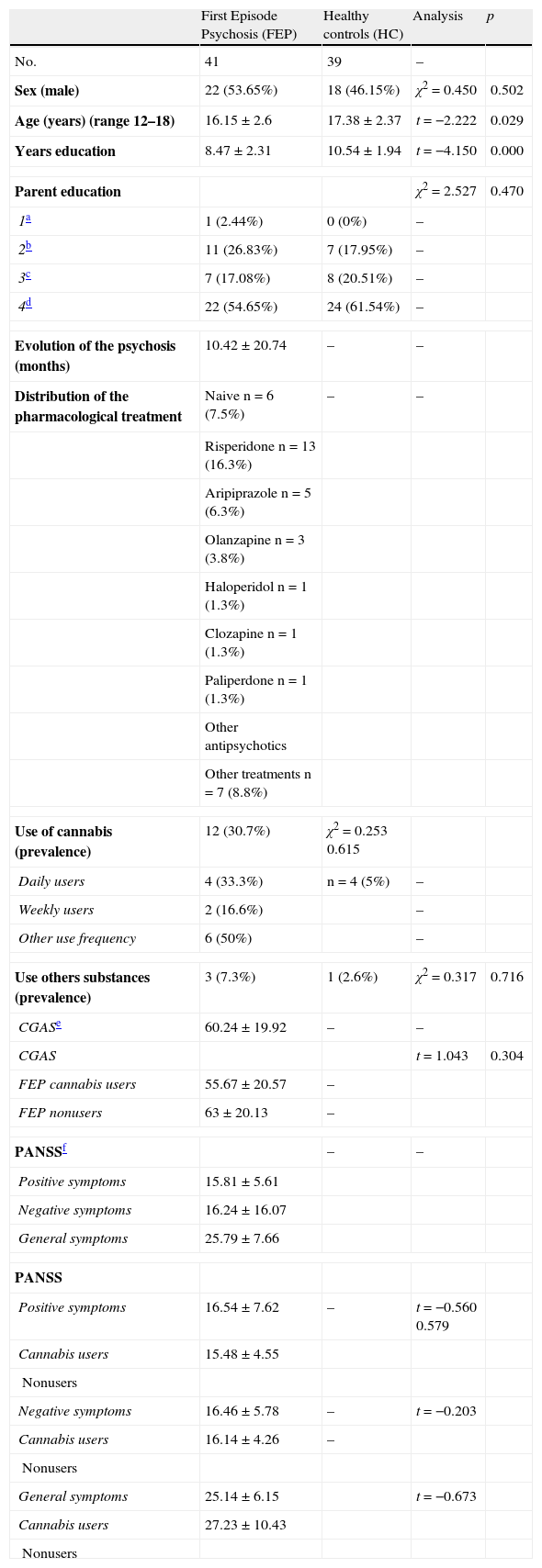
ResultsA. Sociodemographic characteristics of the sampleTable 1 shows the sex, age, education in years, parent education, prevalence of cannabis use, prevalence of other substance use, psychopathology of patients and psychopathology of patients who were cannabis users compared to nonusers, as well as evolution of the psychosis (months) and distribution of pharmacological treatment.
Characteristics of First Episode Psychosis patients and healthy controls.
| First Episode Psychosis (FEP) | Healthy controls (HC) | Analysis | p | |
| No. | 41 | 39 | – | |
| Sex (male) | 22 (53.65%) | 18 (46.15%) | χ2=0.450 | 0.502 |
| Age (years) (range 12–18) | 16.15±2.6 | 17.38±2.37 | t=−2.222 | 0.029 |
| Years education | 8.47±2.31 | 10.54±1.94 | t=−4.150 | 0.000 |
| Parent education | χ2=2.527 | 0.470 | ||
| 1a | 1 (2.44%) | 0 (0%) | – | |
| 2b | 11 (26.83%) | 7 (17.95%) | – | |
| 3c | 7 (17.08%) | 8 (20.51%) | – | |
| 4d | 22 (54.65%) | 24 (61.54%) | – | |
| Evolution of the psychosis (months) | 10.42±20.74 | – | – | |
| Distribution of the pharmacological treatment | Naive n=6 (7.5%) | – | – | |
| Risperidone n=13 (16.3%) | ||||
| Aripiprazole n=5 (6.3%) | ||||
| Olanzapine n=3 (3.8%) | ||||
| Haloperidol n=1 (1.3%) | ||||
| Clozapine n=1 (1.3%) | ||||
| Paliperdone n=1 (1.3%) | ||||
| Other antipsychotics | ||||
| Other treatments n=7 (8.8%) | ||||
| Use of cannabis (prevalence) | 12 (30.7%) | χ2=0.253 0.615 | ||
| Daily users | 4 (33.3%) | n=4 (5%) | – | |
| Weekly users | 2 (16.6%) | – | ||
| Other use frequency | 6 (50%) | – | ||
| Use others substances (prevalence) | 3 (7.3%) | 1 (2.6%) | χ2=0.317 | 0.716 |
| CGASe | 60.24±19.92 | – | – | |
| CGAS | t=1.043 | 0.304 | ||
| FEP cannabis users | 55.67±20.57 | – | ||
| FEP nonusers | 63±20.13 | – | ||
| PANSSf | – | – | ||
| Positive symptoms | 15.81±5.61 | |||
| Negative symptoms | 16.24±16.07 | |||
| General symptoms | 25.79±7.66 | |||
| PANSS | ||||
| Positive symptoms | 16.54±7.62 | – | t=−0.560 0.579 | |
| Cannabis users | 15.48±4.55 | |||
| Nonusers | ||||
| Negative symptoms | 16.46±5.78 | – | t=−0.203 | |
| Cannabis users | 16.14±4.26 | – | ||
| Nonusers | ||||
| General symptoms | 25.14±6.15 | t=−0.673 | ||
| Cannabis users | 27.23±10.43 | |||
| Nonusers | ||||
Significant differences in age and years of education were found between patients and healthy participants, showing that controls were older than patients and also had a higher level of education.
There were no significant differences in the other variables.
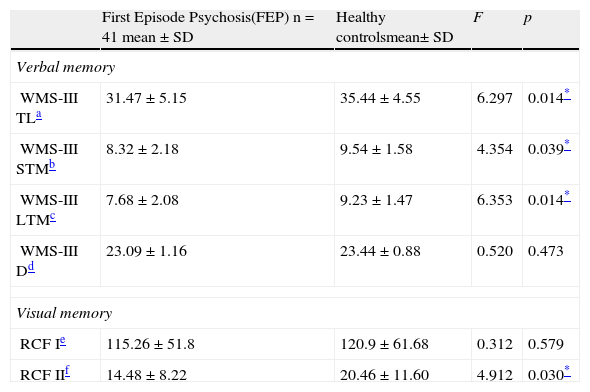
B. Neuropsychological characteristics of First Episode Psychosis and Healthy ControlsComparison of verbal and visual memoryPsychotic subjects had significantly poorer performance than the HC in immediate verbal memory, short-term verbal memory, long-term verbal memory and short-term visual memory (RCFII).
These data are shown by age, education and IQ in Table 2.
Verbal and visual memory results of the First Episode Psychosis patients compared to Healthy Controls.
| First Episode Psychosis(FEP) n=41 mean±SD | Healthy controlsmean±SD | F | p | |
| Verbal memory | ||||
| WMS-III TLa | 31.47±5.15 | 35.44±4.55 | 6.297 | 0.014* |
| WMS-III STMb | 8.32±2.18 | 9.54±1.58 | 4.354 | 0.039* |
| WMS-III LTMc | 7.68±2.08 | 9.23±1.47 | 6.353 | 0.014* |
| WMS-III Dd | 23.09±1.16 | 23.44±0.88 | 0.520 | 0.473 |
| Visual memory | ||||
| RCF Ie | 115.26±51.8 | 120.9±61.68 | 0.312 | 0.579 |
| RCF IIf | 14.48±8.22 | 20.46±11.60 | 4.912 | 0.030* |
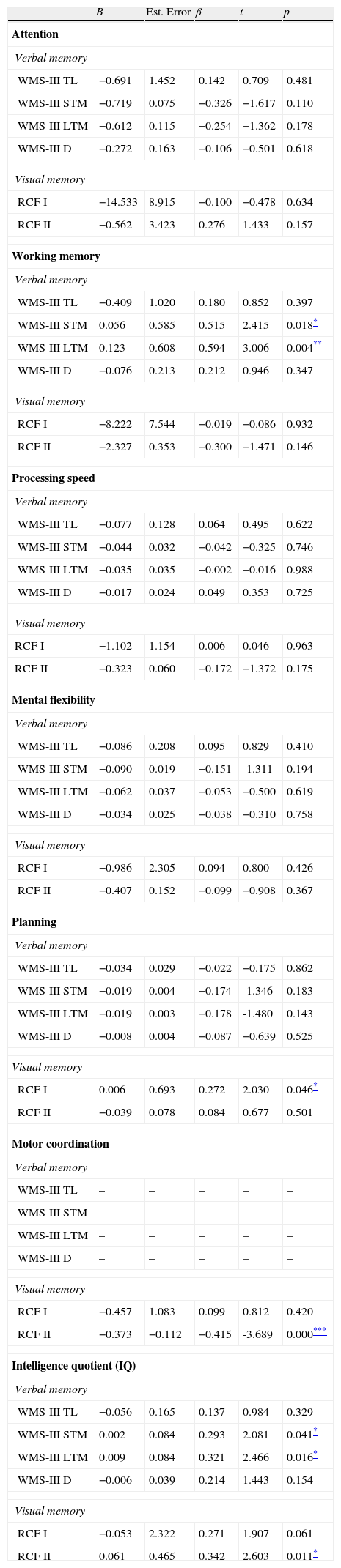
The results on the extent to which the deficits present in the various cognitive domains (Attention, Working Memory, Processing Speed, Mental Flexibility, Planning Ability, Motor Coordination and Intelligence Quotient) can predict or describe impaired verbal and visual memory in patients with FEP, found that all the cognitive domains evaluated influence both verbal and visual memory, except for working memory, which does not interfere with short-term (β=0.515; p=0.018) or long-term (β=0.321; p=0.016) verbal memory, or short-term visual memory(FCRII) (β=0.342; p=0.011).
These results are shown in detail in Table 3.
Analysis of the effect of covariates on verbal and visual memory of the first-episode group compared to healthy controls, adjusted for Attention, Working Memory, Processing Speed, Mental Flexibility, Planning Ability, Motor Coordination and Intelligence Quotient.
| B | Est. Error | β | t | p | |
| Attention | |||||
| Verbal memory | |||||
| WMS-III TL | −0.691 | 1.452 | 0.142 | 0.709 | 0.481 |
| WMS-III STM | −0.719 | 0.075 | −0.326 | −1.617 | 0.110 |
| WMS-III LTM | −0.612 | 0.115 | −0.254 | −1.362 | 0.178 |
| WMS-III D | −0.272 | 0.163 | −0.106 | −0.501 | 0.618 |
| Visual memory | |||||
| RCF I | −14.533 | 8.915 | −0.100 | −0.478 | 0.634 |
| RCF II | −0.562 | 3.423 | 0.276 | 1.433 | 0.157 |
| Working memory | |||||
| Verbal memory | |||||
| WMS-III TL | −0.409 | 1.020 | 0.180 | 0.852 | 0.397 |
| WMS-III STM | 0.056 | 0.585 | 0.515 | 2.415 | 0.018* |
| WMS-III LTM | 0.123 | 0.608 | 0.594 | 3.006 | 0.004** |
| WMS-III D | −0.076 | 0.213 | 0.212 | 0.946 | 0.347 |
| Visual memory | |||||
| RCF I | −8.222 | 7.544 | −0.019 | −0.086 | 0.932 |
| RCF II | −2.327 | 0.353 | −0.300 | −1.471 | 0.146 |
| Processing speed | |||||
| Verbal memory | |||||
| WMS-III TL | −0.077 | 0.128 | 0.064 | 0.495 | 0.622 |
| WMS-III STM | −0.044 | 0.032 | −0.042 | −0.325 | 0.746 |
| WMS-III LTM | −0.035 | 0.035 | −0.002 | −0.016 | 0.988 |
| WMS-III D | −0.017 | 0.024 | 0.049 | 0.353 | 0.725 |
| Visual memory | |||||
| RCF I | −1.102 | 1.154 | 0.006 | 0.046 | 0.963 |
| RCF II | −0.323 | 0.060 | −0.172 | −1.372 | 0.175 |
| Mental flexibility | |||||
| Verbal memory | |||||
| WMS-III TL | −0.086 | 0.208 | 0.095 | 0.829 | 0.410 |
| WMS-III STM | −0.090 | 0.019 | −0.151 | -1.311 | 0.194 |
| WMS-III LTM | −0.062 | 0.037 | −0.053 | −0.500 | 0.619 |
| WMS-III D | −0.034 | 0.025 | −0.038 | −0.310 | 0.758 |
| Visual memory | |||||
| RCF I | −0.986 | 2.305 | 0.094 | 0.800 | 0.426 |
| RCF II | −0.407 | 0.152 | −0.099 | −0.908 | 0.367 |
| Planning | |||||
| Verbal memory | |||||
| WMS-III TL | −0.034 | 0.029 | −0.022 | −0.175 | 0.862 |
| WMS-III STM | −0.019 | 0.004 | −0.174 | -1.346 | 0.183 |
| WMS-III LTM | −0.019 | 0.003 | −0.178 | -1.480 | 0.143 |
| WMS-III D | −0.008 | 0.004 | −0.087 | −0.639 | 0.525 |
| Visual memory | |||||
| RCF I | 0.006 | 0.693 | 0.272 | 2.030 | 0.046* |
| RCF II | −0.039 | 0.078 | 0.084 | 0.677 | 0.501 |
| Motor coordination | |||||
| Verbal memory | |||||
| WMS-III TL | – | – | – | – | – |
| WMS-III STM | – | – | – | – | – |
| WMS-III LTM | – | – | – | – | – |
| WMS-III D | – | – | – | – | – |
| Visual memory | |||||
| RCF I | −0.457 | 1.083 | 0.099 | 0.812 | 0.420 |
| RCF II | −0.373 | −0.112 | −0.415 | -3.689 | 0.000*** |
| Intelligence quotient (IQ) | |||||
| Verbal memory | |||||
| WMS-III TL | −0.056 | 0.165 | 0.137 | 0.984 | 0.329 |
| WMS-III STM | 0.002 | 0.084 | 0.293 | 2.081 | 0.041* |
| WMS-III LTM | 0.009 | 0.084 | 0.321 | 2.466 | 0.016* |
| WMS-III D | −0.006 | 0.039 | 0.214 | 1.443 | 0.154 |
| Visual memory | |||||
| RCF I | −0.053 | 2.322 | 0.271 | 1.907 | 0.061 |
| RCF II | 0.061 | 0.465 | 0.342 | 2.603 | 0.011* |
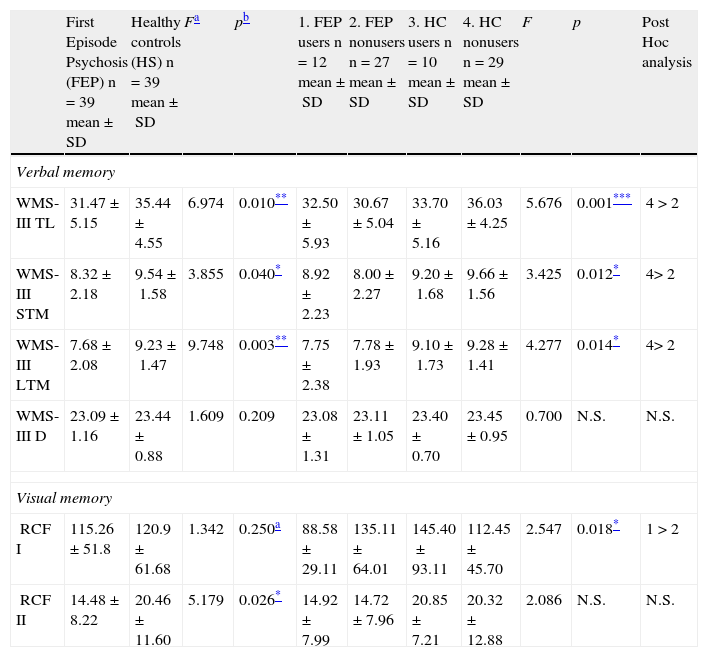
When the results for memory are adjusted for use of cannabis, age, years of education and IQ (covariates), the differences between the psychosis and control groups remain the same for immediate verbal, short-term and long-term memory, as well as short-term visual memory (RCF II) (Table 4), showing poorer performance by patients than controls.
Analysis of the effect of cannabis.
| First Episode Psychosis (FEP) n=39 mean±SD | Healthy controls (HS) n=39 mean±SD | Fa | pb | 1. FEP users n=12 mean±SD | 2. FEP nonusers n=27 mean±SD | 3. HC users n=10 mean±SD | 4. HC nonusers n=29 mean±SD | F | p | Post Hoc analysis | |
| Verbal memory | |||||||||||
| WMS-III TL | 31.47±5.15 | 35.44±4.55 | 6.974 | 0.010** | 32.50±5.93 | 30.67±5.04 | 33.70±5.16 | 36.03±4.25 | 5.676 | 0.001*** | 4>2 |
| WMS-III STM | 8.32±2.18 | 9.54±1.58 | 3.855 | 0.040* | 8.92±2.23 | 8.00±2.27 | 9.20±1.68 | 9.66±1.56 | 3.425 | 0.012* | 4>2 |
| WMS-III LTM | 7.68±2.08 | 9.23±1.47 | 9.748 | 0.003** | 7.75±2.38 | 7.78±1.93 | 9.10±1.73 | 9.28±1.41 | 4.277 | 0.014* | 4>2 |
| WMS-III D | 23.09±1.16 | 23.44±0.88 | 1.609 | 0.209 | 23.08±1.31 | 23.11±1.05 | 23.40±0.70 | 23.45±0.95 | 0.700 | N.S. | N.S. |
| Visual memory | |||||||||||
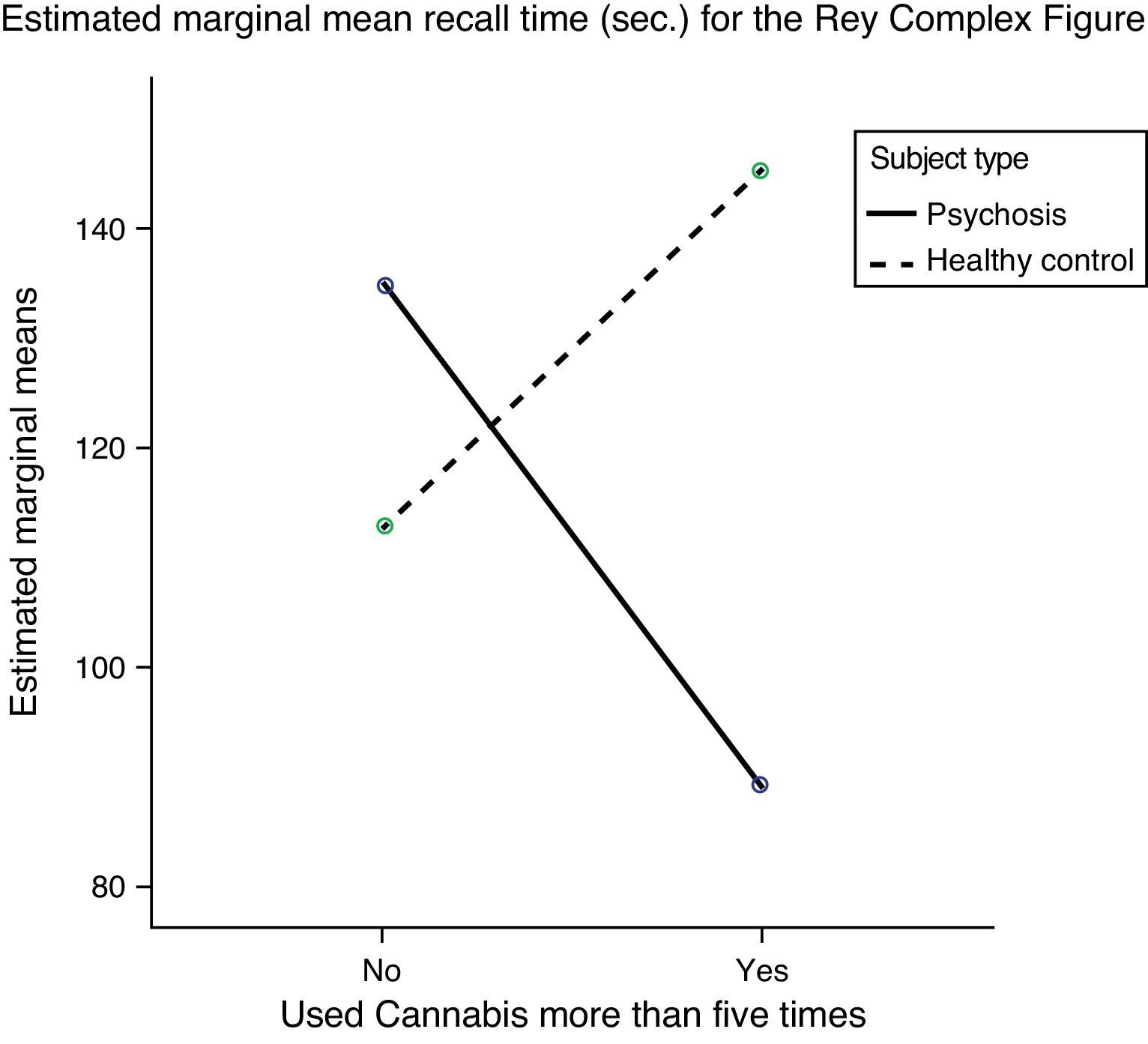
| RCF I | 115.26±51.8 | 120.9±61.68 | 1.342 | 0.250a | 88.58±29.11 | 135.11±64.01 | 145.40±93.11 | 112.45±45.70 | 2.547 | 0.018* | 1>2 |
| RCF II | 14.48±8.22 | 20.46±11.60 | 5.179 | 0.026* | 14.92±7.99 | 14.72±7.96 | 20.85±7.21 | 20.32±12.88 | 2.086 | N.S. | N.S. |
N.S., non-significant.
Short-term visual memory (RCF I), however, which had not shown any significant difference in any of the previous analyses, interacted in a statistically significant manner with type of subject (patient or control) and cannabis use (Fig. 1). The fact that no differences were found in this variable in the preliminary analyses and a statistically significant interaction was found when cannabis use was entered shows that this variable is exerting some type of influence. To analyze this effect, the sample was divided into four groups and a post hoc analysis conducted. These results are shown in Table 4.
DiscussionAs far as we know, this is the first study on cognitive performance in verbal and visual memory in a sample of patients with Child and Adolescent FEP which attempts to find out the influence of other variables, in this case cognitive and environmental that might be affecting memory.
This study shows the existence of immediate, short and long-term verbal memory deficits in patients with FEP compared to a healthy population, while no differences were found in recognition of information stored verbally. Although many studies concentrating on differences in verbal memory have shown the existence of deficits in immediate verbal, short and long-term memory in First-Episode patients,5,6,10,14,16,45–48 the results found for verbal recognition memory are not as consistent, as some authors have found no differences between patients and HC,5,6 while others have found no recognition deficits in FEPs.10,47 The literature to date is not entirely in agreement concerning results on short-term visual memory either. Ueland et al.,17 for example, found significant deterioration in visual memory in FEPs compared to controls using “The Kimura Recurring Figures Test”, however, these differences were not as significant when this domain was evaluated with another test, such as the Weschler Memory Scale-Revised.12 Our data were not consistent in this domain either, as we evaluated short-term memory using two different scales and only found statistically significant differences with one of them. One explanation for these results and the discrepancies among the different studies with respect to verbal and visual memory, could be the type of test (ceiling effect),49,50 as cognitive skill tests are very sensitive and results may vary.
One of the most important findings in this study is that the deficits found in verbal and visual memory in these patients could be predicted by impairment to other cognitive domains as attention, working memory, processing speed, mental flexibility, planning ability, motor coordination and Intelligence Quotient.
As discussed above, there is evidence demonstrating that patients suffering from Child and Adolescent FEP have memory problems.13 Some studies have also shown that they have deficits in such domains as attention,13,18 cognitive flexibility,4,6 working memory,10,14 planning ability,7,16 processing speed,7,48 motor coordination6,8 and intelligence,13,19 so it is important to evaluate their impact on memory in individuals with FEP. Nevertheless, the extent to which memory impairment in such patients could be influenced by problems in these cognitive domains has not yet been examined. Among the very few studies that could be related to these discoveries is the one by Silver et al.,51 which was intended to find out whether the deficits in working memory (verbal and spatial) underly other neuropsychological deficits in schizophrenics. They found a correlation of this cognitive variable with visual orientation, visual retention, executive function, simple motor function, visuomotor coordination, memory for objects and face memory. Cirillo and Seidman52 did a review of studies to show the possible influence or relationship of cognitive variables like intelligence or attention with declarative verbal memory. One of these, by Seidman,53 found deficits in verbal memory compared to HC matched for intelligence. Hawkins et al.54 and Gold et al.55 studied the possible influence of attention on verbal memory, and did not find any association among these variables either. However, other studies have shown that when the effect of working memory is controlled for, the differences found between persons with schizophrenia and HC in free memory tasks disappeared.56 These data provide another contribution to the study of the relationships of different cognitive domains in early psychosis, showing and supporting the idea of the relationship of different cognitive domains, such as verbal and visual memory, attention, working memory, processing speed, mental flexibility, planning and intelligence.
Studies that make it possible to demonstrate the scope and precise nature of the relationships among these cognitive variables thus become truly necessary. Attention, working memory, processing speed, mental flexibility, planning ability, motor coordination and intelligence are abilities necessary for coding, storing and recalling verbal and visual information, and therefore, it would be extremely useful to know whether, in fact, verbal and visual memory are altered in patients with FEP, or if on the contrary, intervention programmes and treatments should be directed at improving other cognitive skills to correct such memory problems.
Another of the findings of this study is the influence that previous use of cannabis has on short-term visual memory in Child and Adolescent FEP. Our results showed that patients who had used cannabis took less time to reproduce the figure from memory than those who had not, to the point where their performance approached that of healthy subjects. However, this effect of improved performance in cannabis users was not observed in the controls. The only study we found that had comparable results is the one done by Yücel et al., published in 2010,7 which found that FEP patients who used cannabis performed better than those who did not in visual memory. However, in that study the sample of patients who used it had a mean age of twenty, and could not be considered children or adolescents.
To date, most of the studies that have attempted to clarify the role of cannabis in the neuropsychological performance of patients with FEP have been done on adult patients.57–59 In fact, only one study was found that concentrated on showing the influence of cannabis use before the appearance of the first episode on cognitive functioning in a sample of children and adolescents in FEP.4 This group found that patients who had taken cannabis had better executive functioning and attention than those who had not, but not verbal memory or working memory. These findings are along the same line as those in this study, since we did not find that use of cannabis influenced verbal memory in patients with early psychosis either.
This paradoxical effect of cannabis has been reported in several studies.4,28,60,61 One of the most recent major studies demonstrating this paradoxical effect, done by James et al., 2011,62 showed that cannabis use in early adolescence increases white and grey matter deficits in adolescent onset schizophrenia.
These findings should be interpreted in the context of several limitations. One of them has to do with the groups formed, which had to be uniform and matched for age and education. In the second place, it would be desirable for the small sample size to be increased. Third, it would be advisable for the same cognitive domain to be measured by different neuropsychological tests to make the results more consistent and reliable, since discrepancies among different studies could be due to the cognitive tests used.50 Another possible limitation is that the same subtest was used to assess different cognitive domains (i.e., subtests from the WISC and WAIS were used to assess individual covariates and IQ). Finally, this study should also have checked the possible effect of other substances.
As future lines of work, the differences between patients who use cannabis and those who do not might be analyzed for positive or negative symptoms63 and premorbid adjustment,64 and finally, studies should have larger sample sizes.
ConclusionIn conclusion, although the patients showed problems in learning, coding, storing and short and long-term recall of verbal information and short-term visual reproduction, delayed recognition of verbal information was not impaired. In the second place, a relationship was found between the cognitive domains of verbal and visual memory, attention, working memory, processing speed, mental flexibility, planning and intelligence. Third, use of cannabis by early onset psychotic patients was associated with better efficiency in recall of the visual information in Child and Adolescent FEP, but there were no significant differences in the accuracy or the detail of the information that was recalled, and therefore, fewer deficiencies than HC. Finally, the data in this study provide additional support for new classifications of mental illness, according to which cognitive impairments are established as additional criteria for the diagnosis of schizophrenia.2 Furthermore, Child and Adolescent FEP is becoming consolidated as an opportunity to find out how the mechanisms involved in illness can affect cognitive function and its development.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed written consent to participate in this study.
Right to privacy and informed consentThe authors acquired the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of these documents.
FundingThis study was supported by Grant GI8374199 (Ayudas económicas para el desarrollo de proyectos de investigación sobre drogodependencias 2007, BOE 263, 2 November 2007), by a grant from the Fundación Alicia Koplowitz, BAE 09/90088, Estancia Formativa de la Junta de Andalucia 2010, Investigacion Biomedica y en Ciencias de Salud en Andalucia 2007.
They had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Conflict of interestThe authors declare that they have no conflicts of interest regarding this study.
Please cite this article as: Moreno-Granados JM, Ferrín M, Salcedo-Marín DM, Ruiz-Veguilla M. Evaluación neuropsicológica de la memoria en un grupo de niños y adolescentes con un primer episodio de psicosis: consumo de Cannabis y «efecto paradójico». Rev Psiquiatr Salud Ment (Barc.). 2014;7:13–24.