Differences in bulimic and impulsive behaviours in Eating Disorders (ED) have been associated with cortico-striatal circuit dysfunction at a neurobiological level. We sought to investigate neo-striatal volume as a biomarker in ED subgroups as well as the possible relationship with trauma history.
Material and methodsWe studied 24 female patients: Anorexia Nervosa AN (n=8), Bulimia Nervosa BN (n=9), comorbid ED with borderline personality disorder (EDc; n=7), and a group of Healthy Controls (n=19). Binge eating behaviours and impulsivity scales were used to characterize our sample as well as Trauma Questionnaires and Magnetic resonance imaging (MRI) volumetric manual measurements of caudate and putamen nuclei (striatum).
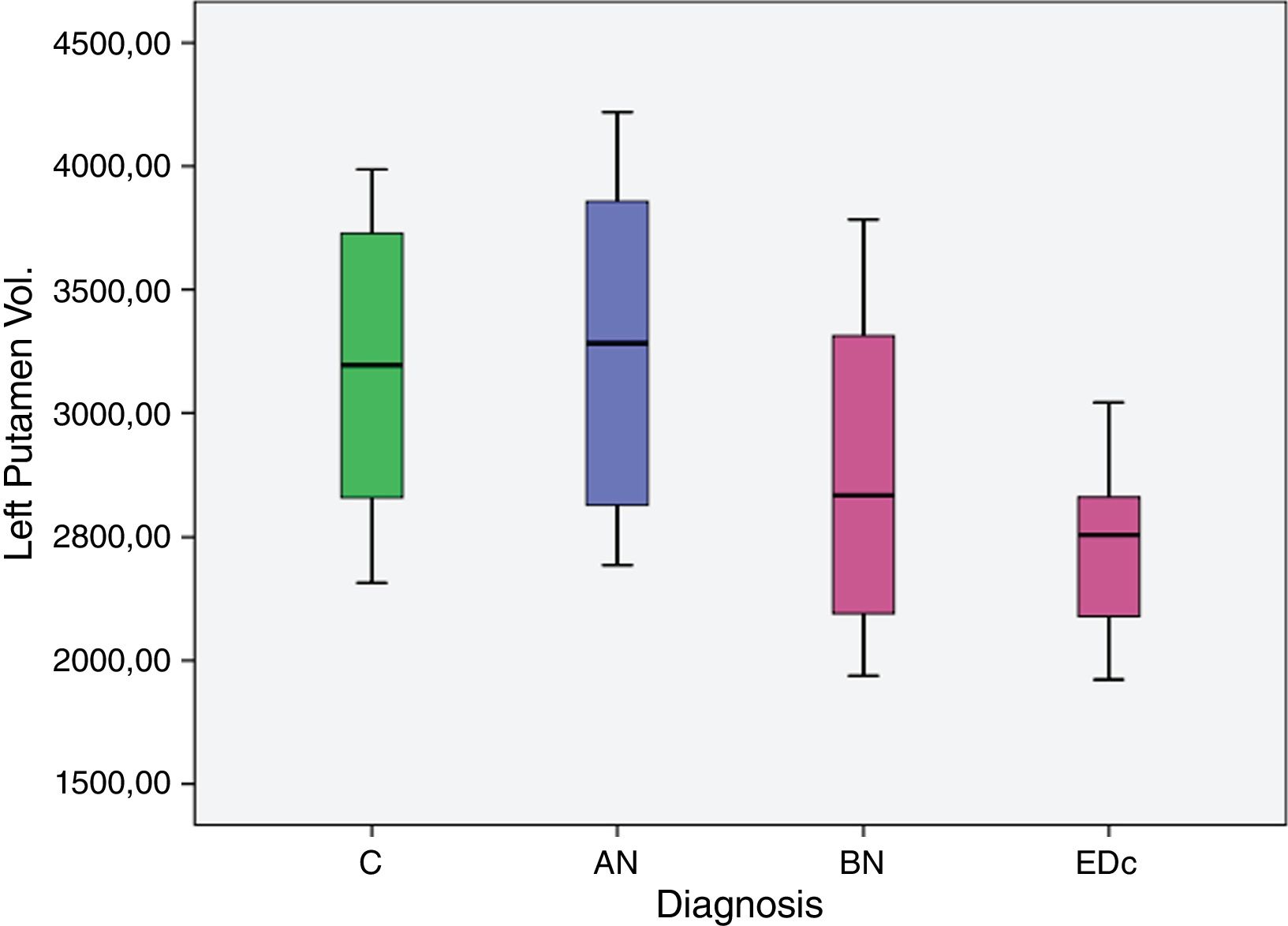
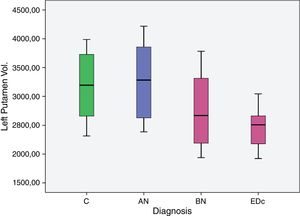
ResultsOur preliminary results showed a significantly larger left putaminal volume in AN compared to the other three groups [C (p=0.008), BN (p<.001) and EDc (p=.001)] and a smaller right putaminal volume in EDc compared to controls (p=.045) and AN (p=.039).
Some negative correlations were found between bilateral putaminal volumes and self-reported general and early traumatization scores.
ConclusionThis pilot study suggested that striatal volumes might differentiate AN from BN and EDc at a neurobiological level with implications for treatment strategies. Larger scale studies should be carried out that allow replication of these data.
Las diferencias en comportamientos bulímicos e impulsivos de los trastornos de la conducta alimentaria (TCA) se han relacionado, desde el punto de vista neurobiológico con una disfunción córtico-estriatal. El objetivo de este estudio fue investigar el volumen neo-estriatal como un biomarcador de los subgrupos de TCA, así como la posible relación con antecedentes traumáticos.
Material y métodosSe estudiaron 24 pacientes con diagnósticos de anorexia nerviosa (AN, n=8); bulimia nerviosa (BN, n=9); TCA comórbido con trastorno límite de la personalidad (TCAc; n=7), y un grupo de controles sanos (n=19). Se utilizaron escalas de impulsividad y comportamiento bulímico para caracterizar a la muestra, así como escalas de trauma y medidas volumétricas de trazado manual de los núcleos caudado y del putamen (estriado) de imágenes de resonancia magnética (RM).
ResultadosNuestros resultados preliminares mostraron un volumen del putamen izquierdo significativamente mayor en las pacientes con AN comparado con el resto de los grupos (C [p<0,008], BN [p<0,001] y TCAc [p<0,001]) y un volumen del putamen derecho menor en el grupo TCAc comparado con los controles. Se encontraron algunas correlaciones negativas entre el volumen del putamen bilateral y algunas puntuaciones auto-referidas de escalas de trauma.
ConclusionesEste estudio piloto sugiere que los volúmenes estriatales podrían diferenciar las pacientes AN, BN y TCAc a un nivel neurobiológico, lo que puede tener implicaciones beneficiosas de cara a las estrategias de tratamiento. Sin embargo, serán necesarios estudios a mayor escala que permitan replicar estos datos.
Anorexia nervosa (AN) and bulimia nervosa (BN), appear to lie on a spectrum of self-regulatory control over feeding behaviours, with excessive control in AN (top-down regulation) and a lack of control in BN (deficit in top-down regulation or predominance of bottom-up functioning).1 Moreover, individuals with AN have difficulty controlling their obsessive thoughts (e.g. preoccupation with thinness, ritualistic behaviours, etc.). Therefore individuals with AN may present with exaggerated cognitive control and seek to reduce negative emotions and anxiety symptoms by restricting food while those with BN may have deficient cognitive control, thus increasing instability and erratic response to appetitive stimuli and obtaining a reduction of negative emotions with binge eating and purging.2–4 Such impulsive eating behaviours are more frequently seen in Eating Disorders (EDs) associated with borderline personality traits.5–7 Based on this “cognitive-control” model, extremes of eating behaviour would emerge from an altered balance of inhibitory processing8 in which cortico-striato-thalamo-cortical re-entrant circuits seem to be involved.1,8–11
There is evidence that the neostriatum (caudate and putamen) at the centre of the cortico-striato-thalamo-cortical circuits, can be considered as the neural relay hub essential in emotional processing, planning, decision-making, response inhibition, set-shifting, identification, planning and implementation of adaptive behaviour.12,13 Many neurocognitive and neuropsychiatric disorders demonstrate functional changes in cortico-striatal circuits which may be structurally characterized by differences in striatal volumes and shape.14 The caudate and putamen have been proposed as structural substrates of neuropsychiatric disorders whether by neuroplastic or pathoplastic changes.15 For example, studies in Obsessive Compulsive Disorder (OCD) have demonstrated abnormally increased activity in the orbito-frontal cortex, anterior cingulate cortex and caudate nuclei16 and volumes in the caudate nucleus correlated significantly and inversely with the severity of tic and OCD symptoms of Tourette's syndrome.17–19 Cortico-striatal dysfunction is also observed in other neurodegenerative disorders such as Fronto-temporal Lobar Degeneration (FTLD), progressive supranuclear palsy or choreo-acanthocytosis.14,20 It can therefore be said that anatomical and functional disturbances in cortico-striatal circuits are involved in the pathophysiology of each of these disorders. Moreover neo-striatal components are ideal candidate structures for analysis due to the highly specific nature of their regional interconnections14,15 and using neuroimaging techniques it is possible to determine their role in the neuropsychiatric disorders.
In conceptualizing preoccupation with body shape and weight and binge eating episodes (and other impulsive behaviours) in ED as similar phenomenological processes to neuropsychiatric obsessive–compulsive and related disorders and impulsive–compulsive spectrum disorders, [e.g., motor urges and intrusive thoughts of Tourette's syndrome21; intrusive thoughts and impaired action cancellation in OCD22; impulsive actions of Attention Deficit and Hyperactivity Disorder (ADHD)23; deficit control in substance-dependent individuals19,24 and even obsessive compulsive symptoms of choreo-acanthocytosis (ChAc)25,26]; we may speculate that common pathophysiological pathways underlie these disorders. There is evidence that these disorders involve disturbances in the cortico-striatal circuits that subserve the capacity for self-regulation1,8,23,24,27 creating a vulnerability for dysregulated appetitive behaviours.1,8,10,11,28 Additionally, these disorders can be considered as being on a compulsivity to impulsivity continuum, characterized by harm avoidance at one end and risk-seeking behaviours at the other8,19,29-31.
Evidence suggests individuals with AN may compensate for dysfunctional reward processing using exaggerated cognitive control2 because of excess prefrontal cortico-striatal circuit activity,2,32 while those with BN and ED with personality disorder comorbidity (EDc), may have deficient cognitive control,33,34 because of lower activation of cortico-striatal circuits1,35,36 similar to what has been seen in studies with Borderline Personality Disorder (BPD),37 thus increasing the instability and erratic responding to appetitive stimuli.
In the context of the similarities between ED and other disorders of the impulsive–compulsive spectrum,29 it is possible that the structural integrity of the neostriatum can inform understanding of the pathophysiology of ED and structural differences in these regions may differentiate ED subgroups.
Traumatic events may play a crucial role in structural brain change.38 Traumatic events appear to determine personality traits and the ability to cope with stressful life events,39 having been linked to various psychiatric disorders such as depression, anxiety and eating disorders.40,41 Furthermore, it has been demonstrated that stressful life events can have an impact on brain structure, morphology and function by modifying key areas of the limbic and cortico-striatal system.42 Impulsive eating disorders such as BN, binge-purging AN and ED with borderline traits, have been more frequently related to trauma history41 so a greater impact on brain structures are expected in these groups.
The aim of the present pilot study was to evaluate whether caudate and putamen volumes would differ among subgroups of ED (AN, BN and ED comorbid, EDc). We also hypothesized that trauma history might, at least in part, also influence these structural changes.
Material and methodsSampleWe studied 24 female patients with AN (restrictive and binge-purging type) (n=8), BN (n=9), comorbid eating disorder and borderline personality disorder- ED+BPD (EDc) (n=7), and a similar group of healthy controls C (n=19), following DSM-IV43 criteria. The EDc group included patients with full BPD diagnosis and comorbid Bulimia Nervosa. All patients were stable, had similar sociodemographic characteristics and were receiving regular ambulatory treatment (Cognitive Behavioural Therapy-CBT, Selective serotonin reuptake inhibitors-SSRI or benzodiazepines). None of them had been taking antipsychotics for the last 6 months; they had not been hospitalized during the last year and had not been diagnosed for more than 10 years.
Healthy women were recruited by advertisement at the University Complutense Madrid and Hospital Clinico San Carlos (HCSC) of the region of Madrid, in an attempt to match them with the patients by age and draw them from the same social background.
MeasuresPatients and controls were interviewed with the Structured Clinical Interview for DSM-IV (SCID I and II)44 to detect any comorbid mental disorders; they also underwent a complete physical examination to exclude major medical disease. None of the participants had a history of head trauma, neurological disease, major medical illness, psychosis or substance use disorders. Clinical subtyping of eating disorders was based on the features present at the time of the study instead of lifetime clinical features.
Impulsivity and bulimic eating behaviours were assessed using both self-report questionnaire measures: (i) impulsivity was assessed using a self-report questionnaire measure, the Barratt Impulsiveness Scale (BIS),45,46 a 30-item questionnaire which assesses impulsive personality traits in three dimensions: attention, motor behaviour and non-planning; and (ii) bulimic behaviours were assessed with a self-rating scale, the Bulimic Investigatory Test, Edinburgh (BITE), a 33-item self-reported questionnaire for the detection and description of binge eating.47–49 For the behavioural assessment of impulsivity, we focused on the general punctuation scale. Finally trauma history was assessed using the Childhood Trauma Questionnaire, CTQ,50 and Trauma History Questionnaire, THQ,51 that were translated into the subject's native language.
Experimental paradigmWe conducted MRI volumetric manual measurements of the putamen and caudate using a previously validated manual tracing method with established reliability using the software ANALYZE 11.0 (Mayo BIR, Rochester, New York, USA).
All subjects were scanned with 1.5 T GE magnetic resonance imaging. High resolution anatomical images were acquired using a 3D T1-weighted turbo field echo pulse sequence. In total 204 contiguous slices were obtained.
Multivariate analysis of co-variance (MANCOVA) using intracranial volume, age and Body Mass Index (BMI) as covariates was used to compare groups (AN, BN, EDc and C) on neo-striatal volumes. The initial sample of 47 was reduced to 43 due to incidental radiological findings and image quality: one patient had hydrocephalus and the other had meningioma. One patient and one control were excluded on poor image quality.
Ethical approval for the study was obtained from the HCSC and Australian National University Ethics Committee. Written informed consent was obtained for all participants.
Image processingImages were transferred to an Intel Apple MacBook Pro computer running OSX 10.5 (Apple Inc, Cupertino, USA), and were checked manually for gross structural abnormalities prior to analysis. The software ANALYZE 11.0 (Mayo BIR, Rochester, NY, USA) was used for image analysis. Images were rescaled to isotropic voxels format (1×1×1 or cubic mm), which were reconstructed via cubic interpolation of the DICOM MRI data. Manual segmentation was axially performed using a standardized view, rigidly aligned in the AC-PC plane. All brain scans were analyzed blindly to all clinical information by trained raters (LL, FAW). A standardized manual tracing protocol was used to trace and quantify the volume of the caudate via tracing its axial outline serially through successive images.52 Intra-rater class correlation was established at .81 for LL on 10 scans (involving 20 comparisons e.g. right and left caudate) and inter-rater class correlation was .84 on 10 scans as above.52 We then used a reference image-based protocol for the putamen, intra-rater class correlation was .86 (FAW, 10 scans, as above) and inter-rater reliability was .84.53 Volumes obtained were normalized in relevant analyses by calculation of total intracranial volume (ICV) for use as a covariate. A stereological point-counting technique manually tracing the intracranial volume measured total ICV with every fourth slice traced. The starting point was randomly chosen from the four most anterior brain slices. The landmarks for delineation and protocol are based upon those previously published.54
Statistical analysisData were analyzed statistically using SPSS (19.0, IBM, Armonk, NY, USA).
Analysis for between-group differences in continuous demographic variables was undertaken with Analysis of Variance (ANOVA). Individual striatal volumes between groups were compared using analysis of covariance (ANCOVA), covarying for ICV, age and BMI. Checks were conducted to ensure there was no violation of normality, linearity, homogeneity of variances, homogeneity of regression slopes and reliable measurement of covariates. To analyze a differential effect of side (left and right) and structure (caudate and putamen) between groups, we undertook a repeated-measures analysis of covariance (RM-ANCOVA) using side and structure as within-subject factors, covarying for ICV, age and BMI.
We used Spearman's correlations to analyze the relationship between trauma scores and striatal volumes within groups.
ResultsDemographic and clinical data are shown in Table 1. Impulsivity and eating behaviour scales supported the classification of our sample: EDc showed higher scores in impulsivity measures and so did bulimic patients compared to AN and controls; bulimic behaviours were more frequently seen in the BN group compared to all other three groups and also in EDc compared to AN and controls.
Demographic variables. Age, Body Mass Index (BMI), Education, Bite and Barratt scales according to diagnosis.
| Variable | AN (N=8) | BN (N=9) | EDc (N=7) | C (N=19) | p | Comparison |
|---|---|---|---|---|---|---|
| Age, years. Mean (SD) | 30.13 (9.25) | 28.44 (11.21) | 32 (10.47) | 23.18 (2.404) | .05 | C<AN & EDc |
| BMI, kg/m2, Mean (SD) | 16.92 (2.80) | 25.90 (7.54) | 19.69 (5.13) | 19.79 (1.54) | .002 | BN>C, EDc & AN |
| Education (years) | 15.14 (2.54) | 14.67 (2.44) | 13.20 (1.78) | 16.33 (1.41) | .16 | C>EDc & BN |
| Barratt | 35 (5.31) | 52.78 (9.92) | 63.43 (15.38) | 33.94 (5.76) | .001 | EDc>BN, AN & C |
| BITE | 13.38 (8.89) | 26.78 (6.45) | 21.71 (7.91) | 3.47 (3.24) | .001 | BN>EDc, AN & C |
AN: anorexia nervosa; BN: bulimia nervosa; EDc: eating disorder comorbid with borderline personality disorder; C: control.
p<.05.
Group comparisons of normally distributed demographic data were applied using univariate analysis of variance (ANOVA) and significant differences between groups are shown.
MANCOVA analysis combining all groups in the model (AN, BN, ED+BPD, and controls), showed a significant difference in bilateral putamen volume among subjects by diagnosis, F (6, 58)=3.913, p=.018. Estimated marginal means by subgroups estimated reflected a significant difference in left putaminal volume, F (3, 29)=3.679, p=.022, and in right putaminal volume, F (3, 29)=3.003, p=.045.
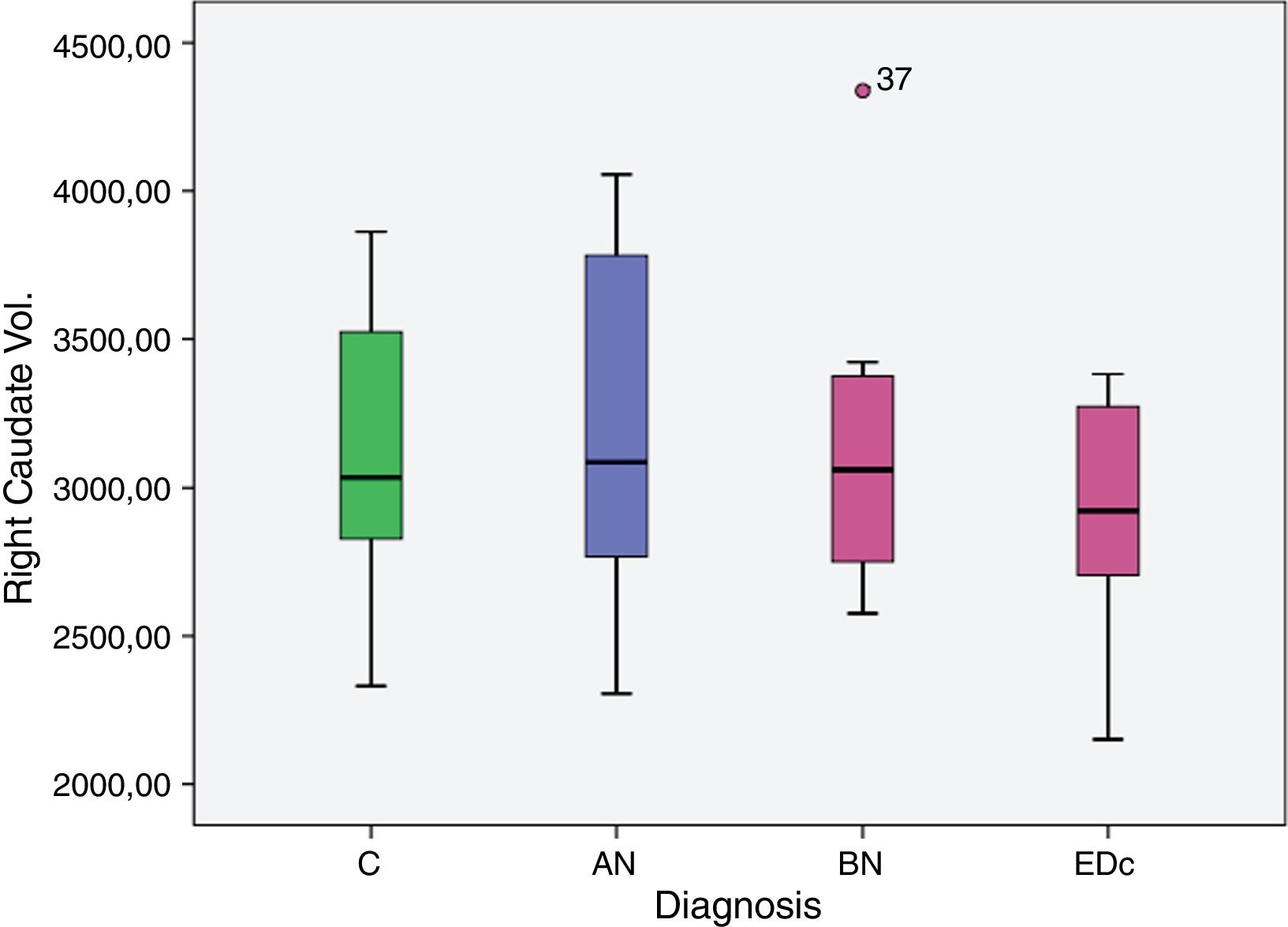
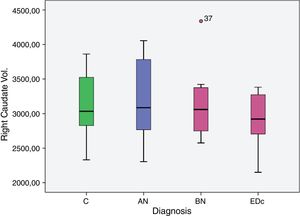
However no significant differences were found in bilateral caudate volume among subjects by diagnosis, F (6, 58)=1.378, p=.239; left caudate volume, F (3, 29)=2.051, p=.129, and in right caudate volume, F (3, 29)=2.119, p=.119.
Estimated marginal means of both right and left caudate and putamen are shown in Table 2.
Estimated marginal means of caudate and putamen volume by subgroups.
| Dependent variable | Diagnosis | Mean (volume in mm3) | Std. error | 95% confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Right caudate vol. | C | 2.956.941 | 102.953 | 2.746.378 | 3.167.504 |
| AN | 3.395.690 | 159.203 | 3.070.084 | 3.721.297 | |
| BN | 3.197.834 | 148.079 | 2.894.978 | 3.500.690 | |
| EDc | 3.273.949 | 159.880 | 2.946.956 | 3.600.941 | |
| Left caudate vol. | C | 3.028.639 | 104.496 | 2.814.922 | 3.242.356 |
| AN | 3.385.468 | 161.588 | 3.054.984 | 3.715.952 | |
| BN | 3.382.473 | 150.298 | 3.075.080 | 3.689.866 | |
| EDc | 3.406.926 | 162.275 | 3.075.036 | 3.738.817 | |
| Right putamen vol. | C | 3.050.741 | 132.783 | 2.779.562 | 3.321.920 |
| AN | 3.121.494 | 201.418 | 2.710.142 | 3.532.845 | |
| BN | 2.762.965 | 186.323 | 2.382.442 | 3.143.487 | |
| EDc | 2.513.387 | 206.939 | 2.090.762 | 2.936.013 | |
| Left putamen vol. | C | 3.041.770 | 128.994 | 2.778.328 | 3.305.211 |
| AN | 3.703.548 | 195.671 | 3.303.934 | 4.103.163 | |
| BN | 2.535.846 | 181.007 | 2.166.182 | 2.905.511 | |
| EDc | 2.703.183 | 201.034 | 2.292.616 | 3.113.750 | |
AN: anorexia nervosa; BN: bulimia nervosa; EDc: eating disorder comorbid with borderline personality disorder; C: control; Std. error: Standard Error; Vol: Volume.
Caudate Volumes. Covariates appearing in the model are evaluated at the following values: ICV=1449.1270313280. Age=27.36. BMI=20.3050.
Data analyzed between disease group differences showed: a greater right putaminal volume in AN compared to EDc (p=.039) and in AN compared to controls (p=.045).
The AN group showed significantly larger (p<.05) left putaminal volume compared to all other three groups [C (p=.008), BN (p=.000) and EDc (p=.001)] as did Controls compared to BN (p=0.037) (see Tables 2 and 3 and Figs. 1 and 2. See Appendix I for details between group analyses.).
Pairwise comparisons of caudate and putamen volume between groups. Estimated Marginal Means by subgroups.
| Dependent variable | Mean difference | Std. error | 95% confidence interval for difference | ||
|---|---|---|---|---|---|
| Lower bound | Upper bound | Sig. | |||
| Right putamen vol | |||||
| AN>EDc | 608.106 | 281.914 | 32.362 | 1.183.851 | .039 |
| C>EDc | 537.354 | 257.338 | 11.799 | 1.062.909 | .045 |
| Left putamen vol | |||||
| AN>C | 661.779 | 234.204 | 183.471 | 1.140.086 | .008 |
| AN>BN | 1.167.702 | 292.082 | 571.191 | 1.764.213 | .000. |
| AN>EDc | 1.000.366 | 273.87 | 441.049 | 1.559.682 | .001 |
| C>BN | 505.923 | 231.107 | 33.941 | 977.906 | .037 |
AN: anorexia nervosa; BN: bulimia nervosa; EDc: Eating Disorder comorbid with borderline personality disorder; C: control; Std. error: Standard Error; Vol: Volume; Sig: Significance. Only significant differences between subgroups are shown in this table. p<.05.
Covariates appearing in the model are evaluated at the following values: Age=27.36. ICV=1449.12; BMI=20.30.
When Trauma History scores (including crime, disasters and physical-sexual history) were analyzed, BN patients showed higher scores in general trauma compared to controls (p=.013); EDc showed higher scores in general trauma compared to the rest of the groups (EDc>AN, p=.004; EDc>BN, p=.034; EDc>C, p=.0001), and no differences were found between AN and BN.
Regarding childhood trauma, the AN group scored more than controls [AN>C (p=.0001)] and more than patients with bulimia nervosa (AN>BN, p=.002) while ED+BPD scored more than controls and patients with bulimia nervosa [EDc>C (p=.0001); EDc>BN (p=.001)].
Within groups, using a Spearman's correlation, the volumes of the putamen (both hemispheres) in combined analysis of ED and control subjects, were negatively correlated (rho: −.506; p=.006) with self-reported early traumatization scores as well as with general trauma scores (rho: −.475; p=.003). By subgroup, general trauma history negatively correlated with right putaminal volume in the BN and EDc group (rho=−.72 for childhood trauma and rho=−.62 for general trauma in BN; rho=−.82 for general trauma in EDc), however p values were not significant. A negative correlation was found in right and left putamen with childhood trauma (rho=−.8 for both left and right putamen) and general trauma (rho=−.812 for right putamen) in the AN group (see Table 4).
Trauma history by subgroups, subtype of trauma and correlation with caudate and putamen volumes.
| Childhood trauma | General trauma | |
|---|---|---|
| C | ||
| N | 15 | 16 |
| Mean±SD | 5.9±2.8 | 1.6±1.3 |
| Median (IQR) | 5 (5–7) | 1 (.3–3) |
| Left put | ρ=−.28 (p=.313) | ρ=−.13 (p=.622) |
| Right put | ρ=−.23 (p=.413) | ρ=−.05 (p=.858) |
| Left caudate | ρ=−.23 (p=.405) | ρ=.19 (p=.482) |
| Right caudate | ρ=−.33 (p=.237) | ρ=.2 (p=.457) |
| AN | ||
| N | 4 | 6 |
| Mean±SD | 27.8±14.7 | 4±4 |
| Median (IQR) | 27 (14.3–42) | 3 (.8–7.8) |
| Left put | ρ=−.8 (p=.2) | ρ=−.46 (p=.354) |
| Right put | ρ=−.8 (p=.2) | ρ=−812 (p=.05) |
| Left caudate | N/A | ρ=−.55 (p=.257) |
| Right caudate | ρ=−.6 (p=.4) | ρ=−.23 (p=.658) |
| BN | ||
| N | 7 | 9 |
| Mean±SD | 12.3±8.8 | 4.3±3.6 |
| Median (IQR) | 13 (4–16) | 3 (2–5.5) |
| Left put | ρ=−.5 (p=0.248) | ρ=.01 (p=.983) |
| Right put | ρ=−.72 (p=0.068) | ρ=.62 (p=.074) |
| Left caudate | ρ=−.64 (p=0.173) | ρ=.4 (p=.329) |
| Right caudate | ρ=−.31 (p=0.504) | ρ=−.06 (p=.881) |
| EDc | ||
| N | 2 | 5 |
| Mean±SD | N/A | 6.8±2.9 |
| Median (IQR) | N/A | 7 (4.5–9) |
| Left put | N/A | ρ=.56 (p=.322) |
| Right put | N/A | ρ=.82 (p=.089) |
| Left caudate | N/A | ρ=−.1 (p=.87) |
| Right caudate | N/A | ρ=−.41 (p=.493) |
AN: anorexia nervosa; BN: bulimia nervosa; EDc: eating disorder comorbid with borderline personality disorder; SD: standard deviation. N/A: not applicable due to the small value of the N in some of the subgroups.
The main findings of this study were: (I) greater volume of the left putamen in patients with AN compared to BN and EDc (II) greater right putamen volume in AN compared to EDc and (III) a larger volume of the left putamen in controls compared to BN.
Previous studies found a decrease of total grey matter volume in adolescents and adults with AN during acute illness and in the recovered state55–59 although others reported that grey matter volumes normalize following recovery.33,59 Reduced caudate and putamen volumes have been seen in recovered restricting-type anorexia and bulimia nervosa but not in a current illness group of patients with AN.11 Also Friederich and colleagues60 reported a decrease in putaminal volume in individuals with AN. Differences may be due to methodology: for example the study by Friederich and colleagues used two different automated methods to measure volumes and only one of these found a decrease in volume in the putamen. Whether or not these anatomical abnormalities reflect the transitory effects of malnourishment is unknown. In contrast, our patients with AN that were stable (had not had any hospitalization during the last year and were on regular ambulatory treatment) and showed an enlarged putamen compared to C, BN and EDc groups with similar sociodemographic characteristics. These changes may be considered a potential consequence of malfunctioning cortico-striatal circuits (hyper or hypoactivation), which may be investigated further as a putative distinguishing marker between the two disorders.
Previous structural MRI data in patients with BN have revealed a significant reduced dorsal putamen compared to controls,11 which is in accordance with our findings.
A decrease in putamen volume (left and right) in the EDc group compared to patients with AN and controls was the most striking and consistently significant difference observed. Because this was not so apparent in other ED subgroups, it might be that the analysis has unveiled a decrease in putamen volume that could be associated with BPD but not with eating disorders. If so, this would be only the second study to investigate putaminal volume in BPD and the first to show a decrease in volume, since only one previous study on BPD has shown an increase in putamen volumes, however substance use disorders comorbidity of the sample may have contributed to these findings.61
Finally, it is also possible that the combination of ED and BPD within the EDc group enhances the effect that either disease alone would have on striatal volume. If this is so, potentially the combination may synergistically amplify the known dysfunctions in dopaminergic system inherent in both ED and BPD,62,63 leading to a greater change in striatal volume; however this will require replication and further investigation to confirm.
From a psychopathological point of view, both groups BN and EDc, have difficulty with impulse control and emotion dysregulation1,64,65 and both EDc and BN demonstrated higher in impulsivity scales (see Table 1). The volumetric reduction of putaminal nuclei in BN and EDc might be explained by malfunctioning cortico-striatal circuits and a failure to engage these circuits appropriately64,66–68 may contribute to impairment in behavioural self-regulation seen in BN (deficits in “top-down” inhibitory control processes). On the other hand, greater volumes of the putamen in patients with AN patients may be linked to their characteristic obsessive psychopathology (rigidity, hypercontrol, compulsivity, ruminative thought) that may arise from hyperactivated fronto-striatal circuits.1,64 However, these structural, functional and psychopathological links cannot be established within this study and are only suggested as possible explanations.
Structural differences in the striatum would likely contribute to ED symptoms of impaired regulation and may differentiate subtypes of ED within the impulsive compulsive spectrum. However, as this is a cross-sectional study, we cannot infer the directionality of effect or causality, or the correlation with clinical characteristics. It may be that degenerative changes in cortico-striatal circuits are leading to a decrease in putaminal volume via deafferentiation.14 If this is so, further structural and functional analysis of the fronto-striatal circuits will elucidate these changes, including the relationship between putaminal volume, length of disease duration and age at first onset.
Considering that putamen volumes in the AN group were greater compared to the more impulsive groups, and that putamen volumes were also enlarged in the control group compared to BN, we could potentially infer a continuum in the following direction: AN>C>BN>EDc. This continuum seems consistent with the fronto-striatal topography model of Obsessive Compulsive Spectrum Disorders (OCSDs) that suggests that the spectrum disorders share striatal pathology as a common attribute, with the clinical phenotype of each disorder determined by the topography of dysfunction within the fronto-striatal circuits.29
In this exploratory approach, both childhood and general trauma history were negatively correlated with right putaminal volume in the BN and EDc group, however N samples were small and p values were not significant. If we take into account that general trauma was more prevalent in BN and EDc (both have difficulty with impulse control), then our results are consistent with previous data that showed correlation between trauma history and diminished striatum, amygdala and prefrontal cortex volumes.69,70 In the AN group a non-significant negative correlation was found in right and left putamen with childhood trauma and general trauma, but p values were not significant. However, these results cannot be interpreted easily due to the small sample size of the subgroups. Moreover, the timeline of these general traumatization experiences was not obtained during assessment, therefore we cannot infer a correlation with changes in striatal volumes over time.
The present findings indicate some future directions to investigate the neurobiological basis of eating disorders psychopathology. However, these results should be regarded as exploratory and larger longitudinal studies should be carried out to help clarify directionality and temporal patterns of change.
LimitationsSome theoretical limitations arise when interpreting these data: (I) the neuropathology underlying this enlargement or atrophy of the striatum is not fully understood,24 in fact blockade of dopamine D2 receptors by antipsychotic drugs has been shown to increase the volume of basal ganglia structures in both animals and humans,71,72 possibly indicating that striatal enlargement is associated with an under-active dopamine system24; (II) there is an overlap of cortico-striatal brain loops implicated in emotion, behaviour and cognition, as well as in the concepts of impulsivity and compulsivity that are increasingly recognized to be linked by shared neuropsychological mechanisms that can switch along the “continuum” over time27,29; (III) structural findings may represent either a cause or a consequence of the symptoms; (IV) lack of some clinical characteristics of the sample such as severity and duration of the disorders may influence the results, therefore the findings should be interpreted with caution.
Methodological limitations: (I) There is a lack of statistical power when comparing striatal volumes among ED subgroups due to the small sample size and so Bonferroni or similar correction for multiple comparisons was not done. Therefore, these results are regarded as exploratory. (II) The lack of longitudinal follow data (i.e. trauma questionnaires).
Funding sourcesThis research received no specific grant from any funding agency, commercial or not-for-profit sectors.
JCLL self-funded computer infrastructure and travel costs to co-supervise and coordinate this research as a component of RMR's doctoral thesis and the Australian United States Scandinavian–Spanish Imaging Exchange (AUSSIE) based at the Australian National University Medical school; he also had partial travel cost support from A.C.T. Health.
Conflict of interestsThe authors declare that they have no conflict of interest.