Core dysfunctions proposed for psychotic disorders include prefrontal cortex (PFC) dopaminergic hypoactivity, executive function (EF) deficits and reduced gray matter in the PFC. The Val variant of COMT Val158Met polymorphism is associated with reduced dopaminergic signaling in the PFC. However, it is unclear how COMT Val158Met modulates PFC gray matter reduction, EF deficits and symptom severity at the time of the first psychotic episode.
MethodsThe effect of COMT on both EF performance and prefrontal volume (PFC-VOL) was tested in 158 first episode psychosis (FEP) patients and 141 healthy controls (HC) matched for age (range 9–35 years), sex, ethnicity, handedness and COMT Val158Met distribution. EF and PFC-VOL were compared between FEP and HC groups within each polymorphism status (Met/Met versus Val carriers) to assess whether COMT influenced diagnostic differences. Next, correlations between PFC-VOL and EF performance were computed, as well as between both variables and other clinical characteristics of interest (PANSS scores, PAS infancy and premorbid IQ) in the FEP sample.
ResultsCOMT influenced the diagnostic differences mainly in PFC-VOL, but also in EF performance. FEP-Val carriers showed lower EF scores and reduced PFC-VOL compared to the HC group but also poorer EF performance than FEP Met/Met. Poorer EF performance was associated with smaller PFC-VOL, and both were related to increased severity of negative symptoms, poorer premorbid adjustment, and lower estimated premorbid IQ in FEP patients.
ConclusionsOur findings suggest that COMT Val158Met polymorphism might contribute to PFC-VOL reductions, executive dysfunctions and symptom severity in FEP patients.
Algunas de las alteraciones descritas en los trastornos psicóticos incluyen una hipoactividad dopaminérgica en la corteza prefrontal (CPF), déficits en la función ejecutiva (FE) y reducción de la materia gris en la CPF. La variante Val del polimorfismo COMT Val158Met se asocia con una menor disponibilidad dopaminérgica en la CPF. Sin embargo, está aún pendiente de determinar la forma en la que COMT modula la materia gris de la CPF, la FE y la gravedad de los síntomas en el momento del primer episodio psicótico (PEP).
MétodosEl efecto de COMT en el rendimiento de la FE y el volumen prefrontal (VOL-CPF) se evaluó en 158 pacientes con PEP y 141 controles sanos (CS) emparejados por edad (9-35 años), sexo, etnia y distribución de COMT. La FE y el VOL-CPF se compararon entre los grupos de PEP y CS, y en función de la variante alélica del polimorfismo (Met/Met versus portadores Val) para evaluar si COMT modula las diferencias diagnósticas. Además, se llevaron a cabo correlaciones entre FE y VOL-CPF, así como entre ambas variables y las puntuaciones en la PANSS, el ajuste premórbido y el CI premórbido.
ResultadosCOMT moduló las diferencias diagnósticas en VOL-CPF y el rendimiento de FE. Los PEP portadores de la variante Val presentaron menores puntuaciones en FE y reducción del VOL-CPF en comparación con el grupo CS, y menor rendimiento de FE que los PEP Met/Met. Un menor rendimiento en FE se asoció con un menor VOL-CPF, y ambas variables estaban relacionadas con un incremento en la gravedad de síntomas negativos, un peor ajuste premórbido y un menor CI premórbido en pacientes con PEP.
ConclusionesNuestros hallazgos evidencian que el polimorfismo COMT Val158Met podría contribuir a la reducción del VOL-CPF, la disfunción ejecutiva y la gravedad de los síntomas en los pacientes con PEP.
Atypical structure and function of the prefrontal cortex (PFC) observed in psychotic disorders might be an intermediate phenotype coupling the effect of genes and neuropsychological impairments.1 Indeed, psychosis-associated cognitive deficits such as executive function (EF)1,2 show a robust association with abnormal PFC structure and function.1,3–8 These PFC alterations are associated with specific susceptibility genes for psychotic disorders, in particular, those involved in the molecular mechanisms of PFC function.2
In healthy and psychotic individuals increased risk for PFC abnormalities and poorer EF performance is consistently related to a functional polymorphism (Val158Met) of the catechol-o-metyltransferase (COMT) gene,2,9–11 although this relationship has not always been found.12–16 COMT gene effect has also found interacting with other environmental factors, such as trauma or cannabis use.17,18 The COMT gene encodes the major enzyme degrading extracellular dopamine (DA) in the PFC.19,20 The single nucleotide polymorphism (SNP) of the COMT gene, the rs4680, results into an amino acid substitution of methionine (Met) to valine (Val) at position 108/158 (Val158Met). The presence of a Val allele increases the enzyme activity leading to increased DA catabolism and therefore an hypodopaminergic state. On the contrary, the low-activity Met allele results in slower inactivation of extracellular DA within the prefrontal region of the brain leading therefore to the contrary hiperdopaminergia state.21–23
Neuropsychological studies report lower scores in Val carriers for different EF domains, including working memory, metal flexibility, attention control, problem solving and response inhibition [see2 for a review]. Moreover, magnetic resonance imaging (MRI) studies report reduced PFC volume (PFC-VOL) and abnormally increased PFC activation during EF tasks in Val carriers patients with schizophrenia.9,24
However, whether the simultaneous presence of a first-episode psychotic disorder and the type of COMT Val158Met polymorphism modulates both PFC structure and EF performance remains unclear.
The current study assesses PFC-VOL and EF performance in a large sample of first episode psychosis (FEP) patients and healthy controls (HC) matched for age, sex, ethnicity and handedness. It has two major advantages over previous studies. Firstly, EF performance was assessed with a comprehensive neuropsychological battery of tests, which covered several EF domains: attention, working memory, mental flexibility, response inhibition and problem solving. The few available studies examining the effect of the COMT Val158Met polymorphism on EF in FEP have usually used a specific neuropsychological test.12,25 However, EF refers to several cognitive processes required for preparing and executing complex behaviors,26 and therefore multiple tasks measuring the EF domains are necessary to validly determine if EF is affected or preserved in FEP. Secondly, compared to chronic psychotic disorders, studying FEP has the advantages of reducing potential effects of several confounding variables on EF performance and PFC-VOL such as patient recruitment bias, the time of antipsychotic exposure (which have several side effect profiles such as obesity, diabetes, hypertension, and cardiovascular morbidity or dystonia) or chronicity.12,13,25
We hypothesize that individuals with FEP and carrying the Val allele will show decreased PFC-VOL and lower scores on EF compared to HC and FEP Met/Met groups; and these neurostructural and neuropsychological deficits will be associated with worse clinical symptomatology severity.
MethodsParticipantsThe sample was selected from a larger group of FEP patients and healthy controls (HC) that belong to a naturalistic, multicenter, 2-year longitudinal Spanish project named ‘Phenotype-genotype and environmental interaction. Application of a predictive model in first psychotic episodes’ (or PEPs study, for its acronym in Spanish)27,28 enrolled at the Spanish Center of Biomedical Network Research on Mental Health (CIBERSAM).29
Inclusion criteria for patients were: (1) age 7–35 years at the time of first evaluation, (2) duration of psychotic symptoms 12 months or less, (3) speaking Spanish correctly, and (4) having signed a written informed consent. Exclusion criteria for patients were: (1) mental retardation according to DSM-IV criteria30 (2) history of head trauma with loss of consciousness, and systemic disease with mental health impact. A sample of HC matched for age and sex was recruited from the same geographic areas as patients. Inclusion criteria for HC were the same as for patients, except for past or present psychotic symptoms. Exclusion criteria were the same as for patients plus (1) past or present psychotic symptoms or major depressive disorder and (2) first-degree relative with history of psychotic disorder.
For the present study we restricted our analysis to subjects with available MRI data, COMT genotype data, and an EF assessment (see the flowchart in Fig. S1, Supplementary Material). In patients, MRI scans were acquired less than 18 months after the onset of positive symptoms, and in all cases, less than six months from the baseline clinical and cognitive assessment – see.31 The final sample included 158 FEP patients and 141 HC, recruited at six different sites across Spain. A description of the main sociodemographic and genotype information per site can be found in Table S1 (Supplementary Material).
The Institutional Review board of each participant's hospital approved the study design. Written informed consent was obtained from all participants and also from parents/legal guardians for children under 16 years of age after being fully informed about the study protocol.
Sociodemographic and clinical assessmentDemographic, diagnostic, clinical, and functional assessments were performed at baseline by psychiatrists with extensive experience in psychotic disorders and trained in the assessment tools. Information was gathered from medical records, interviews with subjects and parents/legal guardians where appropriate, and other relevant informants.
Demographic variables gathered included age at baseline, sex, ethnicity, handedness by means of the Edinburgh Handedness Inventory32 and parental socioeconomic status assessed with the Hollingshead-Redlich Scale.33
Diagnosis was established (according to DSM-IV-TR criteria)30 by the administration of the Spanish version of the Kiddie-Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL) for children,34,35 or the SCID-I for adults.36 To reduce diagnostic instability,37,38 diagnoses were established at the 12 month follow-up, and were grouped as follows: (1) schizophrenia spectrum disorders (SSD), including schizophrenia, schizophreniform and schizoaffective disorders; (2) broad affective spectrum psychoses (AfP), which included bipolar disorder I and depressive episode with psychotic symptoms; (3) other psychoses (OPs), which included brief psychotic disorders, psychoses not otherwise specified, delusional disorder, and substance-induced psychotic disorder.
The participant's socio-academic adjustment in early stages of life was assessed at baseline using the premorbid adjustment scale (PAS).39 PAS infancy was considered in the current study for measuring premorbid adjustment and, indirectly, developmental compromise.40,41 Poor premorbid adjustment has been found to be a good predictor of various poor outcomes in psychosis42–44 and seems to be strongly related to poorer cognitive functioning in FEP patients.45
Severity of symptoms at baseline was measured with the Spanish version of the positive and negative symptom scale (PANSS).46
Premorbid intelligence quotient (IQ) was estimated using the vocabulary subtest of the Spanish version of the Wechsler adult intelligence scale, 3rd edition [Wechsler 1997] or the Wechsler intelligence scale for children, 4th edition [Wechsler 2003], over and under 16 years of age, respectively.
Lastly, daily and cumulative doses of antipsychotic treatment at scan acquisition were calculated for each patient, and doses were converted into chlorpromazine equivalents following pre-established international consensus.47
COMT Val158Met genotyping (rs4680)Blood samples were collected from participants in EDTA tubes (K2EDTA BD Vacutainer EDTA tubes; Becton Dickinson, Franklin Lakes, New Jersey) at the time of the enrollment in the study. DNA extraction was performed with the MagNA Pure LC DNA isolation Kit III and an LC MagNA Pure system (Roche Diagnostics GmbH, Mannheim, Germany). The DNA concentration was determined by absorbance (ND1000, NanoDrop, Wilmington, Delaware); 2.5μg of genomic DNA was sent for genotyping process. COMT Val158Met (rs4680) was genotyped using the GoldenGate® assay with the Veracode genotyping system (Illumina, San Diego, USA) at the Madrid Node of the Spanish National Genotyping Centre (CeGen).
The genotype distribution of the sample followed the Hardy–Weinberg equilibrium (HC: χ2=.00, p=.999; FEP: χ2=1.248, p=.536).
Firstly, COMT Val158Met polymorphism genotypes were categorized into Val/Val, Val/Met and Met/Met genotypes. After demonstrating that within FEP patients and HC group the Val/Val and Val/Met genotypes did not differ in the variables of interest (EF and PFC-VOL; see Table S2, Supplementary Material), COMT Val158Met polymorphism genotypes were categorized into Val carriers (Val/Val+Val/Met genotypes) and Met/Met14 to simplify the statistical analyses.
Cognitive assessment: executive functionA neuropsychological assessment was conducted 4–8 weeks after inclusion in the study by trained and experienced neuropsychologists to ensure a clinical stability. For each site, reliability in administering and scoring the neuropsychological tests was assessed prior to the baseline assessment in an independent sample of 10 subjects (inter-rater reliability>0.85 for all tests). The cognitive characterization of the complete PEP sample can be found elsewhere.45
EF performance was assessed with a comprehensive neuropsychological battery of tests, which covered several EF domains: attention, working memory, mental flexibility, response inhibition and problem solving. Specifically, the neuropsychological tests used were: FAS test,48 Test Barcelona,49 Trail Making Test (TMT)50 and Wisconsin Card Sorting Test (WCST).51Tables S3 and S4 (Supplementary Material) include a detailed description of the neuropsychological tests and subtests used to estimate the composite EF score and the descriptive z scores (based on the mean and standard deviation of the initial control group) obtained by subjects in all the used cognitive tests.
Raw test scores for the EF domains were converted into z-scores (mean=0, standard deviation=1) based on the performance of the control group in two age ranges 7–15 and 16–35 years. All z-scores were calculated such that higher z-scores reflect better performance. The final EF score for each individual was obtained by averaging the normalized scores of the different EF domains.
Neuroimaging assessmentImage acquisition and analysisT1-weighted MRI scans were acquired on six scanner platforms in the PEPs-Img study. Full details about the characteristics of each platform and acquisition protocol are provided in.31 Scans were evaluated for quality prior to image processing, and no scans were deemed of insufficient quality. Image quality was determined using two tools: (1) “Check sample homogeneity” tool in the SPM-VBM8 toolbox (v.r435, http://dbm.neuro.uni-jena.de/vbm/check-samplehomogeneity/) and the FreeSurfer QA tool (v5.3, http://surfer.nmr.mgh.harvard.edu/fswiki/QATools).52,53
Total and regional prefrontal cortex volumes were computed using the FreeSurfer Software Suite (v5.3) with default settings. The FreeSurfer analysis stream includes intensity bias field removal, skull stripping, and construction of surface models of the pial and white surfaces.54,55 Total brain volume (TBV: total gray matter volume+white matter volume), total prefrontal volume (PFC-VOL) and regional prefrontal volumes were obtained in the native neuroanatomical space of the individual. Nine prefrontal regions per hemisphere were studied: superior frontal, caudal and rostral middle frontal, pars opercularis, pars triangularis and pars orbitalis, medial and lateral orbitofrontal, and frontal pole. Bilateral frontal pole, right medial orbitofrontal, right pars opercularis and right pars orbitalis were excluded of the study, since these regions did not met the interscanner reliability criteria.
The interscanner stability of the imaging outputs was studied using data from 6 healthy controls scanned at each site. Full details about this study are in.31
Statistical analysesData were analyzed with MATLAB (R2015b). Before testing the parametric statistical models, the normal distributions of quantitative variables were assessed (Asymmetry<2 and Kurtosis<756,57) and all quantitative variables were found to be normally distributed. Effect sizes (Cohens d) for significant comparisons were computed and reported.
Demographic, genetic and clinical characteristics of the sampleIndependent samples t-tests were computed to assess group differences in quantitative variables age, estimated premorbid intelligence quotient (IQ), antipsychotic dose, and PANSS (positive, negative and total scores)].
χ2 tests were used to test the independency between categorical variables [diagnosis, sex, ethnicity, handedness, parental socioeconomic status and genotypes (COMT Val158Met variant status)].
Effect of diagnosis and COMT Val158Met polymorphism on executive function and prefrontal volumeBefore comparing HC and FEP groups and running correlational analyses, the effect of potential confounding variables was regressed out from EF scores and PFC-VOL. Specifically, age (linear an quadratic effects), sex, and ethnicity were regressed out from EF scores. Note that the quadratic age term was centered to alleviate collinearities with the linear age term. For total and regional prefrontal volume, scanner site was also included in the regression model. We did not considered medication as confounding variable for two main reasons. First, it was a low and non-significant relationship between the variables of interest (EF and PFC-VOL) and cumulative chlorpromazine doses at scan [rPFC-VOL−Dose=−.08 (p=.29); rEF−dose=−.12 (p=.11)]. Second, within the FEP group, Met/Met and Val carriers did not differ in antipsychotic dose at scan (t=.08 p=.94).
Unstandardized residuals were saved and submitted to subsequent analyses.
Firstly, independent samples t-tests were computed setting EF and PFC-VOL residual scores as dependent variables and diagnosis (FEP versus HC) or polymorphism status (Met/Met versus Val carriers) as independent variables. Furthermore, to assess whether COMT polymorphism modulated diagnostic differences, pairwise comparisons were conducted for HC-Met/Met, HC-Val carriers, FEP-Met/Met, and FEP-Val carrier groups. Bonferroni's method was implemented for multiple comparisons corrections (alpha=0.05; significance threshold after Bonferroni's correction: 0.05/6=0.008).
Secondly, to evaluate if groups differences were attributable to brain size, all comparisons were repeated including TBV in the models as confounding variable.
Finally, to assess if reported findings for Val carriers could be generalized to both Val/Val and Val/Met variants, group differences in their EF and PFC-VOL were tested.
Relationships between clinical characteristics, executive function and prefrontal volumeFirstly, we computed the Pearson's correlations between global/regional PFC-VOL measurements and EF performance, as well as between both variables and other clinical characteristics of interest (PANSS scores, PAS infancy and premorbid IQ) in the FEP sample.
ResultsDemographic, genetic and clinical characteristics of the sampleTable S5 shows the demographic and clinical characteristics of the FEP and HC samples. The HC and FEP groups did not differ in age, sex, ethnicity, handedness, or proportion of Met/Met and Val carriers. The FEP group had lower socioeconomic status and estimated premorbid IQ and higher PAS infancy score (i.e. worse premorbid adjustment) than the HC group. Within the FEP group, the 15% was diagnosed with SSD, the 23% with AfP and the 63% with Ops.
Table 1 shows the demographic and clinical characteristics of the FEP and HC samples, divided by polymorphism status (i.e. FEP-Val carriers, HC-Val carriers, FEP-Met/Met and HC-Met/Met). Within the HC and FEP groups, Met/Met and Val carriers did not differ in age, sex, ethnicity, handedness, or estimated premorbid IQ. Within the FEP group, Met/Met and Val carriers did not differ in PANSS scores or cumulative chlorpromazine doses at scan, but Val carriers showed worse premorbid adjustment (i.e. higher PAS infancy scores) than Met/Met and lower socioeconomic status. Finally, genotype frequency distribution of the COMT polymorphism did not show significant differences across the three subgroups of patients (SSD, AfP and Ops; χ2=.67, p=.72).
Demographic and clinical characteristics of the first episode psychosis (FEP) patients and the healthy controls (HC) group, divided by COMT Val158Met polymorphism status.
| HC (n=141) | FEP (n=158) | |||||
|---|---|---|---|---|---|---|
| Frequency (percentage) | Frequency (percentage) | |||||
| Categorical variables | Met-Met (n=27) | Val-carriers (n=114) | Statistical Tests | Met-Met (n=27) | Val carriers (n=131) | Statistical tests |
| Sex | Males=14 (51.9%) | Males=72 (63.2%) | χ2=1.17 (p=0.279) | Males=15 (55.6%) | Males=93 (71%) | χ2=2.47 (p=0.116) |
| Ethnicitya | Caucasian=25 (92.6%) | Caucasian=101 (88.6%) | χ2=0.37 (p=545) | Caucasian=25 (92.6%) | Caucasian=114 (87%) | χ2=0.166, p=0.418 |
| Handednessb | RH=23 (85.2%); LH=2 (7.4%) | RH=101 (90.2%); LH=10 (8.9%) | χ2=4.40 (p=0.11) | RH=24 (88.90%); LH=3 (11.1%) | RH=112 (85.5%); LH=14 (10.7%) | χ2=1.06 (p=0.587) |
| Socioeconomic statusc | (1)=20 (74.1%) | (1)=84 (74.3%) | χ2=001 (p=0.978) | (1)=20 (74.1%) | (1)=66 (51.2%) | χ2=4.74 (p=0.030) |
| Diagnosisd | – | – | SSD=11 (40.7%); AfP=4 (14.8%) | SSD=64 (48.9%); AfP=19 (14.5%) | χ2=0.67 (p=0.715) | |
| Mean (SD) | Mean (SD) | |||||
|---|---|---|---|---|---|---|
| Quantitative variables | Met-Met (n=27) | Val-carriers (n=114) | Statistical tests | Met-Met (n=27) | Val carriers (n=131) | Statistical tests |
| Age (years) | 21.89 (6.05) | 23.92 (5.99) 23.33 (6.29) | t=−1.58(p=0.12) | 23.33 (6.29) | 22.86 (6.14) | t=0.36 (p=0.72) |
| Estimated IQ | 107.59 (13.11) | 107.89 (14.373) | t=−0.100 (p=0.921) | 95.00 (16.05) | 92.40 (15.93) | t=0.77 (p=0.442) |
| PAS infancy | 3.00 (2.32) | 3.61 (2.84) | t=−1.04 (p=0.301) | 5.11 (3.12) | 6.15 (4.05) | t=5.67 (p<0.0005) |
| Antipsychotic dosee | – | – | – | 46,473.62 (46,009.11) | 45,344.92 (74,605.61) | t=0.08 (p=0.94) |
| PANSSf positive | – | – | – | 16.85 (9.03) | 17.20 (7.91) | t=−0.20 (p=0.84) |
| PANSSf negative | – | – | – | 17.41 (9.44) | 18.51 (8.62) | t=−0.60 (p=0.55) |
| PANSSf total | – | – | – | 69.70 (26.27) | 71.47 (25.49) | t=−0.33 (p=0.74) |
Finally, Table S6 shows the demographic and clinical characteristics of the COMT Val158Met polymorphism variants, divided by diagnostic status (i.e. FEP and HC). Within Met/Met and Val carriers, FEP and HC groups did not differ in age, sex, ethnicity and handedness; but in both polymorphism status FEP patients had lower estimated IQ and worse premorbid adjustment (i.e. higher PAS infancy scores). Only for the Val carriers, the HC group showed lower socioeconomic status.
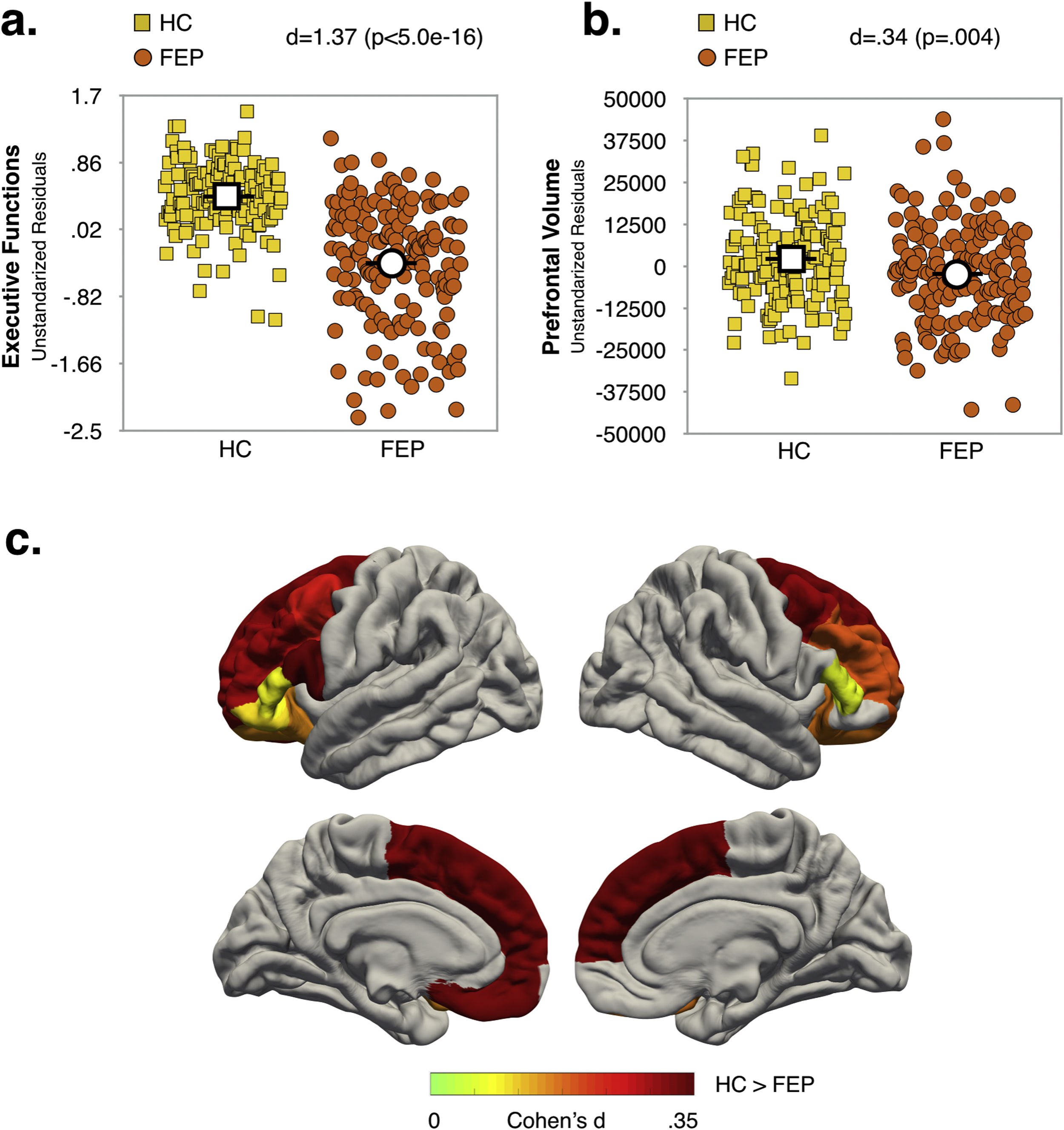
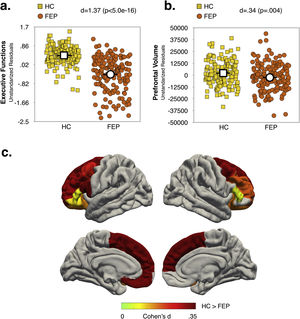
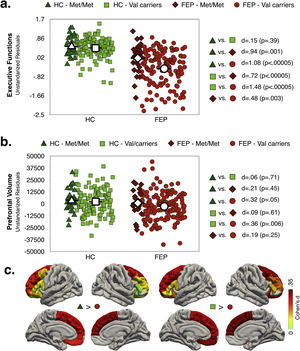
Effect of diagnosis and COMT Val158Met polymorphism status on executive function and prefrontal volumeMain effects of diagnosis and COMT statusAs shown in Fig. 1a and b (see also Table S6, Supplementary Material), the FEP group had lower EF scores (d=1.37; p<.0005) and reduced total PFC-VOL (d=.34; p=.004) relative to HC. The regions with the largest diagnostic effects were the left medial orbitofrontal and bilateral superior frontal cortex (see Fig. 1c, d∼.33; p∼.004). Significant group differences in PFC-VOL remain after controlling for TBV (d=.30, p=.01).
Val carriers had lower EF scores than Met/Met individuals (d=.31; p=.008), but no significant main effect of COMT status on prefrontal volume measurements (i.e. global and regional PFC-VOL) was found (see Table S7, Supplementary Material).
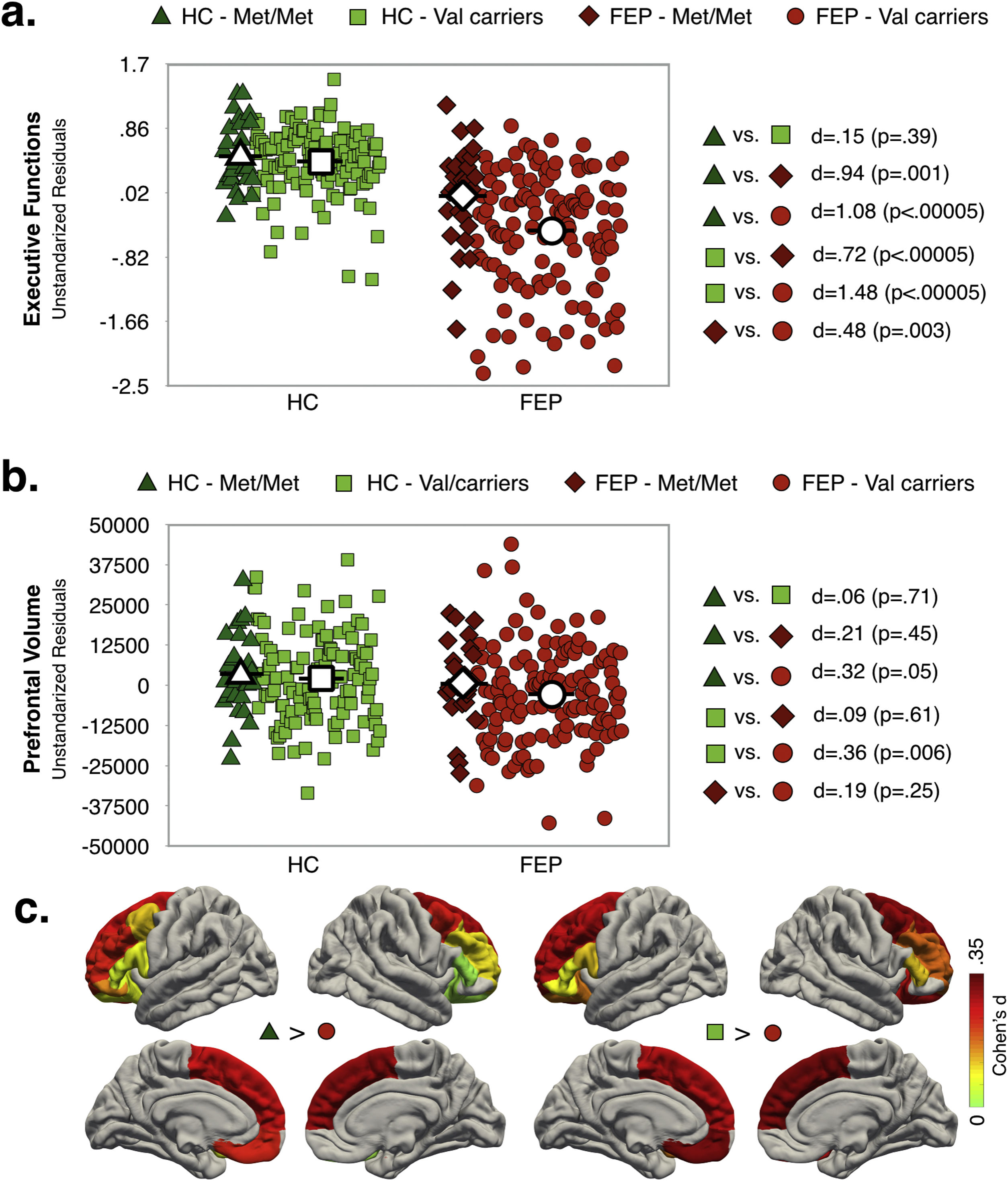
Interaction effect of diagnosis and COMT statusAs shown in Fig. 2a, EF scores were significantly lower in FEP (any genotype) compared to HC group. While for the HC group there were not significant differences between genotype variants in EF performance, for FEP patients, Val carriers showed lower EF scores compared to Met/Met. Note that the highest differences in EF scores were observed between FEP-Val carriers and the both genotypes in the HC group (dHC-Met/Metvs.FEP-Valcarriers=1.08; dHC-Valcarriersvs.FEP-Valcarriers=1.48).
Only FEP-Val carriers had smaller PFC-VOL than the HC group (see Fig. 2b). The region with the largest effect sizes for difference between both genotypes in the HC group and the FEP-Val carriers were found for the right superior frontal cortex (see Fig. 2c and TableS8, dHC-Met/Metvs.FEP-Valcarriers=.42; dHC-Valcarriersvs.FEP-Valcarriers=.36). A similar pattern of results was obtained after controlling for TBV (see Table S9, Supplementary Material)
In other words, COMT Val158Met modulated the diagnostic differences PFC-VOL and EF performance.
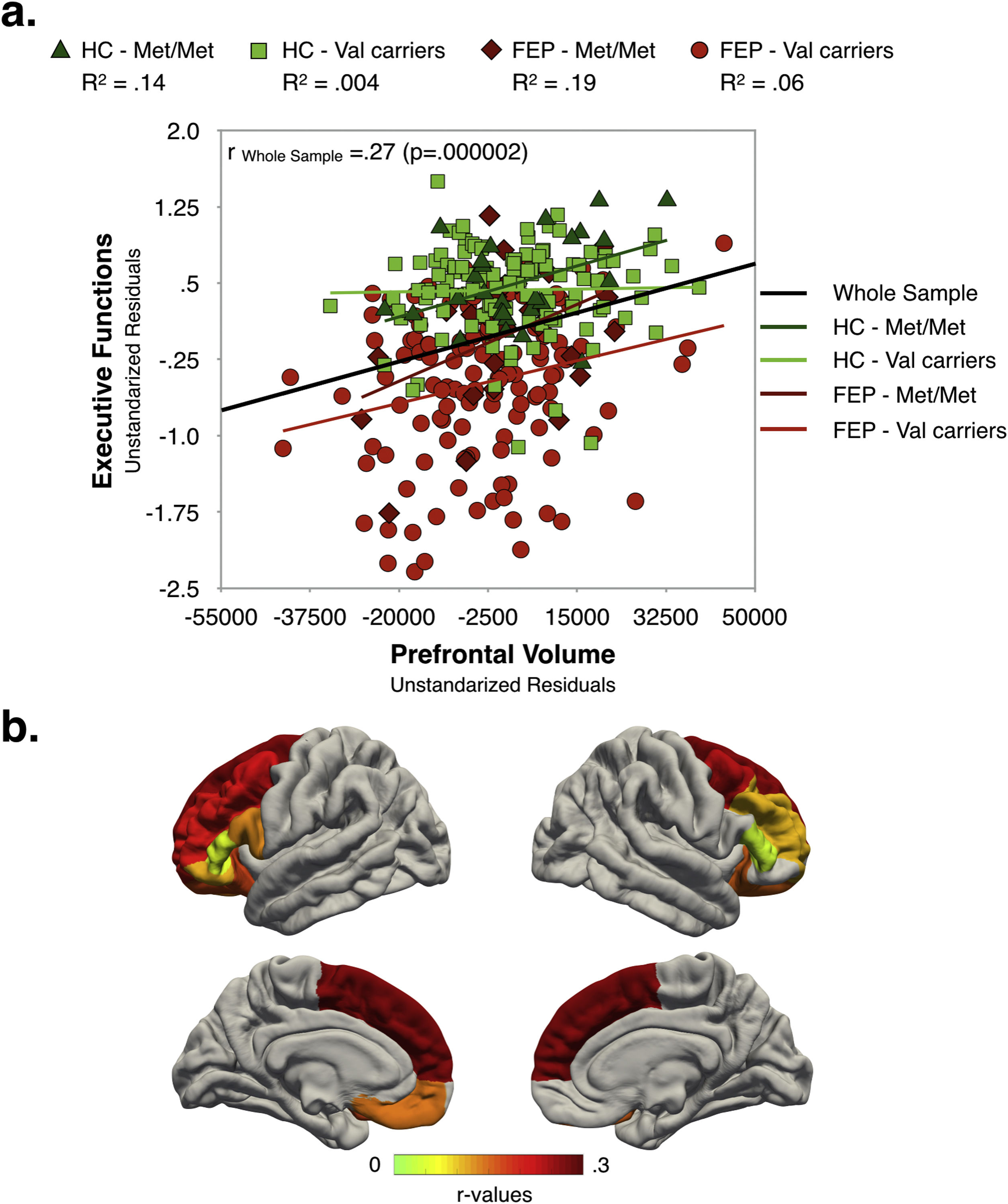
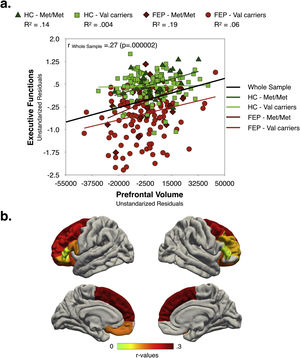
Relationships between clinical characteristics, executive function and prefrontal volumeAs shown in Fig. 3, in the whole sample, better EF performance was associated with larger global PFC-VOL. At a regional level, most volume measurements also displayed a positive correlation with EF scores, with bilateral superior frontal cortex volume being the best regional morphometric predictor of EF performance [r∼0.28 (p<0.0005)] (Fig. 3b and Table S10 in Supplementary Material]. Interestingly, the highest effect sizes were found for Met/Met individuals in both FEP and HC groups.
EF scores were negatively correlated with PANSS negative scores [r=−0.17 (p=0.03)] and PAS infancy score (i.e. the poorer the EF performance the poorer the premorbid adjustment) [r=−0.28 (p=0.0004)], and positively associated with estimated premorbid IQ [r=0.37 (p=0.000002)]. Similarly, total PFC-VOL measurements were negatively correlated with PANSS negative scores [r=−0.16 (p=0.04)] and positively associated with estimated IQ [r=0.34 (p=0.00001)].
DiscussionThe current study demonstrates that the COMT Val158Met polymorphism influences two of the deficits that are already present at the time of a first psychotic episode. Specifically, we found that among FEP patients, those carrying the Val allele (either Val/Val or Val/Met) had poorer EF performance and smaller total and regional prefrontal volumes relative to HC group, and poorer EF performance than FEP Met/Met. We also found that at an individual level, EF scores were associated with prefrontal volume measurements, such that individuals with lower EF scores were those with smaller prefrontal volumes. Moreover, we found that poorer EF performance and smaller PFC-VOL were associated with a worse clinical profile in FEP patients, as denoted by higher PANSS negative score, poorer premorbid adjustment or lower estimated premorbid IQ scores. Taken together, these results suggest that the Val variant of COMT Val158Met polymorphism might confer a risk in FEP patients for showing abnormal phenotypes associated with increased clinical severity.
Our results have showed that EF, measured by a broad battery of tests, is dramatically affected even at the time of the first psychotic episode (large effect size; dFEPvs.HC=1.37). These results are consistent with those studies showing that cognitive impairments are already present at the time of the first episode, including a broad range of cognitive abilities such as EF.58–60 However, results about EF impairment in particular have been inconsistent, probably due to tasks and samples’ study specificities, among other factors.2,61 EF is not a single process but refers to a number of neuropsychological processes allowing for self-control and goal-directed behavior, such as planning, working memory, attention, problem solving, verbal reasoning, inhibition, mental flexibility, set-shifting, multitasking, initiation and monitoring of actions.62,63 Therefore, further studies using and comparing different neuropsychological tasks tapping EF are necessary to determine if EF is affected or preserved at the time of a first psychotic episode.
We also found that in FEP patients, a poorer EF performance was concomitant with smaller gray matter volume in the prefrontal cortex. Moreover, better EF performance was associated with increased PFC-VOL at the individual level, which could be coherent with the reported crucial role of prefrontal cortex in executive tasks. Indeed, previous studies have demonstrated that EF deficits in psychotic disorders are related to abnormal prefrontal structure and function4,64; reviews1–3; and these abnormalities are already present at the time of the first episode.53,65,66 In these lines, worse performance in several EF tasks has been associated with reduced volume and aberrant fMRI activation in prefrontal regions.4,7,8,67–69 Similarly, other genes have shown an association to schizophrenia susceptibility and its symptoms70 That being said, the link between brain structure, function, and behavior is still far from clear, and further studies using multimodal approaches are needed.
One fundamental brain dysfunction proposed for the pathophysiology of psychotic disorders involves prefrontal dopaminergic hypoactivity, and this would be reflected in presence of negative symptoms, cognitive dysfunction and prefrontal cortex morphometric and functional abnormalities.71–73 In coherence with this hypothesis, we found that in our FEP sample prefrontal structural abnormalities and executive dysfunction were influenced by the Val158Met polymorphism of the COMT gene.
Presumably, increased prefrontal DA availability in Met allele individuals derive in a more efficient use of the pF cortex while solving cognitive tasks, as denoted by less prefrontal cortical activation than Val carriers.9 Coherently, Val carriers tend to show poorer performance in prefrontal-mediated cognitive task involving several executive subprocesses74 and reduced regional PFC-VOL.24,75 However, this effect of COMT Val158Met in case–control studies may be present only in psychotic patients,10,24,76–79 only in healthy control individuals,80 in both9,25,74,81,82 or neither,12–16 depending on the sample characteristics (e.g. age, gender and ethnicity distribution], specific task-domain and psychotic disorder studied [e.g. schizophrenia spectrum disorders, affective psychosis or FEP).
In the current study, reduced PFC-VOL and concomitant lower EF performance is observed in FEP Val carriers compared with HC; and better EF performance appear to be associated with greater PFC-VOL. Additionally, while Met/Met and Val carriers individual do not differ in EF performance in the HC group, FEP Met/Met patients show better EF scores than FEP Val carriers. Also, Val/Met and Val/Val do not significantly differ in EF nor in PFC-VOL. Therefore, at the time of the first psychotic episode, both prefrontal structure and cognitive functions seem to be affected mainly in patients carrying a Val allele. Also, other specific characteristics of the present study and sample could explain the found positive results. Importantly, a broad neuropsychological battery of few cognitive tests measuring specific executive subprocesses was used for computing summative EF scores instead of a concrete neuropsychological test estimating a subcomponent of EF by its own, such as working memory (e.g. n-back task), sustained attention (e.g. Continuous Performance Test – CPT) or mental flexibility and problem solving (e.g. Wisconsin Card Sorting Test – WCST). Additionally, a large sample of FEP patients was analyzed, and therefore potential confounding influences of antipsychotic exposure or chronicity on gene-cognition-prefrontal associations were reduced.12,13,25 Also note that FEP and HC with both allelic variants were similar in several relevant variables: age, sex, ethnicity, handedness.
Finally, less impaired EF performance and PFC-VOL in FEP patients seem to be accompanied by less severe negative symptoms, better estimated premorbid IQ and better premorbid adjustment during infancy. This is consistent with previous studies showing that negative symptomsare associated with worse performance in several EF tasks, such as CPT and WCST.83–85 Also, positive correlations between premorbid functioning and EF performance are found for FEP patients.86 Neuroimaging studies show that more severe negative symptomatology in schizophrenia is related to cross-sectional prefrontal volume reductions,87–90 and decreased relative prefrontal cortex glucose metabolism (hypofrontality).91 Furthermore, schizophrenia patients with persistent negative symptoms have gray matter reductions compared to HC in several frontal regions.92,93 Longitudinal studies suggest an association between prefrontal GM loss in FEP patients and negative symptom using different clinical scales (e.g. Brief Psychiatric Rating Scale -BPRS, Positive and Negative Syndrome Scale -PANSS).65,94,95 This linkage between negative symptoms and frontal gray matter reductions is consistent with the physiological hypothesis that negative symptoms of schizophrenia and other psychotic disorders may depend on reduced dopaminergic activity in frontal cortices.96,97
There are several limitations to this study that should be taken into account when interpreting the results. Firstly, we did not have access to full-scale premorbid IQ for the FEP patients. Patients underwent extensive neuropsychological testing, and therefore, to reduce the time of assessment, premorbid IQ was estimated on the basis of two Wechsler Adult Intelligence Scale subtests. Secondly, the influence of antipsychotic and antidepressant treatment on cognitive performance cannot be completed ruled out. However, studying FEP patients reduced potential confounding effects from exposure to pharmacological treatment and other disease effects on cognitive performance and brain structure. Furthermore, (1) non-significant correlations we found between PFC-VOL and EF scores and the cumulative dose of antipsychotics for each individual, and (2) average dose of chlorpromazine equivalents were similar for FEP-Met/Met and FEP-Val carriers. Importantly, the reduced number of subjects in Met/Met group could lead to type II statistical errors. It can be noted that no differences were found in the variables of interest when Val/Val and Val/Met were compared. Finally, due to the multisite nature of the study, our results could have been influenced by differences in MR scanner type. However, to minimize this effect, only regions meeting a proper interscanner reliability (see criteria and procedure in31) were analyzed.
ConclusionsPrefrontal cortex abnormalities, executive dysfunction, and symptoms severity seem to be influenced by the COMT Val158Met polymorphism in FEP. Consistent with idea that dopamine enhances prefrontal neuronal function; COMT Val allele FEP patients show reduced PFC-VOL and greater impairment in frontally mediated executive tasks compared to HC and FEP-Met/Met groups. Also, poorer EF performance and smaller PFC-VOL were associated with a worse clinical profile in FEP patients, as denoted by higher PANSS negative score, poorer premorbid adjustment or lower estimated premorbid IQ scores.
Although the underlying mechanisms for these modulation effects of COMT are not completely understood, PFC structure might therefore be considered as an endophenotype linking the neuropsychological dysfunctions in FEP patients with the effect of specific genes involved in dopaminergic regulation. Clearly, the effects of one polymorphism of a gene could not fully explain the brain structural changes and clinical profile in FEP. However, our findings suggest that COMT Val158Met polymorphism might be useful for identifying high-risk patients at the early stages of psychosis, and thus develop personalized or precision-based strategies to improve their resilience and outcomes.
Authors’ contributionsElisa Rodriguez-Toscano conceived and planned the original idea of the study, collected data, performed the statistical analysis, wrote and approved the manuscript.
Kenia Martínez conceived and planned the original idea of the study, performed the statistical analysis, wrote and approved the manuscript.
David Fraguas contributed to the design of the study, critically reviewed and approved the manuscript.
Joost Janssen contributed to the interpretation of the results, provided a critical feedback, reviewed and approved the manuscript.
Laura Pina-Camacho contributed to the interpretation of the results, critically reviewed and approved the manuscript.
Bárbara Arias contributed to the design of the study, to the interpretation of the results, provided a critical feedback, reviewed and approved the manuscript.
Eduard Vieta supervised the project, critically reviewed and approved the manuscript.
Gisela Mezquida supervised the project, critically reviewed and approved the manuscript.
Silvia Amoretti supervised the project, critically reviewed and approved the manuscript.
Miguel Bernardo supervised the project, critically reviewed and approved the manuscript.
Josefina Castro-Fornieles supervised the project, critically reviewed and approved the manuscript.
Manuel Jesús Cuesta-Zorita supervised the project, critically reviewed and approved the manuscript.
Antonio Lobo supervised the project, critically reviewed and approved the manuscript.
Ana González-Pinto supervised the project, critically reviewed and approved the manuscript.
Iluminada Corripio Collado supervised the project, critically reviewed and approved the manuscript.
Anna Mané supervised the project, critically reviewed and approved the manuscript.
Celso Arango contributed to the design of the study, supervised the project, critically reviewed and approved the manuscript.
Mara Parellada conceived and planned the original idea of the study, supervised the study, wrote and reviewed the manuscript.
Data availabilityAll the data included in the present study is available and can be provided upon request.
FundingThe funding sources did not play any role in the design or writing of the manuscript.
Conflicts of interestDavid Fraguas has been a consultant and/or has received fees from Bristol-Myers-Squibb, EISAI, Janssen, Lundbeck, and Otsuka. David Fraguas and Laura Pina-Camacho have received grant support from Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiveness) and from Fundación Alicia Koplowitz. Eduard Vieta has received grants and served as consultant, advisor or CME speaker for the following entities: Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Janssen, Lundbeck, Novartis, Otsuka, Richter, Sage, Sanofi-Aventis, and Takeda. A González-Pinto has received grants and served as consultant, advisor or CME speaker for the following entities: Almirall, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Ferrer, Johnson & Johnson, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Servier, Shering-Plough, Solvay, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), the Basque Government, the Stanley Medical Research Institute, and Wyeth.
Supported by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO), Instituto de Salud Carlos III (PI12/01303, PI13/02112, PI14/00397, FlammPEPS, SAF16-75500-R), co-financed by ERDF Funds from the European Commission, “A way of making 16 Europe”, CIBERSAM. Madrid Regional Government (S2010/BMD-2422 AGES, AGES-CM-2), European Union Structural Funds, European Union Seventh Framework Program under grant agreements FP7-HEALTH-2009-2.2.1-2-241909 (Project EU-GEI), FP7-HEALTH-2009-2.2.1-3-242114 (Project OPTiMISE), FP7-HEALTH-2013-2.2.1-2-603196 (Project PSYSCAN) and FP7-HEALTH-2013-2.2.1-2-602478 (Project METSY); FP7-HEALTH- F4-2010-241959 (Project PERS), and European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement No 115916; Project PRISM), Fundación Alicia Koplowitz, Fundación Mutua Madrileña and Fundación Familia Alonso. ERA-NET NEURON (Network of European Funding for Neuroscience Research). EV thanks the support of the Spanish Ministry of Science, Innovation and Universities (PI15/00283, PI17/01249, PI17/00997, PI18/00753) integrated into the Plan Nacional de I+D+I y cofinanciado por el ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER); CIBERSAM; and the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya to the Bipolar Disorders Group (2017 SGR 1365) and the project SLT006/17/00357, from PERIS 2016–2020 (Departament de Salut). CERCA Programme/Generalitat de Catalunya. Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2014 SGR 1636).