Selecting the most effective treatment represents a critical challenge with the potential of modifying the long-term prognosis of individuals suffering a first break of psychosis. Head-to-head clinical trials comparing effectiveness among antipsychotic drugs in individuals with a first-episode of non-affective psychosis (FEP) are scarce.
MethodsThe rationale and design of a 3 phases clinical trial (PAFIP-3, NCT02305823) comparing the effectiveness of aripiprazole and risperidone, and to additionally assess the benefits of an early use of clozapine in primary treatment-resistant patients is reported. The design encompasses of 5 work packages (medication algorithm, cognitive functioning, psychoeducation/vocational functioning, imaging and biological markers) addressing critical issues and needs of first episode psychosis individuals and their cares. The primary outcome measure was treatment effectiveness assessed by all-cause treatment discontinuation rate.
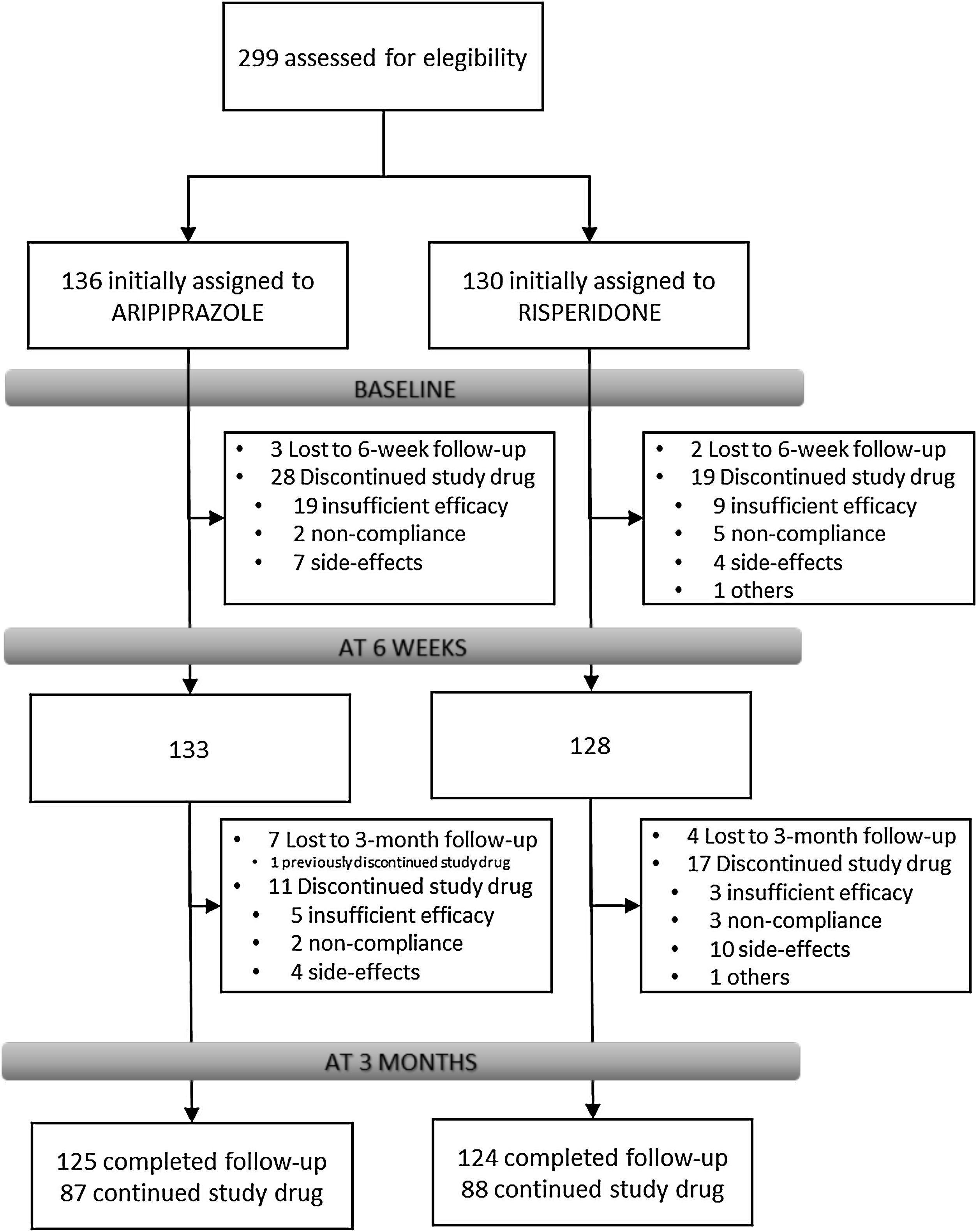
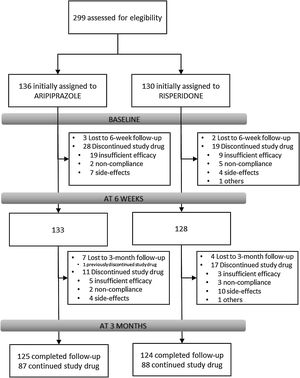
Results266 individuals have been included in the randomization study phase I (risperidone vs. aripiprazole). At 3 months, the retention rate was of 94% (249/266), 48(19.3%) patients have gone through phase II (olanzapine treatment), and 7(2.8%) entered the clozapine phase (phase III).
DiscussionThe PAFIP 3 clinical trial may provide relevant information about clinical guidelines to optimally treat patients with a first episode of non-affective psychosis and the benefits and risks of an early use of clozapine in treatment resistant patients.
Clinicaltrials.gov: NCT02305823.
Selecting the most suitable therapeutic strategies and making appropriate clinical decisions about individuals at the early phases of psychosis is a transcendent undertaking, with undoubtful impact on illness outcome.1 Despite advances in the knowledge on the differential effectiveness patterns among distinct antipsychotic treatments for the early treatment of non-affective psychosis,2 and the critical impact that first antipsychotic choice has for the outcome of the disease,3 randomized clinical trials establishing head-to-head effectiveness comparisons among antipsychotic drugs in individuals with a first-episode of non- affective psychosis (FEP) are scarce.4 Equally scarce are the studies that address the need to establish algorithms that assist clinicians for making decisions on how and when to switch the initially prescribed medication in FEP patients after failing to reach clinical response.5
Clinicians dealing with FEP individuals should be fully aware of the necessity of a rapid titration in order to achieve an adequate dose during the first days of treatment but also to switch or implement treatment-augmentation strategies to prevent for long-term exposure to an antipsychotic that is very unlikely to be efficacious. Lack of clinical efficacy is among the reasons that might prompt people with a FEP to treatment discontinuation.6 Most of schizophrenia patients who do not even show a minimal response (20% reduction in the PANSS/BPRS score) at the 2-week assessment will not show clinical response at 6-week or later, although the application of these results seems to be more appropriate for people who are not in their first episode of non-affective psychosis.2 In a previous research, Agid et al.7 described only a 20% responsiveness among subjects who did not respond to the first-line antipsychotic drug, whereas the efficacy rate of clozapine in these nonresponsive subjects was 75%. Similarly, Kahn and colleagues reported that among first episode individuals who did not exhibit a good response to two lines of antipsychotics, fully an additional 28% achieved remission with clozapine.5 Therefore, early after a lack of response, a second antipsychotic trial and then the use of clozapine may be indicated when other issues such as adherence or co-morbidity have been addressed.
Despite clinical evidence showing the efficacy of clozapine in treatment-resistant patients, it is usually initiated after a lengthy (years) time-lapse from the first diagnosis of treatment resistance.8 During this time, most patients may receive up to 13 antipsychotics, alone or in diverse combinations,9 worsening the disease's prognosis and increasing the side effect burden.10 Clozapine is underemployed with only an estimated 20–50% of eligible patients receiving clozapine.11 In spite of the availability of specialized resources for clozapine treatment, concerns about adherence, side effects, partial compliance with blood monitoring and lack of professional safety, lead many psychiatrists to preclude its prescription.12 Early psychosis intervention services are appropriate for identifying individuals with treatment-resistant schizophrenia and begin on clozapine if indicated and appropriate.13
Research hypothesisProspective studies exploring large sample sizes to compare the effectiveness of widely used second generation antipsychotics (SGAs) to treat people suffering a first episode of non-affective psychosis (FEP) are necessary. The first aim of this clinical trial is to elucidate whether aripiprazole or risperidone may have a distinct effectiveness profile in this population. As a secondary aim, we intend to reveal whether an early switch to olanzapine in case of non-response and, if this change of treatment does not work, the introduction of clozapine in primary resistant patients would be of any benefit at medium or long term.
PAFIP clinic model and standard of care (references)Programa Asistencial de Fases Iniciales de Psicosis (PAFIP) was initiated back in 200114 (PAFIP1 from 2001 to 2005; PAFIP2 from 2005 to 2011). It is a team-based approach grounded on multi-element conceptualization to address the unique needs and recovery goals of individuals suffering a first break of non-affective psychosis and their caregivers.15,16 Component interventions include community-based psychiatric care and evidence-based phase-specific interventions. It is a collaborative and recovery-oriented approach establishing a positive therapeutic alliance and maintaining engagement with clients and their caregivers for at least three years. PAFIP intends to be also sufficiently coordinated with primary medical care, with a focus on preserving physical health of clients.
Study settingPAFIP-3 study was conducted at the outpatient clinic and the inpatient psychiatric unit of the University Hospital Marqués de Valdecilla (Santander), Spain. This hospital is the reference center for a catchment area with a population of around 580,000 people. It provides the only psychiatric acute inpatient unit and the only 24-h psychiatric emergency care service for the whole catchment area. To guarantee the inclusion of all the patients suffering a FEP, regular meetings with mental health care providers in the study catchment area (covering mental health areas of Santander and Laredo) were maintained. The study protocol was approved by the ethics committee of Cantabria. Conforming to international standards for research ethics, this program was approved by the local institutional review board. Patients meeting inclusion criteria provided written informed consent prior to their inclusion to the program.
PAFIP-3 trial designThe PAFIP-3 trial (NCT02305823) consists of 5 work packages (medication algorithm, cognitive functioning, psychoeducation/vocational functioning, imaging and biological markers) addressing critical issues and needs of first episode psychosis individuals and their cares.
Medication algorithmThis work package (Fig. 1) addresses mainly the following aims:
- 1.
To compare head-to-head the effectiveness, efficacy and safety of aripiprazole and risperidone in the treatment of FEP subjects in real-world clinical settings.
- 2.
To elucidate whether non-responders after 3-week adequate treatment with aripiprazole or risperidone, may benefit from a switch to olanzapine (an antipsychotic with a different pharmacological profile).
- 3.
To assess the effectiveness of the early use of clozapine in primary treatment-resistant non-affective psychosis patients.
The following description focuses on the methodology conducted in the antipsychotic treatment trial.
This clinical trial supplies valuable knowledge for making the best possible decisions for the optimal treatment (efficacy and safety) during a critical period such are the early stages after first break of psychosis for the outcome of individuals suffering a FEP.
Neurocognition assessmentsTrained neuropsychologists carried out the neuropsychological assessments after clinical stabilization, which resulted in an average of 10.5 weeks after treatment initiation. Tests were grouped under the following cognitive domains (consistently shown to be impaired in schizophrenia): information processing speed, motor dexterity, working memory, verbal learning, visuospatial abilities, delayed memory, attention, executive function, and theory of mind. A detailed description has been reported elsewhere.17 The aim of this work package was to compare head-to-head neurocognitive effectiveness of aripiprazole and risperidone in the treatment of FEP patients.
Psychoeducation/vocational functioningAll participants were invited to participate in evidence-based phase-specific interventions: psychoeducation for patients and their families, and support for functional recovery based on return to normal daily activities (work/study) with the expectation of a full recovery.
Individual psychoeducation consisted in a six visits intervention, scheduled once a week, addressing the following content: 1. Psychoeducation aims; 2. What is psychosis?; 3. Warning signs; 4. How is psychosis treated?; 5. How to cope with psychotic symptoms? 6. Late suggestions.
In addition, multiple-family group psychoeducation for FEP patients and their families, consisting in a weekly 2-h session during 8 weeks shared with the group members. Socialization and bond strengthening techniques were built into the presentations. Education and group processes were used equally. Patient and family responses to the psychoeducational model were overwhelmingly positive.
In order to help with functional recovery, each case was individually addressed by contacting with public education and leisure centers, and Cantabrian employment service.
Brain imagingBrain imaging was introduced to study the relationship between brain structure features and the use of aripiprazole, risperidone, or olanzapine medications. A magnetic resonance (MR) scan was offered to all patients as soon as they could tolerate it to minimize prior medication exposure. All images were acquired at the Neuroradiology Department of Marqués de Valdecilla University Hospital at the same 3T MRI scanner. Neuroimaging protocol included a high resolution T1-weighted image and a 64 directions DWI sequence. The data extracted from these acquisitions permitted us to extract gray matter morphological features and white matter properties. At 3-year follow-up, patients with MRI at baseline were offered to perform a second MR scan intended to assess the potential effect of pharmacological treatment on biological imaging endophenotypes.
Biological markers of treatment responseThe efforts to discover and develop prognostic biomarkers for treatment efficacy in schizophrenia, based on peripheral blood, plasma, or serum parameters have not led to any core feasible candidate markers so far. Thus, several genetic (GWAS, gene expression, etc.), immunoinflammatory (cytokines profiles, Toll Like Receptors (TLRs), etc.), oxidative (glutathione peroxidase (GPx), thioredoxin/peroxiredoxin (Trx/Prx), etc.), and hormonal (PRL, etc.) biomarkers were recorded and later analyzed in order to further investigate the association of emergent biomarkers with the efficacy of conventional or innovative therapies. We aim to explore likely effects of antipsychotics on D2 receptor-independent mechanisms through indirect biological mechanisms (redox, inflammatory, etc.) that could regulate the mechanisms downstream of the GPC receptors targeted by antipsychotics. These innovative approaches may guide to finding out new explanations for pathophysiological mechanisms and novel approaches for therapy.
Overview of study designParticipantsFrom February 2011 to October 2018, all referrals to PAFIP were screened for patients who met the following criteria: (1) 15–60 years old; (2) living in the catchment area (mental health areas of Santander and Laredo, Cantabria, Spain); (3) experiencing their first episode of psychosis; (4) no prior treatment with antipsychotic medication (antipsychotic naïve) or, if previously treated, a total lifetime of adequate antipsychotic treatment of less than 6 weeks; (5) DSM-IV criteria for brief psychotic disorder, schizophreniform disorder, schizophrenia, psychotic disorder not otherwise specified, or schizoaffective disorder. Patients were excluded for any of the following reasons: (1) meeting DSM-IV criteria for drug dependence; (2) meeting DSM-IV criteria for mental retardation; (3) having a history of neurological disease or head injury. The diagnoses were confirmed using the Structured Clinical Interview for DSM-IV18 carried out by an experienced psychiatrist 6 months on from the baseline visit. Our operational definition for a “first episode of non-affective psychosis” included individuals with a nonaffective psychosis (meeting the inclusion defined above) regardless the duration of untreated psychosis. Of 299 drug-naïve individuals who were initially randomized to treatments, 33 were finally removed from the dataset after verifying they did not fully meet inclusion criteria or removed proper written consent during the first week. Thus, the final sample consisted of 266 participants who were randomly assigned to 2 different antipsychotic treatments: 130 patients were randomly assigned to the risperidone group, while 136 patients were assigned to the aripiprazole group (see Fig. 1). At study intake, out of 266 of individuals 37 (14.2%) reported some prior antipsychotic treatment (mean duration of prior treatment=1.5 weeks, SD=1.3, range=0.4–5.0). Before starting on the initially assigned drug, those participants under current antipsychotic treatment underwent a 2–4-day washout period.
Study designThis is a prospective, randomized, flexible-dose, open-label clinical trial. We used a simple randomization procedure. An automated randomization list was drawn up. Demographic and clinical characteristics, comorbidities, and psychiatric history were collected at intake, along with prior health care resource utilization and patient-reported outcomes. The key outcomes assessed were antipsychotic use and all-cause treatment discontinuation, health care resource utilization, clinical status (symptoms and side effects), symptom remission, relapse rate, suicidality, social, vocational, and cognitive functioning. The severity scale of the Clinical Global Impression (CGI) scale,19 the Brief Psychiatric Rating Scale (BPRS) (expanded version of 24 items),20 the Scale for the Assessment of Positive symptoms (SAPS),21 the Scale for the Assessment of Negative symptoms (SANS),22 the Calgary Depression Scale for Schizophrenia (CDSS),23 and the Young Mania Rating Scale (YMRS)24 were used to evaluate clinical symptomatology. The scale of the Udvalg for Kliniske Undersogelser (UKU) (Committee of Clinical Trials),25 the Simpson-Angus Rating Scale (SARS),26 and the Barnes Akathisia Scale (BAS)27 were used to assess side effects. Clinical assessments and measurements are intended to be completed via face-to-face interview at baseline, 3 weeks, 6 weeks, 12 weeks, 6 months, 12 months and at 3-year follow-up. Those patients who were lost to follow-up were considered as drop-out cases.
Study flowIndividuals with inclusion criteria diagnoses were randomized to open-label aripiprazole or risperidone flexible doses for 3 weeks (phase I). Mean antipsychotic doses expressed as chlorpromazine equivalents (CPZeq; mg/d)28 were as follows: risperidone 3–6mg/d (300–600CPZeq) and aripiprazole 5–30mg/d (100–600CPZeq). Rapid titration schedule (5 days), until optimal dose was reached, was used as a rule unless severe side effects occurred. At the treating psychiatrist's discretion, the dose and type of antipsychotic medication could be changed based on clinical efficacy and the profile of side effects during the follow-up period. Those patients who either did not show at least a 30% reduction of total BPRS score from baseline to 3-week assessment or either suffered severe side effects (UKU scale) that preclude clinicians to maintain the initially prescribed antipsychotic, were switched to olanzapine flexible dose of 5–20mg/d (100–600 CPZeq) for an additional period of 3 weeks (phase II). Those who did not achieve a significant clinical response (minimum of 30% reduction of total BPRS score at intake) despite switching antipsychotic treatment or developed severe adverse events, entered an open label trial with clozapine 100–900mg/d (150–1350 CPZeq) (phase III). Finally, those patients who failed to achieve a significant symptomatic response (as previously defined) after clozapine trail (at least 12 weeks on clozapine with plasma levels above 350ng/mL), may be switched back to treatment as usual according to clinician's criteria. Doses could be adjusted as clinically indicated within the prescribed range to target the minimum effective dose. Certain concomitant medications (lormetazepam and clonazepam) were permitted for the management of agitation, general behavior disturbances, and/or insomnia. Only if clinically significant extrapyramidal symptoms occurred, anticholinergic medication (biperiden at a dose of up to 8mg/day) was allowed. Antidepressants and mood stabilizers were permitted if clinically needed.
MeasurementsPrimary outcome measures: treatment effectivenessThe main outcome of effectiveness was the all-cause treatment discontinuation rate, which is the percentage of all-cause discontinuation of the initially assigned treatment and the mean time to all-cause medication discontinuation. Four reasons for the discontinuation were recorded: (1) nonsufficient or insufficient efficacy, (2) significant side effects, (3) nonadherence, and (4) other causes. Insufficient efficacy was established at the treating physician's judgment only after at least 3 weeks of adequate treatment after failing to reach at least a 30% reduction of total BPRS. Adherence to antipsychotic drugs was assessed by the information obtained from patients, close relatives, and staff involved in the follow-up. Patients were consensually dichotomized into having a good (defined as patients regularly taking at least 90% of prescribed medication) and a poor adherence (medium or poor compliance).2
Secondary outcome measures: efficacy and safetyThe efficacy outcomes were the mean change from baseline to 6 weeks in BPRS, SAPS, and SANS total scores. Additional analyses included changes from baseline to 6 weeks in CGI, YMRS, and CDSS total scores. Patients were defined as responders to the optimum dose of antipsychotic if they had a ≥50% reduction of BPRS total score and a CGI severity-score ≤4 after 6 weeks since the beginning of the treatment. Side effects were evaluated using the UKU side effects rating scale. Only side effects rated as moderate or severe and with a possible causal relationship to medication were recorded. Treatment-emergent akathisia and extrapyramidal symptoms were assessed using BAS and SARS scales.
Statistical analysisAll data were tested for normality (using Shapiro–Wilk test) and equality of variances (using Levene test). To ensure group comparability, baseline sociodemographic and clinical characteristics were tested by 1-way ANOVA or Kruskal–Wallis tests for continuous variables or by chi-squared tests for qualitative variables.
Kaplan–Meier survival curves and log-rank tests were used to assess time to all-cause medication discontinuation at the three phases. Concerning these 2 analyses, patients were followed-up from the inclusion in the study until discontinuation of the initial treatment or censoring. Survival time could be censored by the end of the study period or by lost to follow-up.
For efficacy and safety measures, we performed both intention-to-treat analyses and per-protocol analyses. Differences between groups in the degree of change in clinical scores from baseline were evaluated with ANCOVA after baseline scores were controlled. Comparisons of the discontinuation rates and the prevalence of side effects as well as the use of concomitant treatment between the 2 antipsychotics were carried out, performing chi-squared tests. R 3.6.1 was used for statistical analysis. Statistical tests were 2-tailed with a 95% confidence interval.
Study progress and outlookAs showed in Fig. 1, 266 individuals have been included in the randomization study (phase I) of the clinical trial. At 3 months, the retention rate was of 94% (249/266), 48(19.3%) patients have gone through phase II (olanzapine), and 7(2.8%) entered the clozapine phase. Since the last patient was included in October 2018, short- and medium-term analyses and reports are expected shortly. The 3-year follow-up assessment will be completed in 2021, and long-term outcome results can be expected by 2022.
Our sample size calculation was based on results from previous studies. These studies were comparable with the current study in terms of length of follow-up, intervention used and proposed primary outcome. The plan was to randomize 250 patients (including 20% inflation for dropouts) on risperidone and aripiprazole (1:1). Completion rates at 3-month follow-up of>90% in previous clinical trials from our group have been reported, so we estimated there would be 200 study completers (i.e. 100 completers in each treatment group) at 3-month.
Response was defined as a>30% improvement on the BPRS. To detect a response of 30% in BPRS total score, with 80% power, an alpha of 0.05 and standard deviation of 10, we required 92 participants per group (two-sided).
Authors’ contributorsDr. Jacqueline Mayoral-van Son, Dr. Marcos Gómez-Revuelta, Victor Ortiz-García, Dr. Javier Vázquez- Bourgon, Dr. Rosa Ayesa-Arriola collected clinical data. Jacqueline Mayoral van Son and Marcos Gómez-Revuelta interpreted the results and drafted the manuscript. Victor Ortiz-Garcia de la Foz performed the statistical analyses and drafted the manuscript. Prof. Benedicto Crespo-Facorro interpreted the results and reviewed the manuscript. Dr. Esther Setién-Suero, Dr. Miguel Ruiz-Vegilla, Dr. Nathalia Garrido and Dr. Diana Tordesillas help in the interpretation of clinical data and reviewed the manuscript. The corresponding authors had access to all study data.
All the authors have reviewed the final version of the manuscript and have approved it.
Funding sourceThe present study was carried out at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain.
Unrestricted educational and research grants from AstraZeneca, Pfizer, Bristol-Myers Squibb and Johnson & Johnson provided support for PAFIP activities.
No pharmaceutical industry or institutional sponsors participated in the study conception and design, data collection, analysis and interpretation of the results, or drafting of the manuscript.
Conflicts of interestDr. Mayoral-van-Son, Dr. Gómez-Revuelta, Dr. Ayesa Arriola, Dr. Vázquez-Bourgon, Dr. Miguel Ruiz-Veguilla, Dr. Nathalia Garrido, Dr. Tordesillas-Gutiérrez, Dr. Setién-Suero, and Mr. Ortiz-García de la Foz, report no conflicts of interest. Prof. Crespo-Facorro has received unrestricted research funding from Instituto de Salud Carlos III, MINECO, Gobierno de Cantabria, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), from the 7th European Union Framework Program and Lundbeck. He has also received honoraria for his participation as a consultant and/or as a speaker at educational events from Janssen, Mylan, Lundbeck, and Otsuka Pharmaceuticals.