The neural correlates of the cognitive dysfunction in first-episode psychosis (FEP) are still unclear. The present review and meta-analysis provide an update of the location of the abnormalities in the fMRI-measured brain response to cognitive processes in individuals with FEP.
MethodsSystematic review and voxel-based meta-analysis of cross-sectional fMRI studies comparing neural responses to cognitive tasks between individuals with FEP and healthy controls (HC) according to PRISMA guidelines.
ResultsTwenty-six studies were included, comprising 598 individuals with FEP and 567 HC. Individual studies reported statistically significant hypoactivation in the dorsolateral prefrontal cortex (6 studies), frontal lobe (8 studies), cingulate (6 studies) and insula (5 studies). The meta-analysis showed statistically significant hypoactivation in the left anterior insula, precuneus and bilateral striatum.
ConclusionsWhile the studies tend to highlight frontal hypoactivation during cognitive tasks in FEP, our meta-analytic results show that the left precuneus and insula primarily display aberrant activation in FEP that may be associated with salience attribution to external stimuli and related to deficits in perception and regulation.
Los correlatos neurales de la disfunción cognitiva en el primer episodio psicótico (PEP) aún no están claros. Esta revisión y este metaanálisis proporcionan una actualización de la localización de las anormalidades en la respuesta cerebral medida por fMRI a los procesos cognitivos en individuos con PEP.
MétodosRevisión sistemática y metaanálisis basado en vóxeles de estudios cros-seccionales de fMRI que comparen respuestas neuronales a tareas cognitivas entre individuos con PEP y controles sanos de acuerdo con las guías PRISMA.
ResultadosSe incluyeron 26 estudios, que comprendían 598 individuos con PEP y 567 controles sanos. Los estudios individuales reportaban hipoactivación estadísticamente significativa en la corteza prefrontal dorsolateral (6 estudios), el lóbulo frontal (8 estudios), el cíngulo (6 estudios) y la ínsula (5 estudios). El metaanálisis mostró hipoactivación estadísticamente significativa en la ínsula anterior izquierda, el precúneo y el cuerpo estriado bilateral.
ConclusionesSi bien los estudios tienden a resaltar la hipoactivación frontal durante las tareas cognitivas en PEP, nuestros resultados metaanalíticos muestran que el precúneo izquierdo y la ínsula presentan principalmente una activación aberrante en PEP que puede estar asociada con la atribución de saliencia a estímulos externos y relacionada con déficits en la percepción y la regulación.
First-episode psychosis (FEP) is characterized by the first period experiencing suprathreshold psychotic symptoms in an individual's life. Psychotic disorders are associated with impairment in many areas of life and the risk of all-cause mortality associated with these disorders is more than twice that of the general population.1 Therefore, understanding the mechanisms involved throughout the course of the illness is crucial.2 The clinical symptoms of psychosis have been described and conceptualized as positive and negative. Positive symptoms include hallucinations, delusions, and disorganized thinking, while negative symptoms include affective flattening, apathy, anhedonia and cognitive impairment.3 In addition, recent studies in FEP show that functional deficits of the right middle frontal gyrus (Broadman area, BA, 9) during attentional and memory performance may be central in the pathophysiology of psychosis.4 However, these results must be cautiously interpreted given the complexity and heterogeneity of existing studies.5,6 That said, sufficient evidence exists to support improving and developing tools for early intervention in FEP to reduce dysfunction and enhance the quality of life of patients.7
One instrument used in biomarker research for FEP is functional magnetic resonance imaging (fMRI). Unfortunately, no fMRI technique has proven to be sufficiently specific or sensitive for clinical diagnosis. Furthermore, results from different studies do not appear to be consistent, likely due to heterogeneity of samples, cognitive tasks, neuroimaging methodologies, and statistical analyses. A single fMRI study may provide insights about the abnormalities observed using a specific task in patients with specific characteristics, and thus results may change from one study to another. Therefore, meta-analyses may be useful because they focus on the commonalities (across patients, across tasks, etc.) and discard the specificities. Multivariate patterns of functional dysconnectivity across FEP, as suggested by Fusar-Poli et al.8, and a recent systematic review of functional brain changes in task-based and resting state fMRI suggest impairment of the fronto-temporal pathways are the core issue in FEP.9 These findings align with the classical hypothesis of fronto-temporal dysfunction in chronic patients with schizophrenia.10
Nevertheless, many questions remain regarding the patterns of activation according to the cognitive task fMRI studies in FEP. Thus, the primary question compares the main areas of dysfunction in FEP vs HC. We hypothesized that FEP patients show reduced functional activity in frontal and temporal lobes, across cognitive tasks, compared with HC.
We conducted a systematic review and a meta-analysis using anisotropic effect-size seed-based mapping11 to investigate the patterns of activation according to the cognitive tasks used during fMRI acquisition in FEP patients versus HC with a specific focus on the relevance of methodology, the different cognitive tasks used during scans and the mode of presentation. This review intends to show different methodological biases and activation results to explore their relationships and contribute to the methodological optimization of cognitive fMRI task studies. In addition, we expect to identify and compare cerebral activation correlates of FEP patients and HC during cognitive task performance to clarify the areas most associated with differences in brain activation.
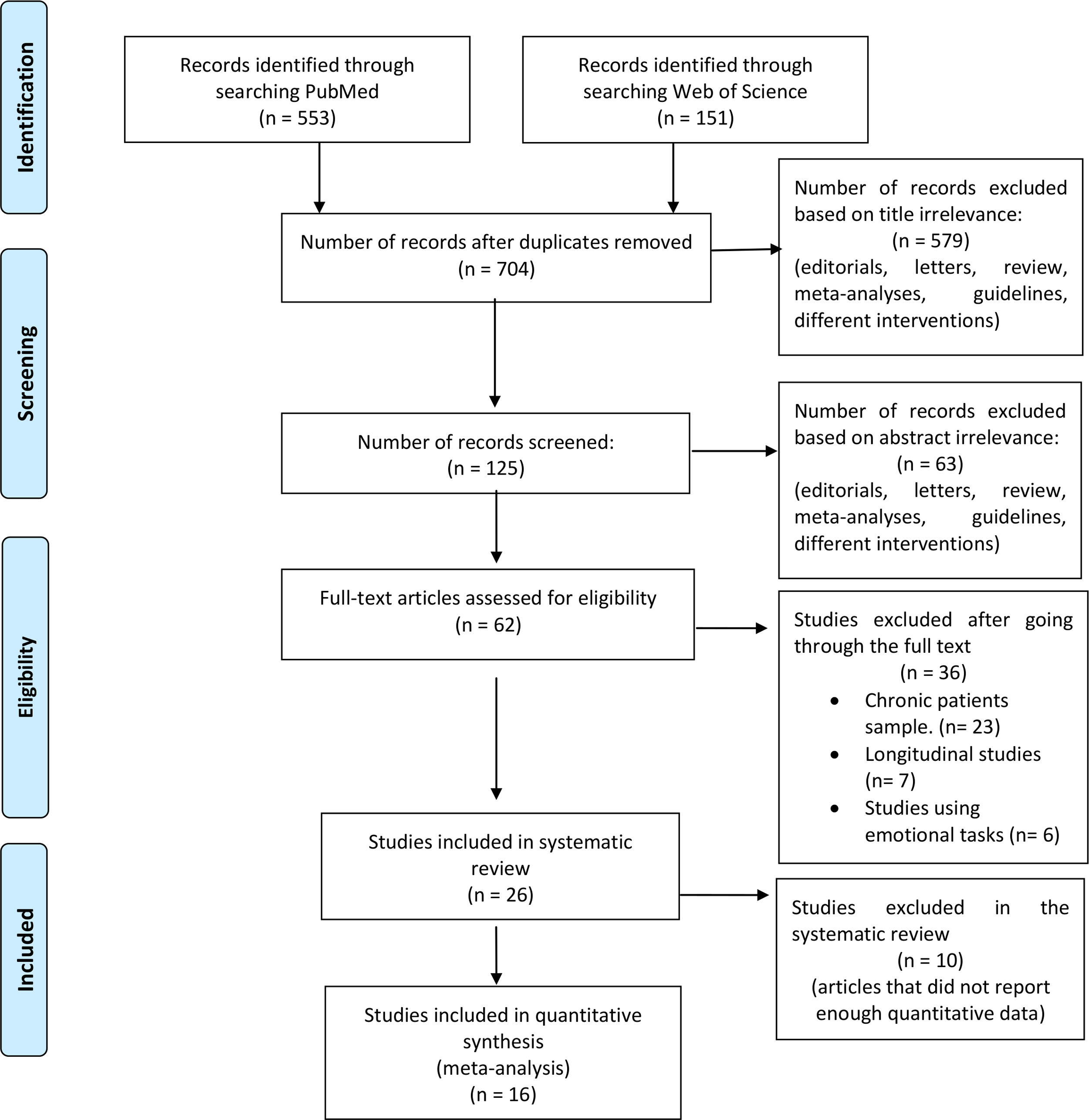
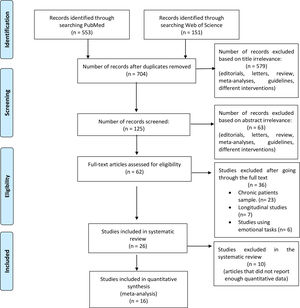
MethodsSearch strategy and study selectionThe systematic review was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) recommendations,12 and the meta-analysis according to the best practice recommendations by Müller et al.13 We searched PubMed and the Web of Science databases to identify functional neuroimaging studies (Fig. 1 for an outline of this process). We used the following key terms: “(fMRI AND (First episode psychosis))”. The automatic searches were accompanied by manually reviewing the references of the eligible articles after the final selection. We identified 704 articles that were published between January 2000 and July 2017.
Inclusion criteria were: (a) studies comparing the blood oxygenation level-dependent (BOLD) response to a cognitive task such as attention, perception, memory, or cognitive control between individuals with a first-episode sample, defined as individuals who had been diagnosed with a psychotic disorder for the first time in their lives; (b) the diagnosis (schizophrenia, schizophreniform disorder, bipolar disorder, schizoaffective disorder, brief psychotic episode, and psychosis not otherwise specified) must be verified by structured clinical interviews; and (c) the BOLD response must be measured using task-based fMRI. Exclusion criteria were: (a) studies using resting state and structural MRI modalities; (b) studies using non-cognitive tasks; (c) reviews and meta-analyses; and (d) studies that included participants with >1 acute psychotic episode. Finally, specific exclusion criteria for the meta-analysis were (a) region of interest and small volume correction results; (b) studies using partial coverage of the gray matter; and (c) studies that do not report the required data (coordinates and t-values of the peaks of abnormal brain response).
Twenty-six studies met the inclusion criteria for the systematic review, 16 of which also met the criteria to be included in the voxel-based meta-analysis (Fig. 1).
Data extractionTwo researchers (PSM and GGM) independently read the full text of each potentially eligible article, and disagreements regarding eligibility criteria were resolved by consensus. The selection of these studies was performed hierarchically.12 A primary screening was performed based on title, a second screening on abstract, and a third screening on a full text review. When data were either unpublished or incomplete, the corresponding author was invited to send additional information.
Relevant data of selected articles were extracted in a predefined structured table (Table 1). The following variables were included in the review: author and year of publication; sample size (FEP patients and HC); sex and mean age of participants; medication, duration of untreated illness, stimulus modality; cognitive task paradigm and summary of brain activation. Moreover, we included an additional table listing the location and activation of brain areas according to the experimental cognitive task applied in the studies. For the meta-analysis, we also extracted the peak coordinates and t-values of the findings.
fMRI studies comparing individuals with a first episode psychosis (FEP) and healthy controls included in the systematic review.
| First author and year | Patients information | Healthy controls | Scales | Medication | DUI | Field strength of scanners | Space coordinates | Software for analysis of fMRI | Stimulus modality | Cognitive task paradigm | Summary of brain activation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Braus et al., 2000* | N=12Age: 25Men: 50%Diagnosis: FES | N=12Age: 28Men: 50%Diagnosis: NA | BPRS | Antipsychotic-Naive | 34 months | 1.5T | Talairach | SPM99 | Visual | Sequential finger opposition | Reduce in motor cortical dysfunction |
| Braus et al., 2002* | N=12Age: 25Men: 50%Diagnosis: FES | N=11Age: 29Men: 55%Diagnosis: NA | BPRS | Antipsychotic-Naive | Not specified | 1.5T | Talairach | SPM99 | Auditory and visual | Information processing | Reduce thalamus, LSTG, and parietal lobe |
| Boksman et al., 2005* | N=10Age: 22Men: 90%Diagnosis: FES | N=10Age: 23Men: 90%Diagnosis: NA | SANSSANP | Antipsychotic-Naive | 17 months | 4.0T | Talairach | SPM99 | Visual | Word fluency | Reduce DLPFC, STG.Increase LFL, anterior cingulate, thalamus, insula, IFL, IOG and FG |
| Tan et al., 2005* | N=11Age: 25Men: 45%Diagnosis: FES | N=11Age: 26Men: 45%Diagnosis: NA | PANSSGAF | 2.3mg/day: risperidone (n=6)11mg/day: olanzapine (n=5) | 2 months | 3.0T | Talairach | Brainvoyager | Visual and verbal | Working memory | Reduce bilateral DLPFC.Increase VLPFC |
| Schneider et al., 2007* | N=48Age: 31Men: 54%Diagnosis: FES (42 paranoid, 2 disorganized, 3 undifferentiated, 1 schizophreniform disorder) | N=57Age: 30Men: 59%Diagnosis: NA | PANSSGAFHAMD | Medication was double blind and presently unknown (risperidone vs. haloperidol) | Not specified | 1.5T | MNI | SPM2 | Visual | Working memory (N-back) | Reduce STG, thalamus, and hippocampusIncrease VLPFC |
| Bleich-Cohen et al., 2007 | N=12Age: 30Men: 50%Diagnosis: FES | N=17Age: 31Men: %Diagnosis: NA | PANSS | 3.2mg/day: risperidone (n=9)700mg/day quetiapine (n=1)16mg/day perphenazine (n=1), 2mg/day haloperidol (n=1) | Not specified | 1.5T | Talairach | Brainvoyager | Auditory | Word Fluency | Reduce LIFG and Wernicke area.Increase RSTS |
| Achim et al., 2007* | N=26Age: 22Men: 69%Diagnosis: FES (15 schizophrenia, 4 schizoaffective disorder, 7 psychosis not otherwise specified | N=20Age: 23Men: 55%Diagnosis: NA | SAPSSAPNHAMD | 2.11mg/day risperidone (n=11), 10.5mg/day olanzapine (n=5), 325mg/day quetiapine (n=2), 300mg/day clozapine (n=1), 1.5mg/day haloperidol (n=1), Mixed (n=2)Antipsychotic-Naïve (n=4) | 18 months | 1.5T | Talairach | SPM2 | Visual and verbal | Encoding strategies; subsequent memory effect; semantic relatedness | Reduce bilateral MTG. |
| Fusar-Poli et al., 2007 | N=10Age: 25Men: 100%Diagnosis: FES | NONE | PANSSNART | 1.7mg/day risperidone63.75mg/day quetiapine | Not specified | 1.5T | Talairach | SCM | Visual | Word Fluency | Reduce DLPFC, cingulate, thalamus and FPC.Increase VLPFC |
| Benetti et al., 2009 | N=10Age: 25Men: 70%Diagnosis: FES | N=14Age: 26Men: 64%Diagnosis: NA | PANSSNART | Antipsychotic-Naïve (n=3)121mg/day chlorpromazine equivalents (n=7) | Not specified | 1.5T | MNI | SPM5 | Visual | Encoding strategies; maintenance; recognition | Increase in encoding SPG, SMG. In maintenance bilateral anterior insula, right anterior cingulate. In recognition Bilateral (IFG and STG), insula and MTG |
| Crossley et al., 2009* | N=10Age: No specifiedMen: No specifiedDiagnosis: FES | N=13Age: No specifiedMen: No specifiedDiagnosis: NA | None | Antipsychotic-Naïve (n=3)1.7mg/day risperidone63.75mg/day quetiapine | 12 months | 1.5T | MNI | SPM5 | Visual | Working memory | Increase in STL and MFG |
| Woodward et al., 2009* | N=15Age: 22Men: 80%Diagnosis: FES | N= 32Age: 22Men: 71%Diagnosis: NA | PANSSGAF | Antipsychotic-Naïve | 5 months | 1.5T | Talairach | SPMs | Visual | Choice reaction time | Increase SMA and MFG |
| Lencer et al., 2011* | N=40Age: 23Men: 70%Diagnosis: FES (21 schizophrenia, 2 schizoaffective disorder, 1 schizophreniform disorder, 13 bipolar I disorder) | N=20Age: 24Men: 50%Diagnosis: NA | PANSSWASI | 2.5mg/day risperidone (n=35), 20mg/day olanzapine (n=1), 15mg/day aripiprazole (n=1) | Not specified | 3.0T | Talairach | AFNI | Visual | Motion processing | Reduce intraparietal sulcus, DLPFC.Increase Dorsomedial thalamus and insula. |
| Purdon et al., 2011 | N=17Age: 21Men: 76%Diagnosis: FES | N=17Age: 22Men: 76%Diagnosis: NA | PANSSGAF | Antipsychotic-Naïve | 4 Months | 1.5T | Talairach | MPRAGE | Visual | Serial reaction time | Reduce Bilateral MFGStriatum-thalamus-cortical circuits.Increase Left STG |
| Guerrero-Pedraza et al., 2012 | N=30Age: 26Men: 70%Diagnosis: FES (7 schizophrenia, 9 schizophreniform disorder, 1 delusional disorder, 3 brief psychosis, 10 unspecified psychosis) | N=28Age: 27Men: 71%Diagnosis: NA | PANSSTAP | 299mg/day chlorpromazine equivalents | 18 months | 1.5T | MNI | FSL | Visual | Working memory (N-back) | Reduce MFC, thalamus and cingulate. Increase DLPFC, VLPFC, insula. |
| Smieskova et al., 2012 | N=21Age: 28Men: 76%Diagnosis: FES | N=20Age: 26Men: 50%Diagnosis: NA | BPRSSANSSANPGAFBSIP | Antipsychotic-naïve (n=7), Antipsychotic free (n=6), Quetiapine (n=5), Paliperidone (n=2)Olanzapine (n=1) | Not specified | 3.0T | MNI | SPM8 | Visual | Working memory (N-back) | Reduce Precuneus, SFG, MFG, IFG, insula |
| Yoon et al., 2012 | N=51Age: 20Men: 76%Diagnosis: FES | N=51Age: 20Men: 51%Diagnosis: NA | GAFSANSSAPSBPRS | Antipsychotic-naïve (n=19)Antipsychotic treatment not specified (n= 32) | 12 months | 1.5T | MNI | SPM5 | Visual | Attentional processing (AX continuous Performance) | Reduce DLPFC |
| Kambeitz-Ilankovic et al., 2013* | N=20Age: 25Men: 70%Diagnosis: FES | N=20Age: 26Men: 70%Diagnosis: NA | PANSSPSYRATS | 252mg/day Chlorpromazine equivalents | 18 months | 3.0T | Talairach | SPM8 | Visual | Attentional processing | Reduce MTG, insula and precuneus |
| Lesh et al., 2013 | N=43Age: 28Men: 79%Diagnosis: FES (41 schizophrenia, 1 schizoaffective, 1 schizophreniform) | N=54Age:Men: 64%Diagnosis: NA | SANSSAPSBPRS | Antipsychotic-naïve (n=15), Atypical antipsychotic (n=27), Typical and Atypical antipsychotic (n=1) | 12 months | 1.5T | MNI | SPM8 | Visual | Attentional processing (AX continuous Performance) | Reduce DLPFC and parietal |
| Schmidt et al., 2013* | N=21Age: 28Men: 76%Diagnosis: FES | N=20Age: 26Men: 50%Diagnosis: NA | BPRSGAFBSIP | Antipsychotic-naïve (n=7), Antipsychotic free (n=6), Quetiapine (n=5) Paliperidone (n=2), Olanzapine (n=1) | Not specified | 3.0T | Talairach | SPM | Visual | Working memory | Reduce MFG and superior parietal lobe |
| Benetti et al., 2015 | N=46Age: 25Men: 59%Diagnosis: FES | N=22Age: 24Men: 50%Diagnosis: NA | PANSSPSYRATS | 197mg/day Chlorpromazine equivalents | Not specified | 3.0T | MNI | SPM8 | Auditory | Word fluency | Reduce LIFG and left MTG.Increase MTG and VLPFC |
| Bendfeld et al., 2015 | N=19Age: 28Men: 76%Diagnosis: FES | N=19Age: 26Men: 50%Diagnosis: NA | BPRSSANSSANPGAFBSIP | Antipsychotic free (n=9), Antipsychotic naïve (n=4), Antipsychotic medicated without specified either mg/day or type of antipsychotic (n=6) | Not specified | 3.0T | MNI | SPM8 | Visual | Working memory and verbal fluency | Reduce Parietal lobe and precuneus.Increase Cingulate and Frontal lobe |
| Buchy et al., 2015 | N=25Age: 24Men: 80%Diagnosis: FES | N=24Age: 25Men: 79%Diagnosis: NA | SANSSAPS | Antipsychotic medicated without specified either mg/day or type of antipsychotic (n=17) | 16 months | 3.0T | MNI | SPM8 | Visual | Memory processing | Increase VLPFC |
| Hawco et al., 2015* | N=26Age: 24Men: 85%Diagnosis: FES | N=24Age: 25Men: 79%Diagnosis: NA | SANSSAPSHAMD | Antipsychotic free (n=26) | 16 months | 3.0T | MNI | SPM8 | Visual | Memory processing | Reduce: frontal lobe, parietal lobe.Increase Cingulate, FG and DLPFC |
| Keedy et al., 2015* | N=21Age: 24Men: 76%Diagnosis: FES | N=21Age: 24Men: 47%Diagnosis: NA | PANSSHAMD | Antipsychotic naïve (n=14) | 1 month | 3.0T | Talairach | AFNI | Visual | Attentional processing | Reduce SFG, insula, SMG, cingulate and bilateral, ILC |
| Raij et al., 2015* | N=20Age: 27Men: 60%Diagnosis: FES (6 schizophrenia, 2 schizophreniform disorder, 2 psychotic disorder not otherwise specified) | N=20Age: 29Men: 60%Diagnosis: NA | BPRS | 462mg/day Chlorpromazine equivalents. | Not specified | 3.0T | MNI | SPM8 | Visual | Attentional processing | Reduce Putamen, cingulate and insula. |
| Schmidt et al., 2016* | N=29Age: 24Men: 65%Diagnosis: FES | N=19Age: 26Men: 52%Diagnosis: NA | BPRS SANSSANPGAF | Antipsychotic medicated without specified mg/day. | Not specified | 3.0T | MNI | SPM8 | Visual | Reward task. | Reduce Insula and cingulate cortex |
FEP: First-Episode Psychosis; FES: First Episode Schizophrenia; NA: Not Applicable; BSIP: Basel Screening Instrument for Psychosis; PSYRATS: Psychotic Symptoms Rating Scale; SPM: Statistical Parametric Mapping; AFNI: Analysis of Functional NeuroImages; MPRAGE: Magnetization-Prepared Rapid Gradient-Echo; PANSS: Positive and Negative Scale Syndrome; SANS: Scale of the Assessments of Negative Syndrome; SAPS: Scale for the Assessment of Positive Syndrome; TAP: Word Accentuation test; DUI: Duration of Untreated Illness; GAF: Global Functional Scale; BPRS: Brief Psychiatry Rating Scale; NART: National Adult Reading Test; HAM-D: Hamilton Rating Scale for Depression; AG: Angular Gyrus; CPT: Continuous Performance Test; DLPFC: Dorsolateral Prefrontal Cortex; FG: Fusiform Gyrus; FPC: Frontal Posterior Cingulate; HPC: Hippocampus; IFG: Inferior Frontal Gyrus; IFL: Inferior frontal lobe; ILC: Inferior Lingual Cortex; IOG: Inferior Occipital Gyrus; LFL: Left Frontal Lobe; LG: Lingual Gyrus; LIFG: Left Inferior Frontal Gyrus; LSTG: Left Superior Temporal Gyrus; LTG: Left temporal Gyrus; MFG: Middle Frontal Gyrus; MOFG: Medial Orbitofrontal gyrus; MNI: Montreal Neurological Institute space coordinates; MTG: Middle Temporal Gyrus; RSTS: Right Superior Temporal Sulcus; SFG: Superior Frontal Gyrus; SMA: Supplementary Motor Area; SMG: Supramarginal gyrus; SPG: Superior Parietal Gyrus; STG: Superior Temporal Gyrus; STL: Superior Temporal Lobe; VLPFC: Ventrolateral Prefrontal Cortex; VS: Ventral Striatal; WASI: Wechsler Abbreviated Scale of Intelligence.
The quantitative approach was carried out meta-analyzing 16 studies with SDM software version 5 using anisotropic effect-size seed-based mapping (AES-SDM, https://www.sdmproject.com/).14 Studies included in the meta-analysis are marked with an asterisk (*) in Table 1.
First, AES-SDM used the coordinates (converted to MNI space) and t-values of the peaks of maximum statistical significance reported in the studies to generate a three-dimensional image of the effect-size of the differences in activation between patients and HC, separately for each study. Specifically, AES-SDM assigned each voxel an effect size that depended on the spatial covariance with the close peaks, of which the effect size was known. Second, a three-dimensional image of the variance of the effect size was generated – again, separately for each study. This step is straightforward because the variance of a given effect size depends only on the effect size and the samples sizes. Third, a standard random-effects meta-analysis was fitted separately for each voxel. Finally, a permutation test for spatial convergence was conducted to detect those regions that showed larger effect sizes between groups compared with most regions.15
We also conducted several complementary analyses. First, for each of the identified clusters in the results, we created the funnel plot of the effect size of the peak with regards to its standard error for visual detection of potential publication bias. Additionally, we quantitatively assessed the potential publication bias with the Egger test, and the heterogeneity with the I2 statistic. Additional sensitivity analyses were performed using a jackknife leave-one-out procedure, which consisted of sequentially removing each study individually and repeating the analysis as many times as studies we had include in the main analysis (i.e., 16 sub-analyses, each including 15 studies). If a region were detected abnormal in all 16 iterations, we concluded that we would have detected this region in the meta-analysis even if any of the studies had not been published. We used a composite threshold for statistical significance (uncorrected voxel P<0.005, peak SDM-Z value >1, plus cluster extent >100 voxels), which is more conservative than the recommended threshold for ES-SDM.11
ResultsA total of 704 records were identified through database searching, with 26 studies meeting eligibility criteria. The pooled sample size in the FEP group was n=598, the mean age was 24 years old (range 20–31), 30% of subjects were women and 80% were receiving antipsychotic medication. The HC group had a sample size of n=567 and a mean age of 25 years old (range 20–34 years old), and 46% of the subjects were women. FEP and HC groups were already age- and sex-matched in all eligible studies.
Characteristics from included studiesThe included studies demonstrated reduced activation of the temporal lobe, parietal lobe, frontal lobe and limbic areas.16–21 Conversely, five studies reported increased activation in the ventro-lateral prefrontal cortex.22–24 Furthermore, nine studies showed reduced and increased activation in different brain regions, including the frontal and prefrontal cortex, insula, temporal lobe, occipital lobe and thalamus.22,25–29
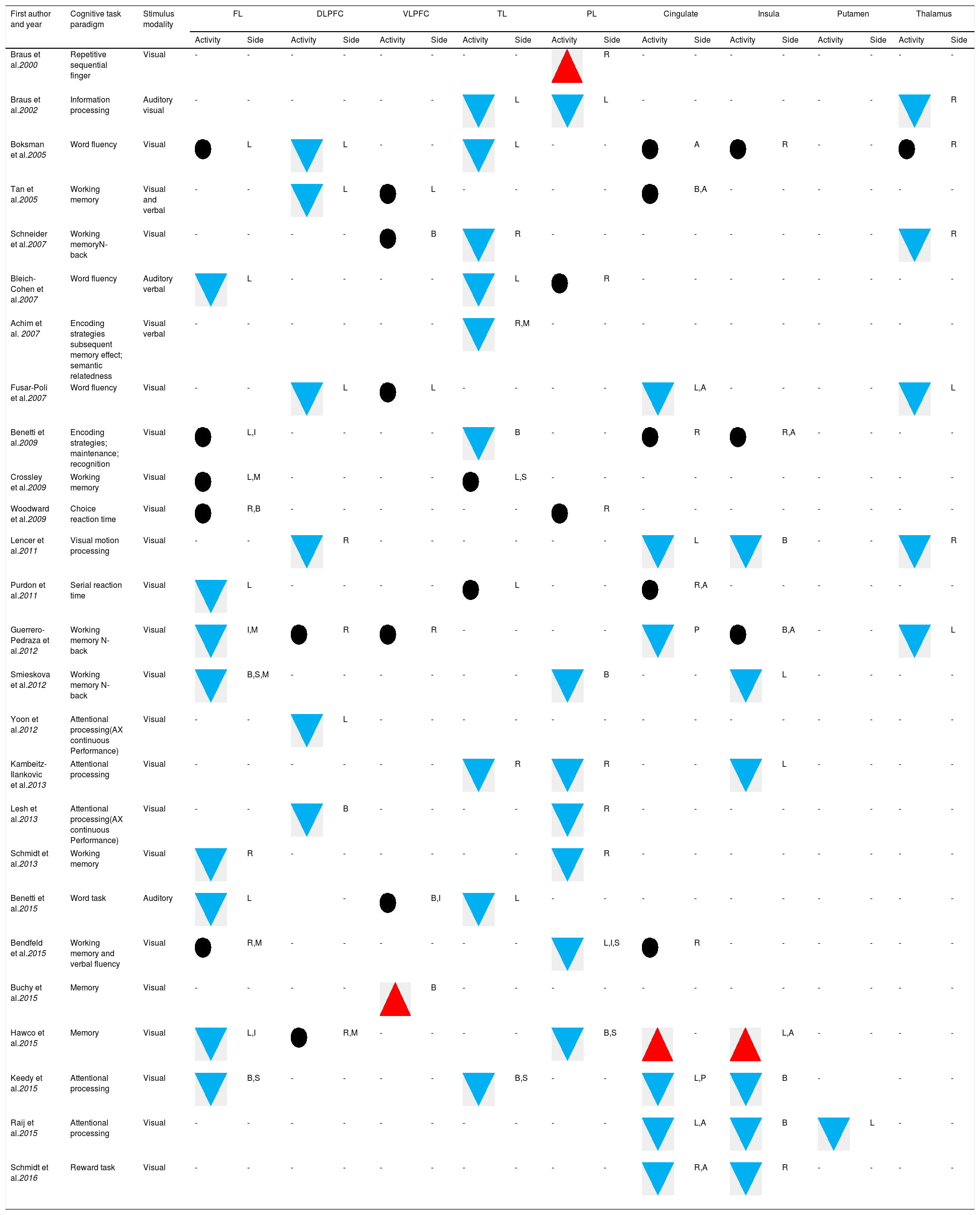
The most significant finding in the systematic review of cognitive task fMRI and brain activation was found in the prefronto-temporal pathways (Table 2).16,23,30–33 Interestingly, activity appears to be decreased in the left inferior frontal gyrus,26,34,35 orbital frontal gyrus,21,27 superior parietal lobe35,36 and thalamus17,20,23,30 in FEP patients compared with HC.
Brain activation abnormalities in individuals with First Episode Psychosis (systematic review).
| First author and year | Cognitive task paradigm | Stimulus modality | FL | DLPFC | VLPFC | TL | PL | Cingulate | Insula | Putamen | Thalamus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity | Side | Activity | Side | Activity | Side | Activity | Side | Activity | Side | Activity | Side | Activity | Side | Activity | Side | Activity | Side | |||
| Braus et al.2000 | Repetitive sequential finger | Visual | - | - | - | - | - | - | - | - | R | - | - | - | - | - | - | - | - | |
| Braus et al.2002 | Information processing | Auditory visual | - | - | - | - | - | - | L | L | - | - | - | - | - | - | R | |||
| Boksman et al.2005 | Word fluency | Visual | L | L | - | - | L | - | - | A | R | - | - | R | ||||||
| Tan et al.2005 | Working memory | Visual and verbal | - | - | L | L | - | - | - | - | B,A | - | - | - | - | - | - | |||
| Schneider et al.2007 | Working memoryN-back | Visual | - | - | - | - | B | R | - | - | - | - | - | - | - | - | R | |||
| Bleich-Cohen et al.2007 | Word fluency | Auditory verbal | L | - | - | - | - | L | R | - | - | - | - | - | - | - | - | |||
| Achim et al. 2007 | Encoding strategies subsequent memory effect; semantic relatedness | Visual verbal | - | - | - | - | - | - | R,M | - | - | - | - | - | - | - | - | - | - | |
| Fusar-Poli et al.2007 | Word fluency | Visual | - | - | L | L | - | - | - | - | L,A | - | - | - | - | L | ||||
| Benetti et al.2009 | Encoding strategies; maintenance; recognition | Visual | L,I | - | - | - | - | B | - | - | R | R,A | - | - | - | - | ||||
| Crossley et al.2009 | Working memory | Visual | L,M | - | - | - | - | L,S | - | - | - | - | - | - | - | - | - | - | ||
| Woodward et al.2009 | Choice reaction time | Visual | R,B | - | - | - | - | - | - | R | - | - | - | - | - | - | - | - | ||
| Lencer et al.2011 | Visual motion processing | Visual | - | - | R | - | - | - | - | - | - | L | B | - | - | R | ||||
| Purdon et al.2011 | Serial reaction time | Visual | L | - | - | - | - | L | - | - | R,A | - | - | - | - | - | - | |||
| Guerrero-Pedraza et al.2012 | Working memory N-back | Visual | I,M | R | R | - | - | - | - | P | B,A | - | - | L | ||||||
| Smieskova et al.2012 | Working memory N-back | Visual | B,S,M | - | - | - | - | - | - | B | - | - | L | - | - | - | - | |||
| Yoon et al.2012 | Attentional processing(AX continuous Performance) | Visual | - | - | L | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Kambeitz-Ilankovic et al.2013 | Attentional processing | Visual | - | - | - | - | - | - | R | R | - | - | L | - | - | - | - | |||
| Lesh et al.2013 | Attentional processing(AX continuous Performance) | Visual | - | - | B | - | - | - | - | R | - | - | - | - | - | - | - | - | ||
| Schmidt et al.2013 | Working memory | Visual | R | - | - | - | - | - | - | R | - | - | - | - | - | - | - | - | ||
| Benetti et al.2015 | Word task | Auditory | L | - | B,I | L | - | - | - | - | - | - | - | - | - | - | ||||
| Bendfeld et al.2015 | Working memory and verbal fluency | Visual | R,M | - | - | - | - | - | - | L,I,S | R | - | - | - | - | - | - | |||
| Buchy et al.2015 | Memory | Visual | - | - | - | - | B | - | - | - | - | - | - | - | - | - | - | - | - | |
| Hawco et al.2015 | Memory | Visual | L,I | R,M | - | - | - | - | B,S | - | L,A | - | - | - | - | |||||
| Keedy et al.2015 | Attentional processing | Visual | B,S | - | - | - | - | B,S | - | - | L,P | B | - | - | - | |||||
| Raij et al.2015 | Attentional processing | Visual | - | - | - | - | - | - | - | - | - | - | L,A | B | L | - | - | |||
| Schmidt et al.2016 | Reward task | Visual | - | - | - | - | - | - | - | - | - | - | R,A | R | - | - | - | - | ||
A: Anterior; B: Bilateral; DLPFC: Dorsolateral prefrontal cortex; FL: Frontal lobe; I: Inferior; L: Left side; M: Medial; P: Posterior; PL: Parietal lobe; R: Right side; S: Superior; TL: Temporal lobe; VLPFC: Ventrolateral prefrontal cortex;
: significant differences (P<0.05) in increased activation; : significant differences (P<0.05) in reduced activation; : non-significant difference; Hyphen (-): indeterminate data. Note. (1) The data were collected based on the accuracy rate reported in the included studies.However, numerous studies reported an increased task-related BOLD activity in the insula29,31 and inferior frontal gyrus31,37 of FEP patients compared with HC.
An Ontario group25 showed that antipsychotic-naïve first-episode patients exhibited relatively lower activation in the prefrontal and anterior cingulate during a word fluency task. Further, Bleich-Cohen et al.26 reported reduced activation in the left inferior frontal gyrus and increased activity in the right superior temporal sulcus in FEP patients compared with HC during an auditory language task.
Achim et al.18 examined encoding strategies and detected reduced activation in the bilateral medial temporal lobes in FEP patients relative to HC. Yoon et al.38 reported decreased activation in the dorsolateral prefrontal cortex (DLPFC), suggesting that neurophysiological markers of illness may not be less evident in FEP patients versus patients with a more established psychotic illness. In addition, Lesh et al.39 determined that FEP patients exhibited reduced activity in the DLPFC and inferior parietal cortex, which was not seen in HC. In contrast, in a study of patients after treatment, Keedy et al.28 reported a marked increase in activity in the DLPFC in FEP, similar to that shown in HC. Another study reported that putamen signaling was lower in the FEP group, and the degree of this alteration was positively correlated with delusion scores and negatively correlated with the antipsychotic equivalent dose,40 which was in accordance with the dysfunction of striate-cortical connectivity.32
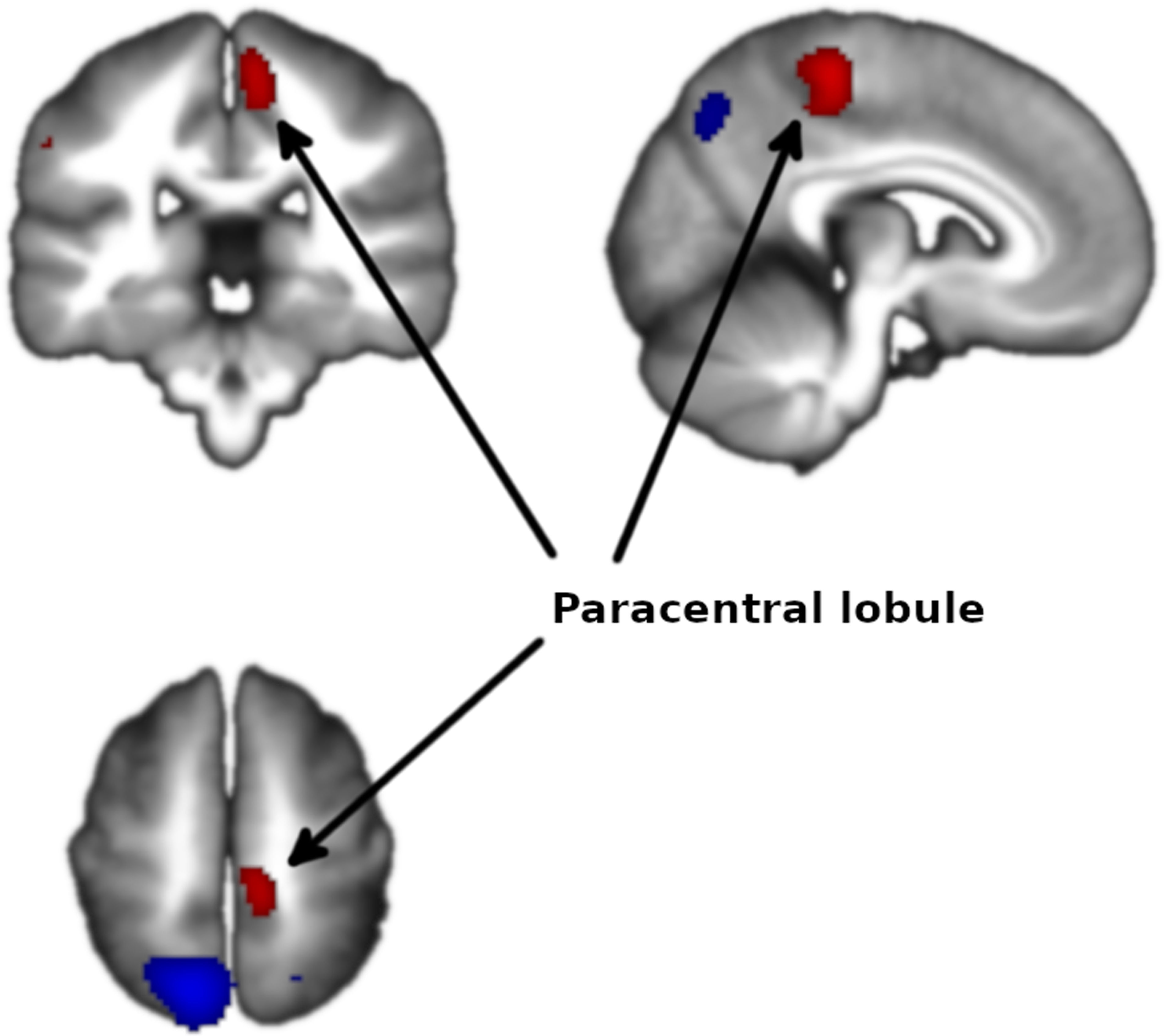
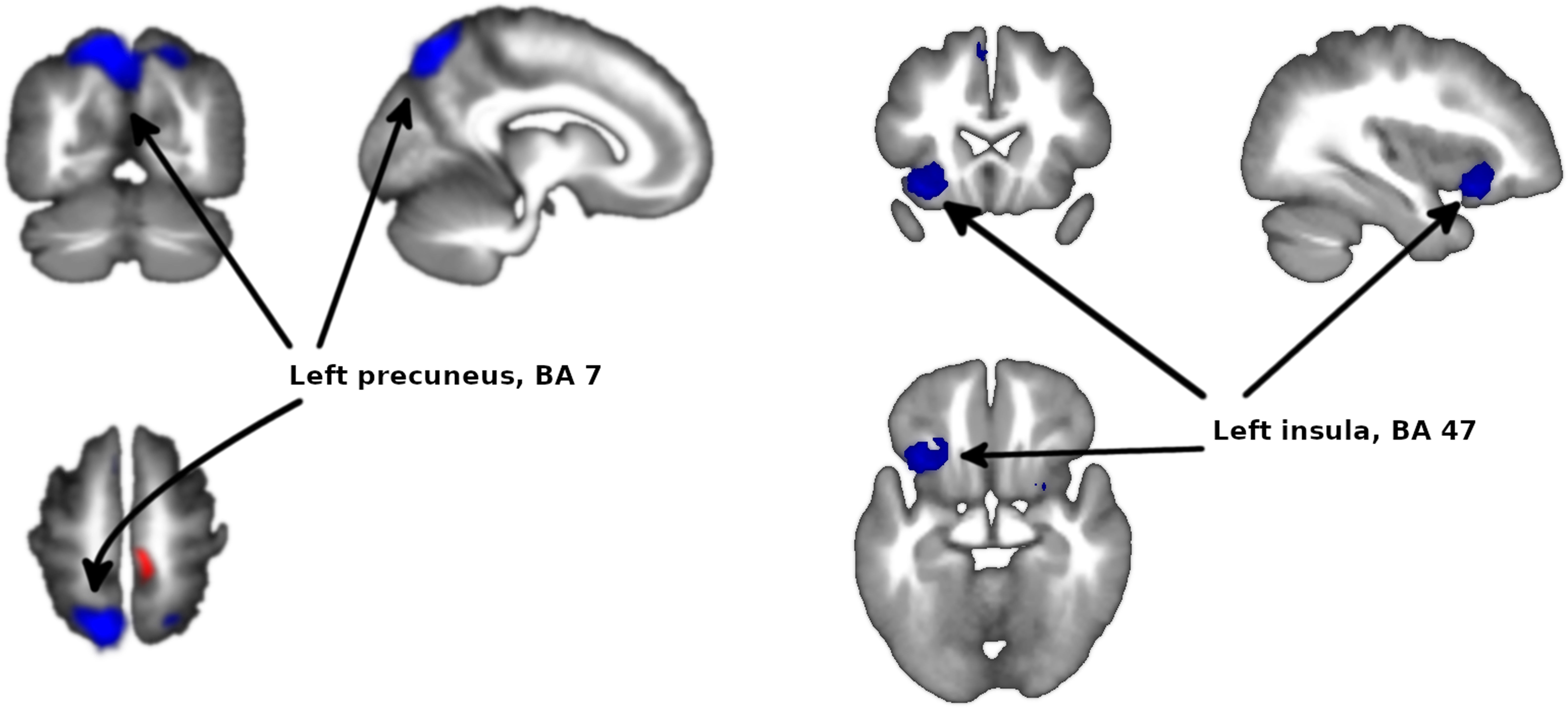
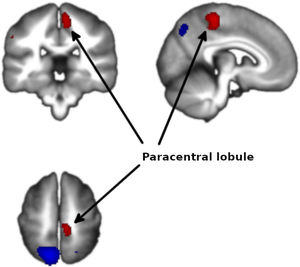
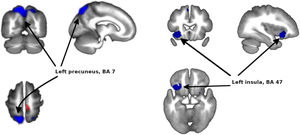
Meta-analysis of functional response to cognitive processing in FEPFour clusters with >100 voxels were identified. Patients with FEP showed statistically significant increased activation in paracentral lobule (MNI 8,−30,58 with Z=1.75 and P=2×10−4) and statistically significant decreased activation in precuneus (BA7, MNI −12,−64,58 with Z=3.351 and P=3×10−7) extending to bilateral superior parietal gyrus, left insula (mostly BA47, MNI −34,18,−12 with Z=2.62 and P=3.7×10−5) extending to left striatum, and right striatum (BA48, MNI 26,4,−4 with Z=1.965 and P=1.4×10−3) (Figs. 2 and 3 and Table 3).
Brain activation abnormalities in individuals with First Episode Psychosis (meta-analysis).
| Peak MNI coordinate | Peak SDM-Z | Peak P | Voxels | Description |
|---|---|---|---|---|
| 8,−30,58 | 1.753 | 0.0002 | 202 | Paracentral lobule |
| −12,−64,58 | −3.351 | 0.0000003 | 2052 | Left precuneus, BA 7 |
| −34,18,−12 | −2.620 | 0.00004 | 492 | Left insula, BA 47 |
| 26,4,−4 | −1.965 | 0.001 | 136 | Right striatum |
Threshold for statistical significance (uncorrected voxel P<0.005, peak SDM-Z value>1, plus cluster extent>100 voxels).
The analysis revealed no heterogeneity (I2=0% in all clusters). In the jackknife analysis, the paracentral lobule and the left insula clusters were present in all sub-analyses except for the sub-analysis excluding Schneider et al.,23 showing that the significance of these results hinges on the inclusion of this study in the meta-analysis. The right striatum cluster was not present in the sub-analyses excluding Schneider et al.23 or Keedy et al.,28 indicating that the significance of these results depends on the inclusion of both studies in the meta-analysis. The left precuneus cluster was present in all the jackknife sub-analysis, indicating that this result is not dependent on the inclusion on any single study in the meta-analysis.
No publication biases could be identified from the Egger tests or the funnel plots (eFigures 1–4). For the activation at the paracentral lobule, the Egger test was statistically significant (P=9×10−3) and the funnel plot showed a slight asymmetry. Larger studies were associated with larger effects, which is the opposite pattern expected in the existence of publication bias. Similarly, for the right striatum deactivation, the Egger test was statistically significant (P=0.016) but the funnel plot showed again asymmetry in the direction opposite to that expected of publication bias.
DiscussionTo the best of our knowledge, this report is the largest review and meta-analysis of studies comparing brain activity between FEP patients and HC.
The primary findings of the systematic review are that there are differences in activation in several brain regions during cognitive tasks between FEP and HC, including in DLPFC, frontal lobe, thalamus, cingulate cortex, precuneus and insula. This is consistent with the classical model of fronto-temporal abnormality being a key issue in schizophrenia.3,41,42 This finding is also commensurate with previous meta-analytic findings on altered frontal activation in chronic patients43 and with a recent study comparing fMRI of FEP patients with chronic patients.44
However, our voxel-based meta-analysis reveals that the frontal lobe might not be the most extensively altered region when comparing FEP patients with HC. Our results showed significant differences in only two brain areas with decreased functional activity in the left insula (BA47) and left precuneus (BA7). These differences in the results between individual studies and the meta-analysis highlight the important effect of methodological differences between studies; as more studies were included, more brain areas appeared to be significantly different between groups. The number of studies included in the systematic review (N=26) is larger than in the meta-analysis (N=16). This assumes that the meta-analysis, as a set of quantitative procedures, generates conclusions that are more accurate, reliable, and more rigorous than those generated from any single study or in a non-quantitative review.45
The present meta-analyses of all 16 studies indicated that the left precuneus, part of the parietal lobe, is the most clearly implicated area.
Functional experiments have shown the precuneus to be part of the default mode network (DMN).46 The precuneus is an association area with wide-spread extra parietal connections, and there is evidence that the fronto-parietal control network is disrupted in psychosis.47 The precuneus has been recently shown to alter the DMN in FEP48 and also in FEP during auditory verbal hallucinations.49 This alteration in DMN intrinsic activity is associated with poor cognitive function.50 In addition, decreased functional connectivity between the hippocampus and precuneus has been demonstrated in unmedicated patients.51 This structure plays an important role in memory retrieval and self-related visuospatial imagery,46 both of which have been shown to be altered in psychosis.
The other main area implicated in the meta-analysis is the left insula, which is a brain area with broad influences on numerous parts of the cortex and limbic system, primarily the amygdala. The insula incorporates external sensory input with the limbic system.52 Many deficits reported in psychosis include insula functions, which may be associated with altered processing of emotions, visual and auditory perceptions and representations of the self.53 In addition, most studies have reported reduced functional activity of the insula in FEP patients relative to HC.28,54,55 We must note that insula hypoactivation lost statistical significance when we excluded the study by Schneider et al.,23 though this may well be an problem of statistical power given that this study had the largest sample size (n=105) in the meta-analysis.
In contrast, several studies reported increased activation in the insula during different cognitive tasks25,29,31,56 and with auditory verbal hallucinations.49 Although there is no clear explanation for these inconsistencies, we can speculate different reasons for either the increased or decreased activation in the insula. First, the results may depend on the clinical state of the patients at the time of the fMRI, such as their cognitive state, positive symptoms, and the duration of the illness. Many studies have used structural MRI to explore the longitudinal course of FEP finding progressive cortical changes after the onset of a FEP, most notably in anterior cingulate cortex and insula.57,58
There are few longitudinal studies of FEP using fMRI. In a systematic review of this issue,59 we showed that most studies reported a hypoactivation in the limbic system, hippocampus and striatum at baseline. At follow-up, almost all studies reported normalization of activation in these regions. Still, fMRI is not currently used in clinical practice as a predictor of treatment response. The primary explanation for this gap is the heterogeneity in the methodology, particularly in the use of different tasks and fMRI acquisition procedures.
The meta-analysis also showed hypoactivation in the striatum, although this result should be taken with more caution given that it failed to reach statistical significance in two subgroup analyses of the jackknife procedure. Once was when excluding the study by Schneider et al.,23 though as we noted earlier this may well be related to statistical power as this was the largest study in the meta-analysis. The other sub-analysis involved the exclusion of Keedy et al.,28 a smaller study with no apparent particularities other than a short duration of untreated illness (Table 1). We speculate that its relevance in the jackknife analysis is mostly due to chance.
Role of methodological differences in the comparability of results across studiesBoth MR equipment hardware (magnet's strength, manufacturer, antenna, etc.)60,61 and software (acquisition sequence, processing and analysis pipeline, etc.)62–67 have impact in final results published. Unfortunately, the full list of parameters, thresholds, and critical values are not usually included in the methods section of published papers; thus, many of these parameters cannot be clearly considered and analyzed separately. This contributes a confounding effect when considering findings from FEP fMRI studies.
It must be noted that this review has some limitations. First, the meta-analysis was limited to studies with t values of activation peaks to provide the quantitative data. Second, we cannot reject the possibility that the medication effects may be confounded by factors such as the length and severity of psychosis, socioeconomic status, or neurocognitive variables. Third, another potential limitation is that many studies chose to include patients only when they were considered stable enough to undergo the imaging procedure and comply with the task requirements, and thus there may be a gap between the onset of the episode and the scan. Unfortunately, few studies reported these data accurately, which prevented a covariate analysis. Similarly, only some studies reported the actual medication doses, thus a meta-regression with chlorpromazine equivalents was not possible. Finally, the review and meta-analyses were limited to studies using cognitive tasks. Results may be different in studies using other tasks68,69. However, the study has several strengths. First, to our knowledge, this is the first meta-analytic study presenting comprehensive evidence to suggest that the frontal lobe might not be the most extensive altered region related to the pathophysiology of the illness at an early stage of psychosis. Second, the strict selection criteria for the meta-analysis resulted in very low heterogeneity. This study improves our understanding of the neurobiology of comparing the response to cognitive tasks in FEP and HC and provides a foundation that will hopefully lead to greater precision and tailoring of the treatment offered to patients.
ConclusionsOur meta-analytic results show that the left precuneus and insula primarily display aberrant activation in FEP that may be associated with salience attribution to external stimuli and related to the deficits in perception and regulation. Further studies with identical technical procedures in larger samples are warranted to obtain clear conclusions regarding differences in task-related brain activation in FEP patients and HC.
Authors’ contributionsPau Soldevila-Matias: Conceptualization, Methodology, Investigation, Formal analysis, Writing – Original draft preparation, Visualization. Anton Albajes-Eizagirre: Conceptualization, Methodology, Formal analysis, Data curation, Writing – Original draft preparation. Joaquim Radua: Formal analysis, Data curation, Writing – Reviewing and Editing, Supervision. Gracián García-Martí: Writing – Original draft preparation. José M. Rubio: Supervision. Diana Tordesillas-Gutierrez: Writing – Original draft preparation. Inmaculada Fuentes-Dura: Writing – Original draft preparation. Aleix Solanes: Writing – Review and Editing. Lydia Fortea: Writing – Review and Editing. Dominic Oliver: Writing – Review and Editing. Julio Sanjuán: Conceptualization, Writing – Reviewing and Editing, Supervision.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was supported by grants from the Conselleria de Educación (PROMETEO/2016/082) and from the Plan Nacional de I+D+i, the Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación and the European Regional Development Fund (FEDER) (CP14/00041, FI16/00311, PI14/00292, PI17/00402, CPII19/00009 and PI19/00394). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.