Neurological correlates of impaired insight in non-affective psychosis remain unclear. This study aimed to review and meta-analyze the studies assessing the grey matter volumetric correlates of impaired insight in non-affective psychosis.
MethodsThis study consisted of a systematic review of 23 studies, and a meta-analysis with SDM-PSI of the 11 studies that were whole-brain and reported maps or peaks of correlation of studies investigating the grey matter volumetric correlates of insight assessments of non-affective psychosis, PubMed and OVID datasets were independently reviewed for articles reporting neuroimaging correlates of insight in non-affective psychosis. Quality assessment was realized following previous methodological approaches for the ABC quality assessment test of imaging studies, based on two main criteria: the statistical power and the multidimensional assessment of insight. Study peaks of correlation between grey matter volume and insight were used to recreate brain correlation maps.
ResultsA total of 418 records were identified through database searching. Of these records, twenty-three magnetic resonance imaging (MRI) studies that used different insight scales were included. The quality of the evidence was high in 11 studies, moderate in nine, and low in three. Patients with reduced insight showed decreases in the frontal, temporal (specifically in superior temporal gyrus), precuneus, cingulate, insula, and occipital lobes cortical grey matter volume. The meta-analysis indicated a positive correlation between grey matter volume and insight in the right insula (i.e., the smaller the grey matter, the lower the insight).
ConclusionSeveral brain areas might be involved in impaired insight in patients with non-affective psychoses. The methodologies employed, such as the applied insight scales, may have contributed to the considerable discrepancies in the findings.
Los correlatos neurológicos de la conciencia de enfermedad en psicosis no afectivas siguen sin estar claros. Este estudio tiene como objetivo revisar y metaanalizar los estudios que evalúan los correlatos volumétricos de la materia gris de la conciencia de enfermedad deficiente en la psicosis no afectiva.
MétodosEste estudio consistió en una revisión sistemática de 23 estudios y un metaanálisis con SDM-PSI de los 11 estudios que examinaron todo el cerebro y reportaron mapas o picos de correlación de estudios que investigan los correlatos volumétricos de materia gris de evaluaciones de insight de psicosis no afectiva. Los conjuntos de datos de PubMed y OVID se revisaron de forma independiente para los artículos que informaban sobre correlaciones de neuroimagen de insight en psicosis no afectiva. La evaluación de la calidad de los estudios de imagen se realizó siguiendo enfoques metodológicos previos usando la prueba de evaluación de la calidad ABC basados en dos criterios principales: el poder estadístico y la evaluación multidimensional del insight. Los picos de correlación del estudio entre el volumen de materia gris y la conciencia de enfermedad fueron utilizados para recrear mapas de correlación cerebral.
ResultadosSe incluyeron veintitrés estudios de imágenes por resonancia magnética (IRM) que utilizaron diferentes escalas de conciencia de enfermedad. La calidad de los estudios revisados fue clasificada como alta en 11 estudios, moderada en 9 estudios y baja en 3 estudios. Los pacientes con insight reducido mostraron disminuciones en el volumen de materia gris cortical de los lóbulos frontal, temporal (específicamente en la circunvolución temporal superior), precúneo, cingulado, ínsula y lóbulo occipital. El metaanálisis mostró una correlación positiva entre el volumen de materia gris y la conciencia de enfermedad en la ínsula derecha (es decir, cuanto más pequeña es la materia gris, menor es el insight).
ConclusionesVarias áreas del cerebro pueden estar involucradas en la conciencia de enfermedad en pacientes con psicosis no afectivas. Tanto las metodologías empleadas como las escalas para valorar la conciencia de enfermedad aplicadas pueden haber contribuido a las considerables discrepancias en los hallazgos publicados.
Schizophrenia is a severe psychiatric disorder characterized by positive (delusions and hallucinations) and negative symptoms (affect, anhedonia, avolition, alogia, and social withdrawal), cognitive impairment, and poor insight, that severely impair patients’ everyday life.1,2 Insight is commonly interpreted as the patients’ ability to understand their own illness, but it is much more. It can also be defined as understanding how much the illness affects personal,3 occupational and social functioning, as well as managing the illness itself. The word ‘insight’ refers to a complex concept that should not be considered as an isolated symptom that is present or absent in a binary way. Conversely, it may be more appropriate to refer to insight as a continuum of feeling, influenced by several internal and external stimuli.3 Poor illness insight has been linked to low treatment adherence, more severe clinical outcomes, and severe cognitive and social cognition deficits, and functional performance.4–7 Therefore, improving and understanding insight physiopathology may be a milestone in diagnosis and treatment management for patients affected by schizophrenia. A patient's insight into his own illness in schizophrenia is a phenomenon at the clinical level that includes several dimensions, such as acknowledging the illness and of the symptoms, symptom attributions, beliefs about the need for treatment, and awareness of the consequence of illness and treatment.8,9 Phenomenologically, the lack of insight is one of the most prevalent symptoms of schizophrenia.10 The importance of understanding impaired insight in schizophrenia stems from poor treatment adherence,11 and its association with low cognitive and social functioning,12 high relapse rates, involuntary hospitalization,13 and overuse of emergency services. Furthermore, poor insight has been associated with increased suicide risk.14 One of the main hypotheses attributed a lack of insight to a defense mechanism, which may help patients adapt to their symptoms.15 Another clinical hypothesis focused on the independence insight from the underlying positive and negative symptoms.8 More recent frameworks suggested that the lack of insight could be a manifestation of the illness itself with explicit neurobiological correlates.16 Within this realm, neuroimaging studies indicated diverse brain alterations in patients with versus without impaired insight, suggesting specific neurobiological patterns underpinning lack of insight.17 Nevertheless, findings from magnetic resonance imaging (MRI) studies bear inconsistencies.18–20 The current neurobiological basis of understanding insight is based on the anosognosia model, suggesting the implication of several distributed cortical correlates of insight; related alterations have been reported in dorsolateral prefrontal, orbitofrontal, anterior cingulate, and parietal cortex.21 Conversely, methodological evaluation of insight has improved, and several scales of assessment have been developed. For a brief time, the Scale Unawareness of Mental Disorders (SUMD)22 was the most popular instrument, followed closely by the Scale Assessment of Insight-Expended (SAI-E)23 and the Birchwood Insight Scale (BIS).24 In addition, some studies assessed insight using a single item of the well-established Positive and Negative Symptom Scale (PANSS lack of Insight and Judgement item (PANSS G12)).25–28
With one exception,29 studies have assessed brain imaging correlates of impaired insight in patients with chronic schizophrenia.
Moreover, a previous work investigated the relationship between insight in first episode psychosis (FEP) and the executive functions deficit related to poor insight.30 Tordesillas-Gutierrez et al.29 observed smaller grey matter volumes in the right occipital lobe in first-episode patients (FEP) with poor insight compared to FEP with good insight. These alterations in the right hemisphere aligned with the right hemisphere dysfunction, which is suggested by the anosognosia model.31 The rest of the studies have mainly included patients with chronic schizophrenia. The long duration of the illness introduces a possible bias due to multiple confounders, such as long-term use of antipsychotic drugs, extended periods of substance use, and psychiatric comorbidity for these findings.32 Therefore, studies of FEP patients are of special interest for the understanding of the causes and the clinical impact of lack of insight.
Considering the heterogeneity for methodological approaches including insight scales, the patient samples and MRI techniques, the aim of this systematic review was meta-analyze grey matter volumetric data for the lack of insight in non-affective psychosis.
MethodsWe followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for the reporting of this systematic review.33 The search strategy is available in the protocol registered with PROSPERO (registration number CRD42020148216).
Search strategyTwo authors (PSM and DTG) independently searched PubMed and OVID databases for research published until March 2020. Reference lists from included articles and past reviews were searched for additional studies, and eight references from these articles were added for later evaluation.
Article screeningTwo researchers (PSM and DTG) independently screened the titles and abstracts of the initially identified articles and subsequently read the articles that met the eligibility criteria. In case of disagreement, a consensus was reached after discussion or the participation of a third reviewer (ESS).
Inclusion and exclusion criteriaFor inclusion, we considered all the original published articles in English that investigated the brain grey matter volume correlates of impaired insight in schizophrenia, schizophreniform disorder, brief psychotic episode and other non-affective psychosis using MRI whole-brain voxel-based morphometry (VBM). The diagnosis must be verified by structured clinical interviews. We excluded non-original papers, such as review papers, abstracts, meeting letters, letters to editors without original scientific content, and commentaries, as well as case reports and non-peer-reviewed articles. Articles that only explore cognitive insight were excluded. Furthermore, studies that used functional or spectroscopy magnetic resonance and theoretical models were excluded. Additional exclusion criteria for the meta-analysis were: (1) peak coordinates were not reported, or t-maps were not available; (2) studies restricting the analysis to a priori small volume corrections; (3) studies reporting findings from multiple ROIs instead of the whole brain.
Data collection and extractionExtracted data included author, year of publication of the study, number of subjects (patients and controls), studied population, age of the patients, insight symptom scale, insight dimension measured, MRI data protocol, statistical design, and key findings (Table 1). Authors were contacted in case of missing data.
Summary of characteristics of included studies.
| Author, year | Patients/controls | Sample | Age patientsMean (SD) | Neuroimaging technique | ISS | MRI data protocol | Statistical threshold | Key findings |
|---|---|---|---|---|---|---|---|---|
| Flashman et al., 2001 | 30/13 | Chronic schizophrenia/healthy | AP: 36.4 (14.9)/UP: 33.9 (9.9) | sMRI | SUMD (1)Awareness of illness and symptoms | 1.5TeslaBRAINS | p<0.05Corrected | No differences between aware and unaware patients after controlling for intracranial volumes, bilateral frontal, temporal, and parietal lobe tissue volumes. |
| Flashman et al., 2000 | 15 | Chronic schizophrenia | 31.9 (11) | sMRI | SUMD (1)Awareness of current episode symptoms | 1.5TeslaROI frontal lobe | p<0.01Corrected | Negative correlation between grey matter volumes in bilateral middle frontal gyri, L ACC and unawareness and misattribution of illness. |
| Shad et al., 2006 | 14/21 | FEP/healthy | 16.23 (2.49) | sMRI | SUMD (2)Average total scores on awareness and attribution | 1.5TeslaBRAINS | p<0.05Corrected | Negative correlations between awareness and R DLPFC as well as attribution and R medial OFC. No correlations between awareness/attribution and L DLFPC and L medial OFC, R or L lateral OFC volumes. |
| Bassit et al., 2007 | 50/30 | Chronic Schizophrenia/healthy | 31.7 (7.1) | sMRI | SUMD (1)Sum of awareness and attribution of symptoms | 1.5TeslaSPM2 | p<0.05Corrected | No correlation between unawareness and misattribution of illness and grey or white matter volumes |
| Bergé et al., 2011 | 21/20 | FEP/healthy | 24.81 (4.3) | sMRI | SUMD (1)Three initial global items | 1.5TeslaSPM 5 | p<0.001Uncorrected | Negative correlation between global items of SUMD scores and grey mater volumes in cerebellum bilaterally, R inferior temporal gyrus, R and L medial superior frontal gyrus, and R inferior frontal opercular gyrus. |
| Buchy et al., 2009 | 54 | FEP | 23.4 (3.7) | sMRI | SUMD (2)Item 1, awareness of mental disorder | 1.5Tesla | p<0.05Corrected | No correlations were detected between unawareness and misattribution of illness and hippocampus volume in R or L hemisphere. |
| Buchy et al., 2011 | 79 | FEP | 23.3 (3.7) | sMRI | SUMD (2)Awareness of illness and composite awareness of treatment need/awareness of treatment efficacy scores | 1.5TeslaCIVET: Cortical thickness | p<0.05Corrected | Negative correlations between awareness of illness and cortical thickness in the L DLPFC (middle frontal gyrus), L IFG, L ITG, L & R precentral gyrus and R IOG. For awareness of treatment need/efficacy negative correlations with cortical thickness in L DLPFC, L medial frontal gyrus, L precuneus, L paracentral lobule, L SMG, L superior, middle and inferior temporal gyri, L parahippocampal gyrus, the L MOG, the R IFG, R precuneus, R parietal & paracentral lobules, R supramarginal gyrus, R superior temporal gyrus, R parahippocampal gyrus, R fusiform & lingual gyri. |
| Buchy et al., 2012 | 52 | FEP | 23.2 (3.8) | sMRI | SUMD (2)Attribution scores aggregated | 1.5TeslaCIVET: Cortical thickness | (p<0.05)Corrected | Negative correlations between hallucination misattribution and L frontal, L inferior temporal and L middle occipital gyri, L precental gyrus, L cingulate gyrus and L parahippocampal gyrus. Positive correlations between hallucination misattribution and R superior, inferior and medial temporal, R inferior parietal lobule, R cingulate gyrus and R parahippocampal gyrus. Negative correlations between delusion misattribution and L frontal gyri. Positive correlations between delusion misattribution in L & R frontal, parietal, temporal and occipital gyri, L & R cingulate gyrus, L uncus, R cuneus. Negative correlations between flat affect misattribution and L frontal gyri, L pre- and postcentral gyrus, L inferior temporal gyrus, L middle occipitalis gyrus, L precuneus, L parahippocampal gyrus. Positive correlations between flat affect attribution and R frontal gyrus, R cuneus. Negative correlations between asociality misattribution and L frontal gyri, L inferior parietal lobule, L parahippocampal gyrus and R precentral gyrus. Positive correlations between asociality misattribution and R ACC and R superior temporal gyrus. |
| Raij et al., 2012 | 21/16 | Chronic schizophrenia/healthy | 24.4 | sMRI/fMRI | SUMD (1)Clinical insight | 3TeslaSPM 8 | p<0.001Corrected | No structural patterns for insight. Negative correlation between insight and activation strengths in L posterior cingulate cortex and L medial prefrontal cortex and R frontopolar cortex. |
| McFarland et al., 2013 | 32 FEP/30 chronic | FEP/chronic schizophrenia | 27.8 (7.6)/35.1 (8.7) | sMRI | SUMD (1)Awareness and symptom misattribution | 1.5TeslaSnPM, ROI nucle caudate | p<0.05Corrected | In FEP negative correlation between symptom misattribution and grey matter excess in the R and L caudate, R thalamus, L insula, L putamen and L cerebellum. No correlation between awareness and grey matter in patients with chronic schizophrenia. |
| Buchy et al., 2017 | 128 | FEP | 24.2 | sMRI | SUMD (2)Sum of questions 1, 2a and 2b. | 1.5TeslaCIVET: CT | p<0.05Uncorrected | No correlation between insight and cortical thickness at baseline to one year and two years follow up. |
| Tordesillas et al., 2018* | 108/77 | FEP/healthy | 26.92 (5.4) | sMRI | SUMD (2)Awareness of a mental disorder | 1.5TeslaSPM5 | p<0.05Corrected | Patients with poor versus good insight differed for grey matter volume in R occipital, cuneus and temporal cortical regions. |
| Morgan et al., 2010 | 82/91 | FEP/healthy | 27.15 (7.58) | sMRI | SAI-ECombinedSAI-E scores | 1.5TeslaAFNI | p<0.01Corrected | No correlations between symptom relabelling or total insight scores and volumetric measures for total, regional GM or lateral ventricular volume for the analysis within patients. Compared to patients with some relabelling ability (n=62), patients lacking relabelling ability (n=20) had reduced GM volumes in L insula, a cluster extending from R putamen to R STG & precentral gyrus, a cluster extending from L precentral gyrus to L STG, a bilateral cluster extending from the posterior cingulate gyrus to superior parietal lobe and a cluster extending from R precuneus to the R medial occipital gyrus. |
| Gerretsen et al., 2015* | 18 | Chronic schizophrenia | 41.7 (5.1) | sMRI/fMRI | SAI-EIllness awareness scores | 1.5TeslaCIVET: CT; LONI: ROI | p<0.05Corrected | After correction for multiple comparisons no correlation between illness awareness and cortical thickness in L angular gyrus. Peak brain activity in the left TPO was positively associated with impaired illness awareness. |
| Emami et al., 2016* | 66/31 | Chronic schizophrenia/healthy | 34.94 (7.96) | sMRI | SAI-EItem-7 | 3TeslaCIVET: CT; LONI: ROI | p<0.05Corrected | No cortical thickness differences between the low- and high-insight patients in the frontal lobe region, or in the superior temporal gyrus on either hemisphere. |
| Asmal et al., 2016 | 92/93 | FEP/healthy | 24.68 (6.75) | sMRI | BISsymptom attribution’ subscale score | 3TeslaFreesurfer: CT frontal. ROI | p<0.01Corrected | Negative correlation between relabelling symptoms and cortical thickness in L and R rostral middle frontal, L and R par triangularis L ACC, R superior frontal. Association between poor symptom attribution and L rostral middle frontal region and L ACC. L medial OFC, bilateral superior frontal, L frontal pole, R rostral middle frontal, R lateral OFC and R superior frontal regions. R rostral middle frontal, L caudal anterior cingulated. |
| Sapara et al., 2007 | 40/20 | Chronic schizophrenia/healthy | 13.45 (10.93) | sMRI | BIS SAI-EInsight into symptoms, illness, need for treatment and consequences. | 1.5TeslaSPM 8 | p<0.05Corrected | Positive correlation in patients with poor illness insight shown less grey matter volume than patients with good illness insight in the inferior frontal and precentral gyri, superior and middle temporal gyri, parahippocampus, cuneus and cerebellum of both cerebral hemispheres. |
| Sapara et al., 2016* | 28/20 | Chronic schizophrenia/healthy | 39 (10.51) | sMRI | BIS“Preserved insight” defined as a minimum score of 13 (out of 14) and “impaired insight” as a score of 8 or below. | 1.5TeslaManual ROI | p<0.05Corrected | Positive correlation between BIS sub-scale of the level of insight into illness and the total prefrontal (IFG, OFG, SFG, MFG) grey matter volumes in both hemispheres. Patients with smaller total prefrontal grey matter volumes were less aware of having a mental illness. SAI-E reflected these relationships most strongly. |
| Cooke et al., 2008* | 52/30 | Chronic schizophrenia/healthy | 38.35 (9.89) | sMRI | BIS SAI-ECombined: four factors of insight, awareness of and attribution to Illness, recognition of the need for medication, awareness of problems and symptom re-labelling | 1.5TeslaSPM2 | p<0.001Uncorrected | Positive correlation in patients with low score on Awareness of and Attribution to Illness shown reduce grey matter volume in L STG and MTG as well as R ITG. In Awareness of Problems there was an association between lower score and reduce grey matter volume in the L precuneus. Symptom Re-labelling shown that there was an association between low score and reduce grey matter volume in the R STG. |
| Ha et al., 2004* | 35/35 | Chronic schizophrenia/healthy | 27.8 (6.2) | sMRI | PANSSG12 | 1.5TeslaSPM 99 | p<0.001 Uncorrected | Positive correlation in patients with higher score on lack of judgement and insight (G12) from the general psychopathology scale demonstrated further grey matter volume reductions in L and R cingulate gyrus and inferior temporal regions including the lateral fusiform gyri. |
| Gerretsen et al., 2013 | 52 | Chronic schizophrenia | 41.5 (14.5) | sMRI | PANSSG12 | 1.5TeslaSPM8 | p<0.001Uncorrected | Positive correlation between patients with higher score on lack of judgement and illness (G12) and reduce R grey matter volumes in the anteroinferior temporal lobe, OFC, and MPO. Only anteroinferior temporal lobe survived p<0.05 corrected. DBM showed associations between lack of illness awareness and reduce R grey matter volumes in the DLPFC, angular gyrus/parietal lobe, and insula. |
| Shad et al., 2006 | 35 | FEP | 25.36 | sMRI | HDRS-Item 22 | 1.5TeslaMRI | p<0.05Corrected | Negative correlation between HDRS-item 22 scores and GM in R DLPFC, but not L DLPFC, L & R hippocampus. |
| Palaniyappan et al., 2011 | 57 | Chronic schizophrenia | 26.10 (7.49) | sMRI | SSPISItem 20 | 3TeslaFreesurfer | p<0.05Corrected | Negative correlation found in R posterior insula area associated with the degree of insight. |
Abbreviations: AFNI: analysis of functional neuroimages; ACC: anterior cingulate cortex; AP: aware patients; BIS: Birchwood Insight Scale; BRAINS: Brain Research Analysis of Images Networks and Systems; CIVET: image processing pipeline for fully automated volumetric; corticometric and morphometric analysis of brain imaging data; CT: cortical thickness; DLPFC: dorso-lateral prefrontal cortex; DBM: deformation-based morphometry; DLPFC: dorso-lateral prefrontal cortex; FEP: first episode psychosis; GM: grey matter; HC: healthy controls; HDRS: Hamilton Depression Rating Scale; HPC: hippocampal area; IFG: inferior frontal gyrus; IOG: inferior occipital gyrus; ISS: insight symptom scale; ITG: inferior temporal gyrus; MFG: middle frontal gyrus; MOG: middle occipital gyrus; MPC: medial prefrontal cortex; MTG: middle temporal Gs; OFC: orbitofrontal cortex; ROI: region of interest; PANSS: Positive and Negative Syndrome Scale; SAI-E: Expanded Schedule of Assessment of Insight; SFG: superior frontal gyrus; SMG: supramarginal gyrus; sMRI: Structural Magnetic Resonance Imaging; SnPM: Statistical Non-Parametric Mapping; SPM: Statistical Parametric Mapping; SSPIS: Symptoms and Signs in Psychotic Illness Scale; STG: superior temporal gyrus; SUMD: Scale to Assess Unawareness of Mental Disorder; UP: unaware patients. (1) Amador et al. (1993); (2) Amador et al. (1994).
Following previous methodological approaches for the ABC quality assessment test of imaging studies,34 we based the study on two main criteria: the statistical power (i.e., sample size) and the multidimensional assessment of insight (i.e., the assessment of different aspects of insight). The rationale for the selection of the sample size as a quality criterion was previously discussed.34 The assessment of different aspects of insight is expected to provide a more sophisticated measure than a single item. The sample sizes were classified as follows: category 1: less than 15 participants per study group; category 2: 15–30 participants per study group and category 3: more than 30 participants per group.
The assessment of insight of studies was categorized as if it was (1) unidimensional (i.e., the inclusion of one single item of a scales in the analysis) and (2) multidimensional covering different aspects of insight (regardless of the number of the applied scales). Totaling the scores, a maximum score of 5 was considered to be quality A (good), while 4 was quality B (moderate), and 3 or below quality C (low).
Meta-analytic methodsTo pool the VBM data, we used Seed-based d Mapping with Permutation of Subject Images (SDM-PSI).35,36 The main advantage of this method is that it directly tests whether there are correlations between VBM and insight, rather than conducting indirect tests such as whether peaks tend to converge in some regions more than in others.37 Additionally, it is unique in both allowing the powerful inclusion of original statistical maps instead of peak coordinates,38 and in allowing the combination of VBM and cortical thickness studies.39 First, we converted all coordinates to a common MNI space. Second, we created the maps of the lower and upper bounds of possible effect sizes for each study based on the level of statistical significance, the coordinates and effect size of the reported peaks, and the anisotropic covariance between adjacent voxels.40 Third, we imputed multiple effect size maps (and the corresponding variance maps) for each study,36 restricted to the FreeSurfer mask.39 Fourth, we combined the images of each dataset of imputed effect size maps using a standard random-effects meta-analysis. Fifth, we combined the meta-analytic maps resulting from the various imputations using Rubin's rules.41 Finally, we imputed the subject images for each study, and we assessed the statistical significance via a permutation test of the subject images.36 We considered statistically significant those voxels with threshold-free cluster enhancement (TFCE)42 familywise error rate (FWER) <0.05, and trend-level those voxel with uncorrected p<0.001 and cluster-extend of 10 voxels. We also assessed the between-study heterogeneity with the I2 statistic (<50% is considered absence of substantial heterogeneity) and conducted Egger tests to evaluate potential publication bias in the findings.
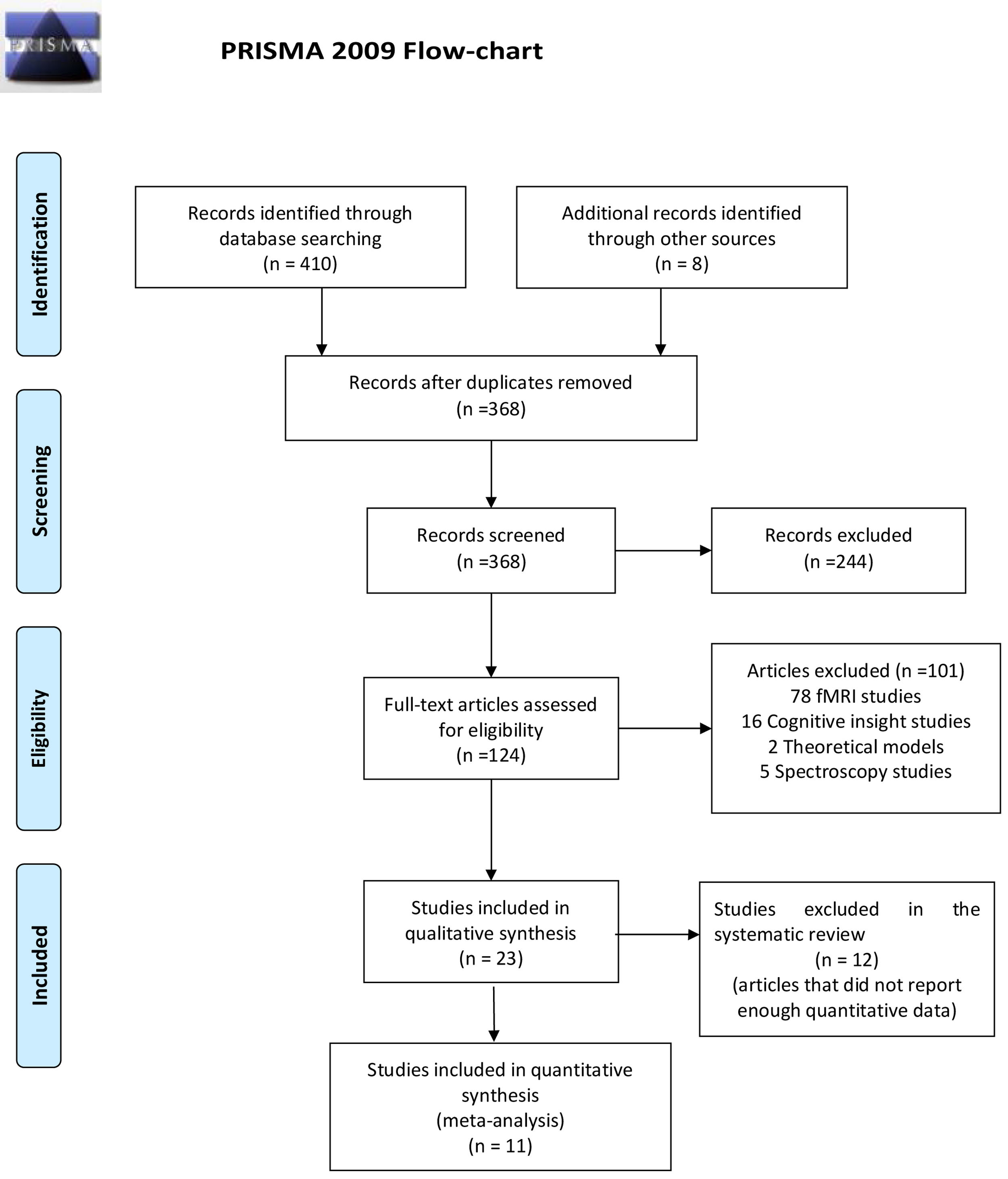
ResultsReview flowThe flowchart of the systematic review is shown in Fig. 1.
Of the 418 articles selected based on the search criteria, we found 50 duplicated, which were then excluded. After reading the title and abstracts, 124 articles were selected as potentially relevant, and 244 studies were excluded because they did not identify a direct relationship between clinical insight, clinical assessment, and GMV. Of these 124 articles, 101 were excluded because they were functional magnetic resonance imagining (fMRI) studies (n=78), cognitive insight (n=16), spectroscopy studies (n=5), and theoretical models (n=2). Finally, 23 articles with a total of 697 FEP, 572 chronic patients and 569 healthy controls were included (Fig. 1). Twelve articles used the SUMD scale,19,29,43–52 three articles used the SAI-E scale,53–55 two articles used the BIS scale,56,57 two articles used BIS and SAI-E scales,58,59 two articles used the PANSS G12 scale,60,61 one article used Hamilton Depression Rating Scale (HDRS)62 and another article used Symptoms and Signs in Psychotic Illness scale (SSPIS).63
Correlations between assessment scales ratings and grey matter volumeRelationship between The Scale to Assess Unawareness of Mental Disorder (SUMD) and grey matter volume alterationsOf the 23 studies reviewed, 10 used the SUMD for the assessment of insight. The early work of Flashman et al.,43,44 assessed the brain imaging correlates of insight deficits in chronic patients using a region of interest approach (ROI).
In their first study in 2000, they investigated whether there were any differences in brain and intracranial volumes of frontal, temporal, and parietal lobe between patients with good and poor insight reporting no differences.44 In their second study, inverse correlations were found between the level of unawareness and grey matter volume in bilateral middle frontal gyrus, right gyrus rectus, and left anterior cingulate gyrus. Furthermore, smaller volumes of both hemispheres’ superior frontal gyrus were associated with higher misattribution scores.43
In an FEP cohort Shad et al.,45 awareness of symptoms was inversely associated with dorso-lateral prefrontal cortex (DLPFC) volume. Moreover, structural alteration in orbitofrontal cortex (OFC) was correlated with the average score on attribution symptoms. Nevertheless, the same study found no GMV differences between control and patients.
Likewise, Bassitt et al.,46 using voxel brain morphometry found no differences between 50 chronic patients and 30 healthy subjects. Conversely, Bergé et al.,47 in FEP patients found a significant inverse correlation between insight impairment and GMV volume in several brain areas including the cerebellum, right inferior temporal gyrus, superior frontal gyrus and right inferior frontal gyrus in 21 drug naïve FEP patients.
Between 2010 and 2017, Buchy and colleagues published several papers on different brain areas in the context of insight in a FEP sample. In 2010, they explored the hippocampus volume in association with verbal memory and cognitive insight without reporting any association.48 A later study reported that poor awareness of illness was associated with regional thinning in left middle frontal and inferior temporal gyrus; on the contrary, unawareness of treatment need and efficacy was associated with cortical thinning in precuneus, left medial frontal gyrus, and temporal gyri.49 Moreover, this group identified an association between thickness in OFC in delusion misattribution, which was associated with thickness in FEP.50 Finally, a longitudinal study on cognitive and neuroimaging predictors of clinical insight reported no interaction in cortical thickness and baseline, one- or two-year follow-up assessments of insight in FEP.
The study by Raij et al.51 in patients with chronic psychosis, found no correlation between brain volumes and insight impairment. This was similar to the study of McFarland et al.,52 which also did not find any significant associations between GMV in any area of the whole brain and the measures of insight in FEP patients. Interestingly, the same article showed a significant association between the insight deficits and symptom misattribution with grey matter thickness in bilateral caudate, right thalamus, left insula, putamen, and cerebellum in FEP patients.
Relationship between Schedule of Assessment of Insight-Expanded (SAI-E) and grey matter volumeThree volumetric studies included the SAI-E for the assessment of insight. One sample investigated a FEP sample53; patients without symptom relabeling ability differed with regard to GMV in the posterior cingulate gyrus and right precuneus compared to patients with relabeling ability. Conversely, Gerretsen et al.,54 comparing aware versus unaware chronic patients reported cortical thickness in left angular gyrus for the illness denial. In chronic psychosis, Emami et al.,55 evidence indicates thinner parahippocampal volume and superior temporal gyrus showed poor insight in patients compared to healthy subjects.
Relationship between Positive and Negative Syndrome Scale (PANSS) and grey matter volumeThe study by Ha et al.,60 found lower grey matter volumes in the left posterior and the right anterior cingulate gyrus using PANSS G1264 in chronic paranoid patients.
Relationship between Birchwood Insight Scale (BIS) and grey matter volumeThe larger sample study by Asmal et al.,56 pointed to a correlation between symptom attribution measured with BIS and frontal cortical thickness. Similarly, the study by Sapara et al.,57 compared stable schizophrenia patients with good or poor insight with healthy controls. The group with good insight showed larger GMV in inferior frontal and precentral gyri, superior and middle temporal gyri, parahippocampus, cuneus, and bilateral cerebellum compared to patients with poor insight showed grey matter reductions compared to healthy controls and awareness patients in the above-mentioned brain areas.
Relationship between BIS and SAI-E scales and grey matter volumeThere were two studies in which BIS and SAI-E were both administered. First, Sapara et al.58 conducted a study with a group of twenty-eight stable chronic patients, examining whether there were differences in prefrontal GMV related to poor insight; smaller prefrontal GMV was associated with a lower level of insight only in males. The second study, run by Cooke et al.59 found lower grey matter volumes in the temporal and parietal regions that have been implicated in self-monitoring, working memory and access to internal mental states are associated with poor insight in a large chronic sample.
Assessment of quality of included studiesEleven studies were classified as high quality (A: sum score 5), nine studies as moderate quality (B: sum score 4) and three studies of low quality (C: sum score 2 or 3), as illustrated in Table 2. Regarding sample size, 15 studies had adequate statistical power, five studies moderate and three studies poor statistical power. In terms of insight assessment, four trials used values of single items for insight evaluation, whereas 19 studies used scales that covered different dimensions of insight.
Quality assessment of included studies according to ABC scale.
| Study | Sample size | Insight dimension | Quality level |
|---|---|---|---|
| Asmal et al. (2016) | 3 | 2 | A |
| Bassit et al. (2007) | 3 | 2 | A |
| Bergé et al. (2011) | 2 | 2 | B |
| Buchy et al. (2010) | 3 | 1 | B |
| Buchy et al. (2011) | 3 | 2 | A |
| Buchy et al. (2012) | 3 | 2 | A |
| Buchy et al. (2017) | 3 | 2 | A |
| Cooke et al. (2008) | 3 | 2 | A |
| Emami et al. (2016) | 3 | 2 | A |
| Flashman et al. (2000) | 1 | 2 | C |
| Flashman et al. (2001) | 1 | 2 | C |
| Gerretsen et al. (2013) | 3 | 1 | B |
| Gerretsen et al. (2015) | 2 | 2 | B |
| Ha et al. (2004) | 3 | 1 | B |
| McFarland et al. (2013) | 3 | 2 | A |
| Morgan et al. (2010) | 2 | 2 | B |
| Palaniyappan et al. (2011) | 3 | 2 | A |
| Raij et al. (2012) | 2 | 2 | B |
| Sapara et al. (2016) | 2 | 2 | B |
| Sapara et al. (2007) | 3 | 2 | A |
| Shad et al. (2006) | 3 | 1 | B |
| Shad et al. (2007) | 1 | 2 | C |
| Tordesillas et al. (2018) | 3 | 2 | A |
Notes. Sample size: 1 <15 participants/group, 2=15–30 participants/group, 3 >30 participants per group. Insight dimension measured: 1=unidimensional, 2=multidimensional. Quality level: A (sum score 5)=good, B (sum score 4)=medium. C=(sum score 3 or 2)=low.
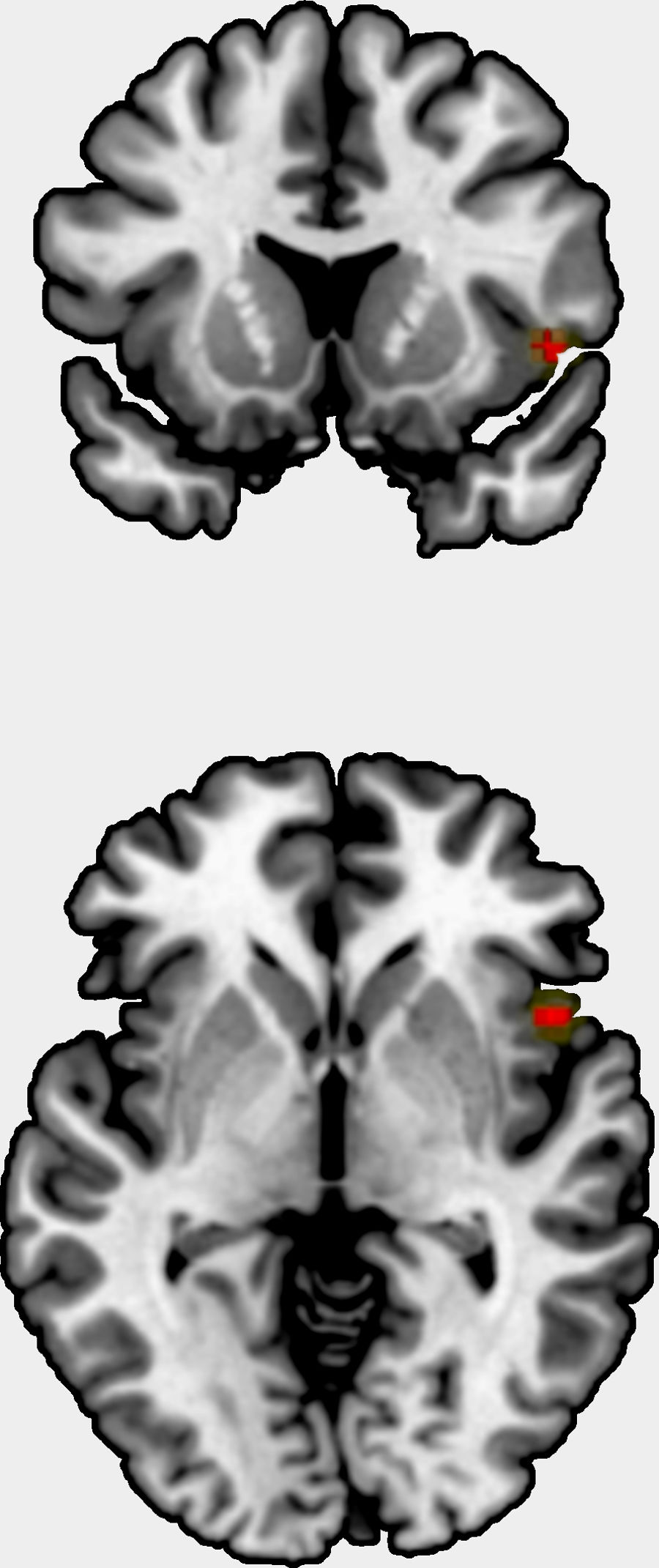
We could include 11 studies to the meta-analysis, comprising one original statistical map,65 eight studies reporting several peaks, and three studies with no findings. The studies comprised 710 patients, and the original statistical map 264 patients. The meta-analysis showed a positive correlation between grey matter volume/cortical thickness and insight in right insula, Brodmann area 48 (peak at MNI=[48,14, −2], Z=3.8, uncorrected p=0.00006, Fig. 2). This result showed neither substantial heterogeneity (I2=2.4%) nor hints of publication bias (Egger's test p=0.79), and it was not statistically significant after FWER correction for multiple voxel testing.
DiscussionTo the best of our knowledge, this report is the largest review concerning studies on insight and GMV findings in non-affective psychosis using magnetic resonance imaging (MRI).
The meta-analysis showed an uncorrected positive correlation between grey matter volume/cortical thickness and insight in the right insula. As a major component of the “limbic integration cortex,” the insula is widely interconnected with cortical and limbic areas.66
The insula is a key component of a general salience network,67 prompting us to believe that insular dysfunction and alterations between network interactions might be characteristic of schizophrenia. The decrease in grey matter volume has been associated with neurological soft signs in ultra-high risk subjects.68 As such, because the insula plays a role in self awareness,69 it could also be linked to a patient's ability to recognize that his or her symptoms stem from the illness.
In the systematic review of 23 studies, 15 revealed that there are mainly two brain areas affected: The frontal and temporal lobes. That said, the studies also indicated that reductions were reported in midline, precuneus, insula, and occipital cortical volumes. Each brain area is discussed separately below.
Frontal lobeOur systematic review highlights the consistently reported involvement of altered prefrontal and frontal structures in lack of insight in patients with psychosis.43,47,49,50,58,62 Several results emerge from these studies, which have used cortical thickness techniques to observe regional thinning in left middle frontal associated with poor insight.49,54–57 This finding might support the conclusion that smaller frontal volumes characterize patients with poor insight.43,45,47,57,62,70
Furthermore, a meta-analysis showed that reduced DLPFC is altered and associated with positive and negative symptoms; Mintz et al.,28 claimed that the lack of insight is part of psychopathological conditions. In this framework, reduced volumes of OFC provided evidence that decreased prefrontal volumes lead to lower levels of clinical insight.58,71 However, this is inconsistent with the notion that increases in GMV in drug naïve FEP involved the prefrontal cortex, including OFC.72 In line with these findings, the alteration of OFC related to poor insight might be associated with the disability and loss of attribution because of the illness itself and its related psychotic complications. The involvement of specific patterns in dorsolateral prefrontal cortex and orbitofrontal cortex related to clinical insight may account for the implication of their associated basic cognitive process, such as working memory and cognitive control decision-making, respectively.18,45
Temporal lobeA trend of differences in temporal cortex volumes associated with clearly poor insight in FEP and chronic patients.43,59–61 Indeed, volume reduction in superior temporal cortex is compatible with empirical studies of functional deficits and thought disorder in psychosis patients.73 However, it is unclear if hippocampus alterations in FEP and chronic patients are associated with other altered cognitive functions affecting the lack of insight. One possible explanation for these alterations in light of the temporal lobe implication can be that it plays a specific role in awareness and relabeling factors, which might affect the ability of insight and attribution.60,61
Parietal and occipital lobesAuthors found that awareness of problems, poor symptom relabeling, and awareness of treatment effects were associated with cortical thinning in left precuneus.49,53,59
Also, Cooke et al.59 found that awareness of symptoms was related to a decrease in GMV in lateral parietal gyri, and Gerretsen et al.54 claimed that illness denial was correlated with cortical thinning of the left angular gyrus and illness awareness to parietal lobe asymmetry. From these studies, the ones that show reduction in the precuneus are also those with the larger sample sizes or the ones conducted in FEP patients.
From the first neuroimaging studies, the parietal lobe has been associated with psychotic experiences.74 More recent studies identified disturbed parieto-occipital functional connectivity as related to schizophrenia.75,76
Precuneus showed alterations in both unaffected siblings and in patients with schizophrenia when related to healthy controls,77 and a recently published article indicated abnormal regional homogeneity and correlation with clinical symptoms in drug naïve first episode patients.78
The occipital lobe showed poor insight in four studies that used non-driven whole brain analysis,29,53,57,59 both in chronic and FEP subjects. Reduction in the inferior and middle occipital gyrus has been linked to the age of the patient at the onset and diagnosis.29,79 Both Morgan et al. and Tordesillas et al.29,53 found reductions in the right cuneus in large groups of FEP subjects.
Other brain regionsAlterations in midline cortical volumes, such as anterior cingulate regions, with bilateral reduction, have been associated with poor insight.60 Previously, neurobiological abnormalities in cingulate cortex have been linked to phenomenology of psychiatric illness,80 and related to altered self-monitoring.81 This fact might explain the inability to assess mental experiences. In addition, inverse correlations between unawareness and anterior cingulate gyrus volume were described.43 Our systematic review further showed two studies throughout region of interest approaches found thinner bilateral insula cortex in patients with poor insight,52,55 and also meanly in right posterior insula.63 These results may stem from the established function of the insula in its emotional and cognitive modulation.82 Importantly, evidence from neuroanatomical studies suggests that certain insula might address misattributed internal information to an external source.83 In this sense, Crespo-Facorro et al.,84 reported that reduction in insular GMV and cortical surface size are associated with the severity of psychotic symptoms in schizophrenia.
Reviewing the studies that included 50 or more patients closely, we found that those were the studies with the most widespread findings, including frontal, temporal, and occipital areas. That could mean that large, homogeneous samples are needed to show subtle differences in patients with insight deficits. Nonetheless, the scales showed high levels of correlation,85 it might mean that they are measuring different dimensions of insight. These results suggest that merging different techniques is needed, acknowledging that cortical thickness might be one of the most sensitive aspects to measure cortical alteration rather than volumetric studies.86,87
Integration of structural correlates of disturbances on insightThis systematic review showed that disturbances on insight dimensions in non-affective psychosis patients were significantly related to a great variety of MRI brain structural abnormalities. Two main domains of reductions in grey matter were focused on cortical (frontal, temporal, parietal and occipital) and midline structures (anterior cingulate, precuneus and insula regions) accounted for most associations. 17 out of 23 studies reported significant reductions, six showed non-significant associations and only one study found significant increase of grey matter abnormalities in areas or midline structures (Table 1).
Thus, no total concordance among studies was found even though the associations between insight dimensions and cortical areas and midline structures were replicated in more than 2 studies. Studies examining laterality of abnormalities reported predominantly left-side abnormalities (7 left-side and 2 right-side abnormalities).
Differences due to the use of insight scaleSome of the heterogeneity in the findings can be attributed to the heterogeneity of the scales used to assess insight, despite the insight scales used were highly intercorrelated.88 In some studies, researchers chose to use specific parts of scales. If we take SUMD since it is the most used scale, we observed that six studies used the long version versus five studies that used the short version. This is relevant since both versions differ in contents and interpretation.89 Furthermore, although awareness of mental illness was a common variable to measure insight, a wide number of scores had been used. As Dumas et al.,89 express in their review “The use of modified versions of the SUMD (…) may affect psychometric properties such as validity” and may explain the inconsistencies of findings between studies using the SUMD scales. Specifically, five studies using this scale did report significant grey matter reductions associated with disturbances on insight dimensions, while six did not.
Structural abnormalities in grey matter may pave the way to functional or anatomically interregional disconnections in non-affective psychosis.90 Since brain structural abnormalities showed a great overlap with abnormalities reported in functional studies in non-affective psychosis, such as fMRI and diffusion tensor imaging (DTI) studies.91
Moreover, a network comprising frontal, temporal, thalamic and striatal regions are among the most frequently reported structural abnormalities in non-affective psychosis90 that are highly interconnected with grey matter of midline and subcortical structures.92,93
These areas are main hubs of large-scale brain networks serving numerous complex processes,94 including clinical insight. Moreover, the involvement of widespread structural brain abnormalities may account for the complexity of different clinical domains of non-affective psychosis and regarding insight domains.95
The correlates of insight in non-affective psychosis extend beyond the imaging findings reviewed here. For example, changes of the HPA axis (hypothalamus–pituitary–adrenal) might be present in patients with first episode psychotic disorders.96 There is also a study focusing on increased cortisol levels that were positively associated with poor insight in women with the first psychotic episodes.97 At the cellular level, impaired insight is related to decreased oligodendrocyte clusters in parietal brain regions98 and associated with genetic basis for insight impairments in schizophrenia Xavier et al.99 provided preliminary molecular evidence from both polygenic scores and specific candidate regions linking schizophrenia genetic liability to poor insight.
Although our findings summarize the relationships among clinical insight and structural abnormalities in non-affective psychotic patients, the study has some limitations, which to an extent derive from the limitations of the included studies. First, there is no consensus regarding the brain areas related to poor insight in non-affective psychosis, as there are many articles with no significant results. Several of these articles had limited statistical power and findings need to be considered with caution. Issues related to the administration of different insight scales may also contribute to the discrepancies. Moreover, one should also consider the variety of neuroimaging techniques used. Furthermore, methodological options such as the use of a low field MRI scanner or relatively liberal statistical threshold need to be considered.
Among the strengths of this review is, to our knowledge, the large size of the study systematic effort to summarize the data on the associations between grey matter and insight in patients with non-affective psychosis both chronic and FEP.
Future perspectivesWe suggest the assessment of different dimensions of insight using standardized scales without modification. In this scenario, results from different studies could be better meta-analyzable and dissect the neural structures involved in each insight dimension.
ConclusionIn conclusion, this article argues that alterations in frontal and temporal regions were more frequently replicated in the context of lacking insight. However, there are several candidate areas, apart from fronto-temporal pathways, that might play a role in illness insight in non-affective psychosis, such as cingulate, insula, precuneus, and occipital lobe. Further large-scales research following the improvement of semiautomatic techniques in longitudinal samples is required to promote knowledge on GMV decreases associated with insight.
FundingThis study was supported by grants from the Consellería de Educación (PROMETEO/2016/082) and Carlos III Health Institute (ISCiii PI17/00402) cofunded by The European Union through FEDER funds. Funding sources had no further role in study design, review, analysis or interpretation of the data.
Conflict of interestsThe authors declare that they have no conflict of interest.