Subclinical psychotic symptoms are associated to negative life outcomes in the general population, but their relationship with cognitive performance is still not well understood. Assessing the relationship between performance in cognitive domains and subclinical psychotic symptoms in the general population may also help understand the handicap attributed to clinical psychosis, in which these alterations are present.
MethodsSubclinical and cognitive assessments were obtained in 203 participants from the general population by means of the Community Assessment of Psychic Experiences, the Brief Assessment of Cognition in Schizophrenia, the Wechsler Adults Intelligence Scale and the Wisconsin Card Sorting Test. The positive and negative subclinical symptoms and their relationship with age and cognition were examined, followed by assessing the influence of subclinical depression scores on the possible relationships between those subclinical psychotic symptoms and cognitive deficits.
ResultsInverse relationships were found between frequency in the Community Assessment of Psychic Experiences positive dimension and motor speed, and frequency and distress in the Community Assessment of Psychic Experiences negative dimension and motor speed. A direct relationship was also found between distress scores of the positive dimension and executive functions. Both positive and negative subclinical symptoms were related to depression scores.
ConclusionsPsychotic symptoms, similar to those in the clinical population, may be associated with cognitive deficits in the general population.
En la población general, los síntomas psicóticos subclínicos se han asociado con mayores dificultades funcionales en la vida real, pero desconocemos si estos síntomas están asociados a un peor rendimiento cognitivo. El estudio de la relación entre las alteraciones cognitivas y estos síntomas puede, además, ayudarnos a comprender mejor las dificultades que presentan los pacientes con psicosis, en los que estas alteraciones cognitivas están presentes.
MétodosRealizamos evaluaciones clínicas y cognitivas en 203 sujetos de la población general mediante los instrumentos Community Assessment of Psychic Experiences, Brief Assessment of Cognition in Schizophrenia, Wechsler Adult Intelligence Scale y Wisconsin Card Sorting Test. Se evaluó la relación de los síntomas psicóticos subclínicos positivos y negativos con la edad y el rendimiento cognitivo. Además, se evaluó la influencia de los síntomas depresivos subclínicos sobre la posible relación entre síntomas positivos y negativos subclínicos y las alteraciones cognitivas.
ResultadosEncontramos una relación inversa del rendimiento en la prueba de velocidad motora tanto con la frecuencia de síntomas positivos como con el distrés y la frecuencia de los síntomas negativos. También encontramos una relación directa entre el distrés de los síntomas positivos y el rendimiento en función ejecutiva. La puntuación en síntomas depresivos subclínicos se asoció con ambas escalas subclínicas, positiva y negativa.
ConclusionesLos síntomas psicóticos subclínicos están relacionados con déficits cognitivos en la población general, similares a los observados en poblaciones clínicas.
Subclinical psychotic symptoms (SPS), such as delusions or hallucinations which are not so serious or involving in nature to require clinical care, are prevalent in the general population, and particularly in young people, and may be associated with individual cognitive performance. Their possible relationship with relevant cognitive impairment in clinical psychosis has therefore been the subject of several research studies. An inverse relationship between the score on the positive scale of the Community Assessment of Psychic Experiences (CAPE) instrument and verbal fluency was thus reported in a sample of the general population, ranging between 17 and 77 years of age.1 This same group later researched the relationship between the scoring of the same questionnaire and the variables of episodic memory and speed of information processing, finding a weak but significant association between negative subclinical symptoms and processing speed.2 More recently, another group, with a larger sample and using an IT application, reported the association of working memory with subclinical psychotic (strange experiences) and depressive symptoms.3
The above mentioned studies have focused, a priori, on specific cognitive domains to assess their relationship with subclinical psychosis. However, as far as we are aware, few groups have used more extended neuropsychological batteries to this end. Among the latter, in one study with a non clinical adolescent population sample (with and without psychotic symptoms), the authors found there was a link between positive symptoms and processing speed.4 However, the findings from another more recent study show that the score on the scales of positive and negative subclinical psychotic symptoms are related to cognitive performance, using the Measurement and Treatment Research to Improve Cognition in Schizophrenia battery. Its authors found that the subjects with higher scores on the positive scale and to a lesser extent also on the negative scale, showed they had a higher performance in several cognitive domains.5
Assessment of the relationship between SPS in the general population and performance in different cognitive domains may also contribute to a better understanding of the role of cognitive alterations of patients with psychosis. In this clinical syndrome the relationship between the symptoms and the cognitive dysfunction is complex, and it would therefore, a priori, be more promising in the general population to focus attention on their relationship with just one or few cognitive dimensions. Several different associations have been reported to this effect between symptoms and cognition in schizophrenia. On the one hand, negative symptoms have been linked with poorer cognitive performance in verbal fluency, working memory,6 verbal learning, visual or verbal memory,7 long-term memory and the capacity for conceptual thinking.8 On the other hand, symptoms of disorganisation were associated with a short-term lack of attention and memory.8 Furthermore, 2 meta-analyses revealed that negative dimensions and disorganisation had a stronger relationship with cognitive impairment than the positive dimension.9,10 Finally, positive symptoms in schizophrenia have been related to auditory processing deficits.11 Examination of the relationship between SPS and cognitive performance in the general population in dimensions related to psychosis may help us to understand the role of cognitive deficit in the symptoms of psychotic patients on avoiding factors such as chronicity or taking anti-psychotics, which have been associated with different cognitive impairment.12
Among the possible elements of confusion whose influence must be controlled in order to accurately assess the relationship between SPS and cognition are subclinical depressive symptoms. These may contribute to poorer performance through a direct effect on cognition13 or as a consequence of the lack of motivation and commitment in performing the task. SPS have in fact been associated with symptoms of depression in the general population.14,15 Measurement of these subclinical depressive symptoms may be made using the CAPE questionnaire, which also assesses SPS.
The aim of our study was to determine the relationship between SPS and cognitive performance of the general population. To do this, we assessed the relationship of impaired cognitive domains in schizophrenia (assessed using the Brief Assessment of Cognition in Schizophrenia– [BACS] tool and the Wisconsin Card Sorting Test [WCST]) classification and age with frequency and distress of positive and negative SPS (measured through the CAPE questionnaire) in the general population, controlling the possible influence of subclinical depressive symptoms on this possible association.
MethodsParticipantsPosters and press adverts were used to help select 203 participants (aged between 18 and 61) from the general population. All the participants received a small incentive for their cooperation to cover transport costs. Participants with a total IQ under 70 were excluded, as were those with a background of neurological disease or brain trauma with loss of consciousness, substance abuse, except tobacco, or the presence of any psychiatric diagnosis, psychiatric drugs or a family history of psychosis. During the initial interview data were collected by means of a self-report and an analysis of toxins in the urine was carried out to rule out current substance abuse. Data on current employment were classified as “occupied” (currently working/studying) or “unoccupied” (unemployed/retired). Differentiation was made between participants with and without access to further education studies.
All participants signed the informed consent form after reading all the information on the research study. The ethical and research committees of the university hospitals of Valladolid and Álava approved the study in compliance with the WMA International Code of Medical Ethics (Helsinki 1975 Declaration, review of 2008).
Clinical and cognitive assessmentClinical scores were obtained using the Spanish version of the CAPE-42 questionnaire, a self-administered validated and reliable tool.16,17 During the development of this tool, individuals responded to 42 questions on their normal psychiatric state of mind. They pointed out the presence of subclinical positive symptoms (reference ideas, paranoid notions or senso-perceptive impairment, among others), subclinical negative symptoms (such as emotional withdrawal or alogia) and subclinical depressive symptoms (sadness, apathy and others). Frequency of presentation and distress were calculated for each individual symptom. The scores obtained were used to assess their relationship with performance in cognitive tests. For each question, a score varying between 1 and 4 in frequency was given (where 1 meant never and 4 almost always, on the scale of frequency) and distress (were 1 meant does not cause upset and 4 meant it is very upsetting) for the subclinical positive, negative and depressive symptoms. No clinical cut off point was defined. The frequency and distress scales of the depressive symptoms were also used for statistical analysis.
We assessed the cognitive performance of participants in the relevant dimensions using the Spanish version of the Brief Assessment of Cognition in Schizophrenia (BACS) scale.18 These dimensions were verbal memory (the sum of words from a list recalled in 5 consecutive attempts), working memory (retention of digits and repetition in rising order), motor speed (files correctly introduced into a recipient in one minute), verbal fluency (mean of words generated for a category and words which begin with a certain letter), speed of processing and attention (symbol key) and executive function/resolution of problems (Tower of London test). The participants also carried out the Wisconsin Card Sorting Test, which evaluated aspects such as completed categories and percentage of perseverative errors in this test, in the statistical calculations. IQ calculation was made using the abbreviated version of the third edition of the Wechsler Adult Intelligence Scale (similarities, number key, incomplete figures and retention of digits).19
Statistical analysisThe relationship between positive and negative SPS and cognitive performance was analysed using step-wise multivariate linear regression. Scores on distress and the frequency of positive and negative SPS from the CAPE questionnaire were regarded as dependent variables and the results of the cognitive tests and age were regarded as independent variables, with analysis of normality and homoscedasticity of residuals. Statistical calculation was made using the number of completed categories, the percentage of Wisconsin Card Sorting Test perserverative errors, the direct scores of the BACS subscales and the CAPE questionnaire.
In a second step we assessed the influence of depressive symptoms as a factor ruling confusion over any possible relationship SPS and cognitive performance. To this end, we repeated the regression line models with the inclusion of frequency and distress of the depressive dimension among the independent variables, together with the results of cognitive tests.
Finally, we used the Pearson linear correlation coefficients to study the association between frequency and distress scores in depressive symptoms with performance in cognitive tests which were identified as SPS predictors in prior analysis.
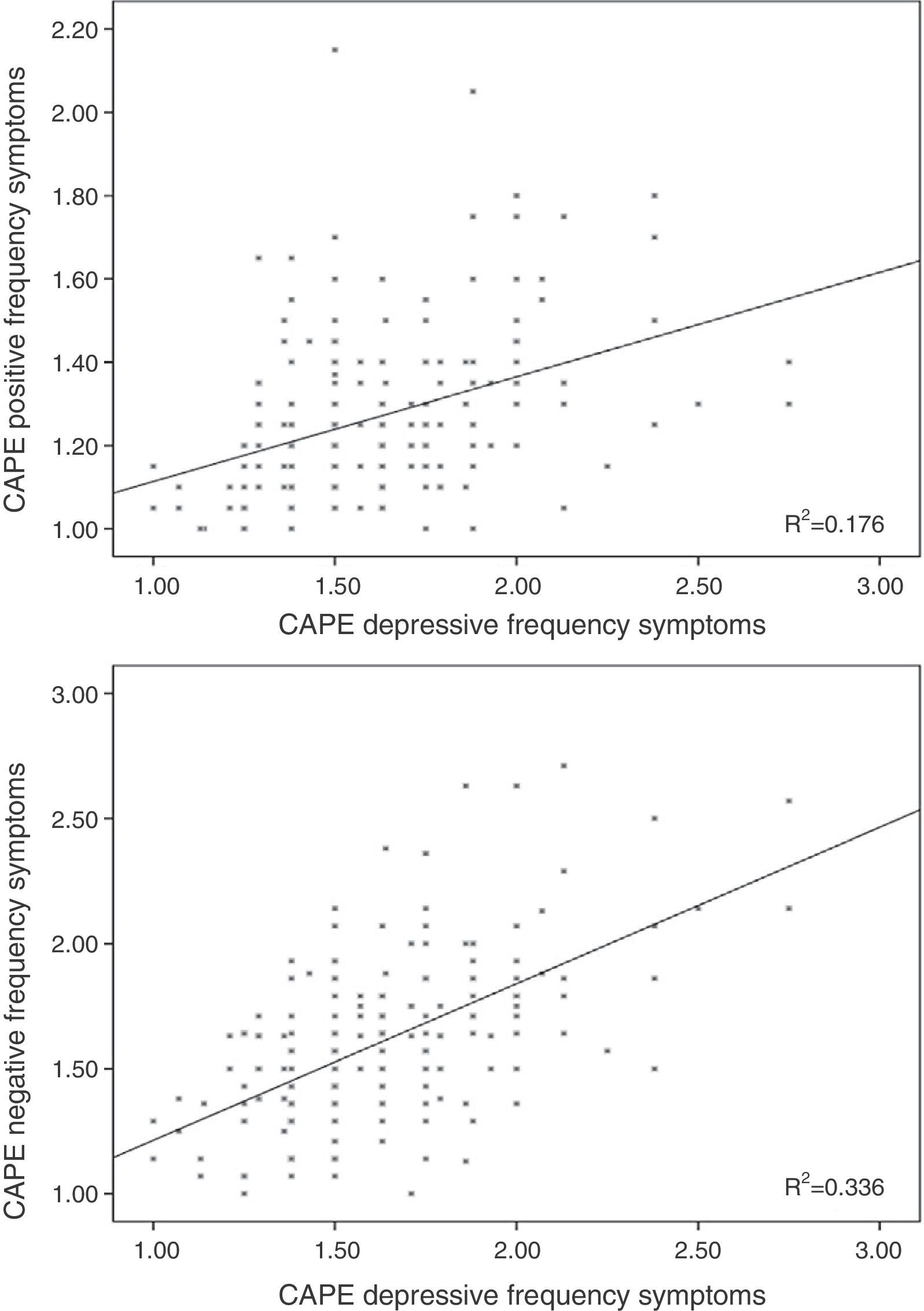
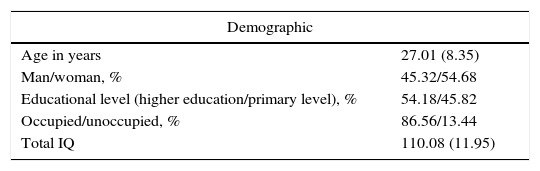
Results96 participants scored 1.50 or above in frequency in the total subscale of the CAPE questionnaire (5 scored 2 or more), whilst 98 scored 2 or more in distress on the same scale (11 scored 3 or above). The mean scores for each subscale are shown in Table 1, and the individual values are represented in Figures 1–3.
Demographic, clinical and cognitive data.
| Demographic | |
|---|---|
| Age in years | 27.01 (8.35) |
| Man/woman, % | 45.32/54.68 |
| Educational level (higher education/primary level), % | 54.18/45.82 |
| Occupied/unoccupied, % | 86.56/13.44 |
| Total IQ | 110.08 (11.95) |
| Clinical (CAPE questionnaire) | Frequency | Distress |
|---|---|---|
| Positive symptoms | 1.26 (0.18) | 1.61 (0.56) |
| Negative symptoms | 1.59 (0.29) | 2.26 (0.65) |
| Depressive symptoms | 1.60 (0.33) | 2.14 (0.68) |
| Total | 1.49 (0.22) | 2.03 (0.53) |
| Cognitive (BACS scale) | |
|---|---|
| Verbal memory | 54.31 (8.63) |
| Working memory | 22.26 (3.76) |
| Motor speed | 67.92 (14.77) |
| Verbal fluency | 23.67 (9.06) |
| Processing speed | 66.71 (12.44) |
| Executive function | 19.29 (14.89) |
Data are expressed as mean (standard deviation), except where indicated. The BACS verbal memory score is the sum total of 5 attempts and in the verbal fluency test this is shown as a mean between the category results and letters.
A significant correlation was observed between the corresponding scores on distress and frequency for positive (r=0.157; p=0.029) and negative (r=0.292; p<0.001) SPS scales.
Relationship between the score on the positive subscale of the Community Assessment of Psychic Experiences and cognitive performanceLinear regression without including depressive symptomsFrequency scores on the subscale of CAPE positive symptoms was inversely related to working memory performance (β=−0.190; t=−2.96; p=0.003) and motor speed (β=−0.149; t=−2.09; p=0.038), and directly with performance in verbal fluency of the BACS (β=0.83; t=2.51; p=0.01; for model R2=0.0783; F=5.66; df=3.197; p=0.001; Table 2).
Summary of the multiple regression results when the Community Assessment of Psychic Experience depression scales are included.
| Pos. S. Freq. | Pos. S. Dist. | Neg. S. Freq. | Neg. S. Dist. | |
|---|---|---|---|---|
| Dep. S. Freq. | β=0.370; t=5.32; p<0.001 | β=0.509; t=8.17; p<0.001 | β=0.123; t=2.24; p=0.026 | |
| Dep. S. Dist. | β=0.155; t=2.23; p=0.026 | β=0.569; t=9.56; p<0.001 | β=0.206; t=3.32; p=0.01 | β=0.601; t=10.93; p<0.001 |
| Mot. S | β=−0.166; t=−2.61; p=0.01 | β=−0.159; t=−2.79; p=0.006 | β=−0.225; t=−4.37; p<0.001 | |
| Ex. F. | β=0.158; t=2.62; p=0.009 | |||
| Age | β=−0.189; t=−3.68; p<0.001 | |||
| Model | R2=0.223; F=18.85; p<0.001 | R2=0.342; F=48.34; p<0.001 | R2=0.387; F=90.68; p<0.001 | R2=0.499; F=47.56; p<0.001 |
Ex. F.: executive function/problem resolving; Dep. S. Dist.: depressive symptom distress Dep. S. Freq.: depressive symptom frequency; Neg. S. Dist.: negative symptom distress; Neg. S. Freq.: negative symptom frequency; Pos. S. Freq.: positive symptom frequency; Pos. S. Dist.: positive symptoms distress; Mot. S: motor speed.
Each column represents the dependent variables. The rows show the independent variables selected as predictors for each dependent variable. The last row shows the complete model data for each of the dependent variables.
No other cognitive variable nor age were significantly associated with frequency or distress in the positive subscale.
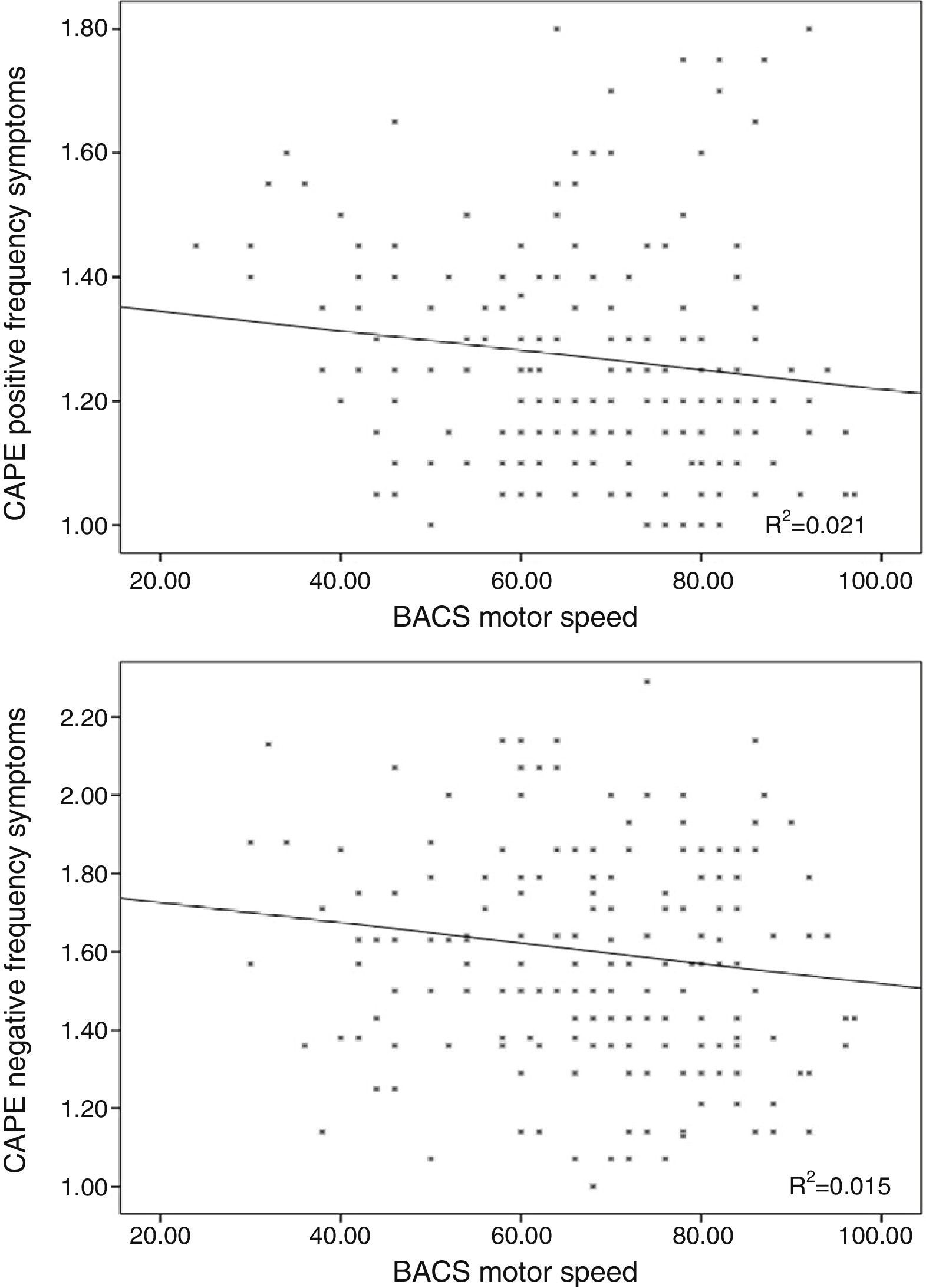
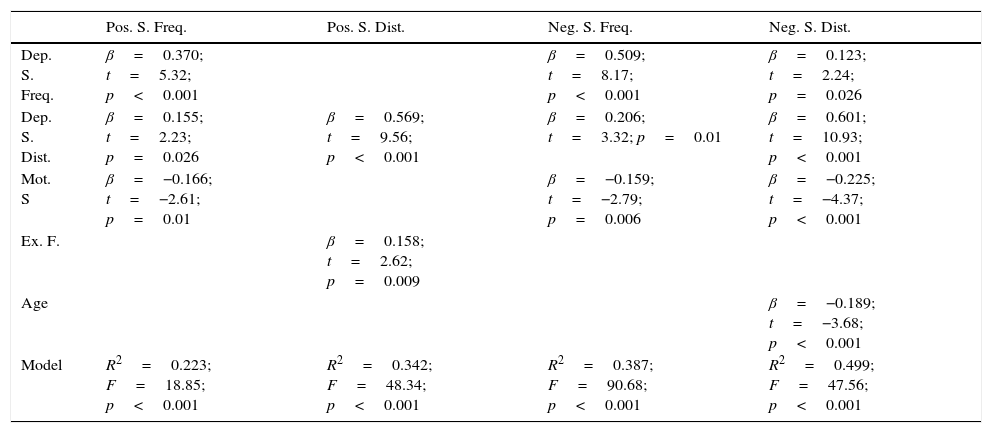
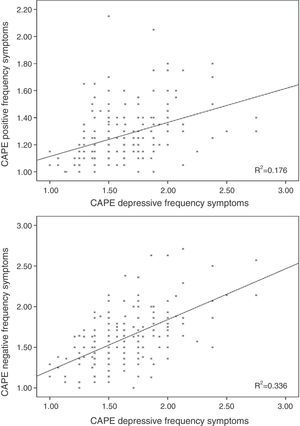
Linear regression including the depressive symptomsThe frequency score in the positive symptom subscale was directly related to the scores in the depressive symptoms for frequency (β=0.370; t=5.32; p<0.001; Fig. 1) and distress (β=0.155; t=2.23; p=0.026) and with the motor speed task of the BACS (β=−0.166; t=−2.61; p=0.01; Fig. 2; for model R2=0.223; F=18.85; df=3.196; p<0.001; Table 2), being excluded from the working memory model and the verbal fluency of the BACS.
Distress of the positive symptoms was directly associated with distress in the depressive symptoms (β=0.569; t=9.56; p<0.001) and performance in the problem resolution task (β=0.158; t=2.62; p=0.009; for model R2=0.342; F=48.34; df=2.189; p<0.001; Table 2).
Relationship between the negative scale of the Community Assessment of Psychic Experiences and cognitive performanceLinear regression excluding the depressive symptom variablesThe frequency scores in the negative symptom subscale showed no relationship with age or with cognitive variables. The distress of the negative symptoms was inversely related with age (β=−0.203; t=−2.95; p=0.004) and motor speed (β=−0.176; t=−2.55; p=0.011; R2=0.074; F=7.75; df=2.195; p=0.001).
Linear regression including the depressive symptoms variablesNegative symptom frequency was directly related with frequency (β=0.509; t=8.17; p<0.001; Fig. 1) and distress (β=0.206; t=3.32; p=0.01) of depressive symptoms, and inversely with BACS motor speed (β=−0.159; t=−.79; p=0.006; Fig. 2; for model R2=0.387; F=90.68; df=2.196; p<0.001; Table 2).
Similarly, the distress of negative symptoms was directly related to frequency (β=0.123; t=2.24; p=0.026) and the distress of depressive symptoms (β=0.601; t=10.93; p<0.001), and inversely with age (β=−0.189; t=−3.68; p<0.001) and motor speed (β=−0.225; t=−4.37; p<0.001; for model R2=0.499; F=47.56; df=4.191; p<0.001; Table 2).
Analysis of correlation between the predictive factorsFrequency and distress of depressive symptoms were moderately correlated (r=0.414; df=201; p<0.001).
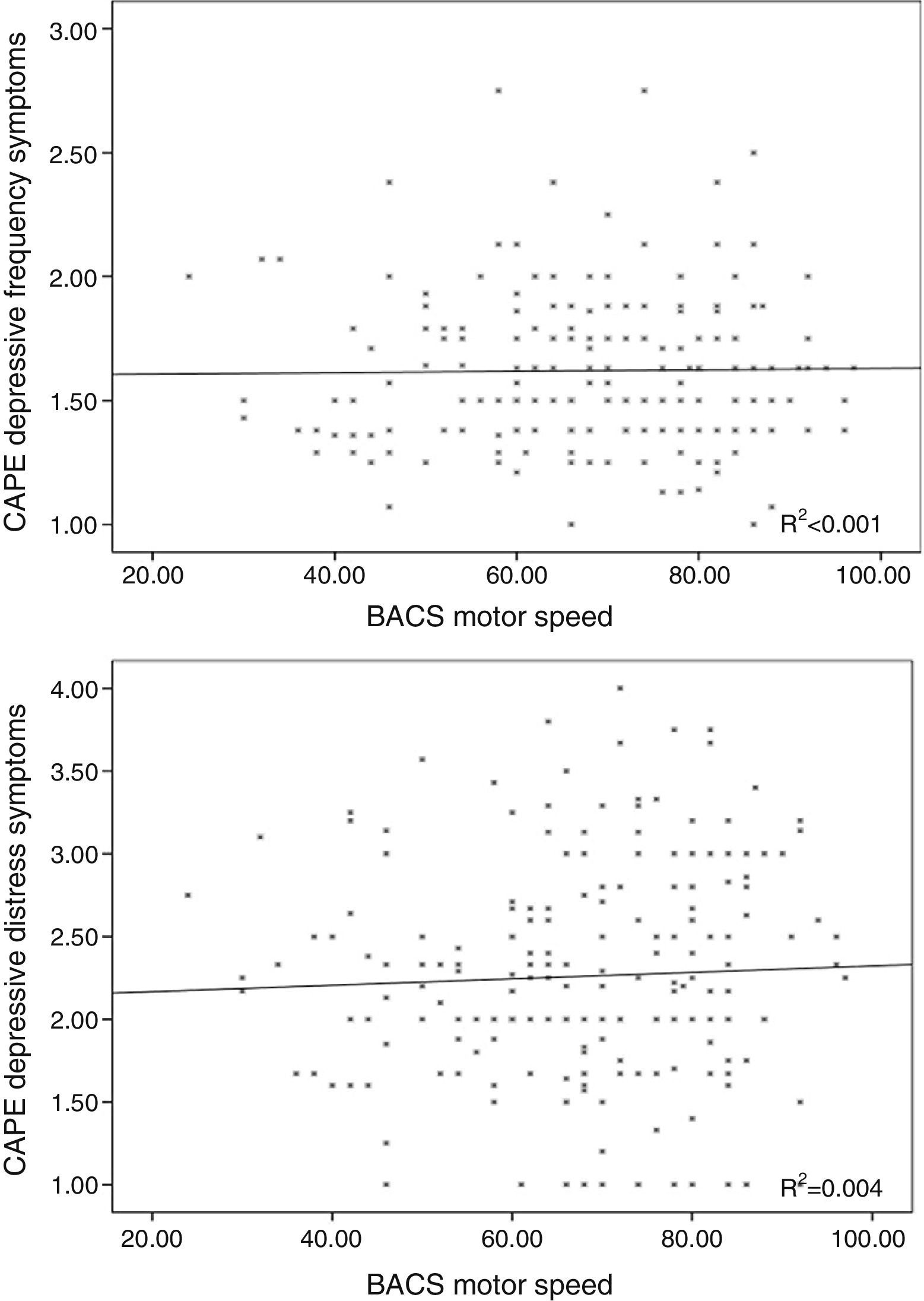
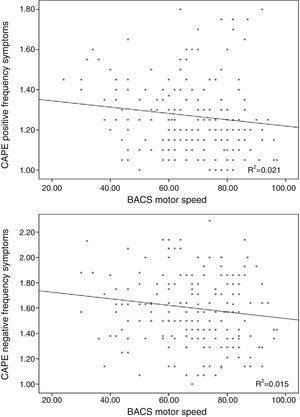
The frequency of depressive symptoms was significantly related with working memory performance (r=−0.163; p=0.02), but not with age (r=0.059; p=0.406), motor speed (r=0.026; p=0.71; Fig. 3) or verbal fluency (r=0.081; p=0.244).
Distress in depressive symptoms was not associated with age (r=−0.033; p=0.642), motor speed (r=0.044; p=0.532; Fig. 3), problem resolution (r=0.001; p=0.990) or verbal fluency (r=−0.053; p=0.446).
DiscussionOur aim was to assess the relationship between SPS and impaired cognitive domains in schizophrenia. We observed a direct relationship between the frequency and distress of subclinical depressive symptoms and subclinical positive (frequency) and negative (frequency and distress) symptoms. After controlling the effect of these depressive symptoms, motor speed continued to be directly associated with the frequency of subclinical positive symptoms and with the frequency and distress of negative symptoms. Motor speed performance was separate from frequency and distress of depressive symptoms.
Relationship between subclinical psychotic symptoms and depressive symptomsThe association between depressive symptoms and positive subclinical symptoms has been previously reported.14,15,20 Our results show a strong association between both dimensions, although we cannot conclude that this relationship is causal. The presence of SPS has been associated with poor social relationships,21 which may subsequently lead to sadness and other depressive symptoms. One alternative explanation would be that the depressive feelings may contribute to a feeling of a threatening environment,22 since self-referential thoughts sometimes form part of the cognitive distortions in depression.23,24
We find there is a strong relationship between the scores in subclinical depressive and negative symptoms. It may be interpreted that similar experiences may have generated similar response in participant in both dimensions of the CAPE questionnaire, bearing in mind their self-administered nature: participants, for example, may have been confused the anhedonism of the depressive experiences with the emotional withdrawal of negative experiences. With regards to this, the subclinical depressive symptoms coincide longitudinally with the negatives, but temporary changes may not be predicted,15 which, together with our results, proves that both dimensions are different but that participants are unable to distinguish them with a self-administered questionnaire.
Relationship between subclinical psychotic symptoms and motor speedThe relationship between motor speed and positive and negative SPS was separate from its association with depressive symptoms. This is in keeping with previous data. A similar relationship between negative symptoms and a slower processing speed (which would include the motor speed task) was found in an out-patient sample of adolescent population with a high risk of psychosis.25
The results in clinical populations also partially support our findings. Motor impairment has been reported in patients with schizophrenia who had had no prior treatment26 or in the first episode.27 A sample of over 200 patients with a first psychotic episode showed an inverse relationship between the scores in positive symptoms and motor speed28 Furthermore, in schizophrenia, a lower motor speed has been associated with ventricular dilatation,29 which was in turn frequently associated with negative symptoms.30 In the same way, negative symptoms (assessed with the CAPE questionnaire) showed a significant relationship with cognitive performance in adolescents with schizoprenia31 and in clinical samples.6,7 These data therefore support the relationship between a lower motor speed and the positive and negative syndrome of the clinical populations. In this context, our findings would support a similar relationship between symptomatology and cognitive variation both in clinical and non-clinical samples.
However, there are also results which do not repeat the relationship between the motor speed task and the positive symptoms in clinical samples, both in adolescents with a risk of psychosis32 and in adults with non emotional psychosis33 and in first-onset psychosis patients.34 However, in this latter study a significant relationship was found between the negative symptoms and the processing speed.
From the relationship described between the motor speed and SPS, one can speculate about the existence of a common neurobiological substratum for the motor slow-down and these subclinical symptoms which could be linked to a dopamine imbalance. This idea is based on the fact that the dopamine neurotransmission is involved in both the motor function and the attribution of aberrant salience,35 related with both positive and negative symptoms in psychotic patients.36 Our findings therefore appear to be coherent with the idea that certain imbalances of the dopamine neurotransmission could increase vulnerability to SPS in the general population.
Relationship between subclinical psychotic symptoms and problem resolvingIn our participants we found there was a direct relationship between planning tasks and problem solving (Tower of London) and the distress caused to subjects by the subclinical positive symptoms. This proved that participants with better executive functions would experience SPS with greater distress, possibly anticipating more intensely the negative consequences of those subclinical psychotic symptoms, and/or showing greater insight into these experiences. Better cognitive performance in other areas, reflected in a better overall cognitive function, could be associated with greater secondary anguish in the face of these experiences, which would be better detected in larger samples.
Other relationshipsOther relationships have been described between cognition and SPS, which have not been repeated in our research. In a large sample of young adults, Ziermans3 reported an inverse association between a poorer working memory and strange experiences (measured as a subset of positive symptoms from the CAPE questionnaire). This same author, however, did not explore other cognitive domains. In the said study, the deficit of the working memory was also associated with depressive symptoms, but the author did not assess the relationship between positive and depressive symptoms. However, we believe that a regression model aimed at assessing the relationship between these dimensions and which includes working memory as a separate variable should also include depressive symptoms in the model. In fact, according to our results there was an inverse relationship between the working memory and positive symptoms, but said relationship disappeared when they had to take depressive symptoms into account. The lack of inclusion of depressive symptoms in previous studies could, therefore, contribute to the differences between their results and ours. Another study, in young adolescents (11–13 years of age) with psychotic symptoms, observed that processing speed was slower.4 This result was not present in our sample, possibly due to the differences between the selected samples and the methods of assessment used. The same group also had lower scores in non-verbal working memory tests in participants with psychotic symptoms. Similarly, in our study we also observed an inverse relationship between working memory and positive subclinical symptoms prior to the inclusion of depressive symptoms in the model.
LimitationsOne of the limitations of our study was the relatively small sample size, although the cognitive assessment made is larger than in similar studies. A more detailed assessment could be made using broader cognitive assessment tools, such as Measurement and Treatment Research to Improve Cognition in Schizophrenia. Moreover, the symptoms of depression were assessed using the e CAPE questionnaire, which could not distinguish them sufficiently from subclinical negative psychotic symptoms. Separate assessment of depression may help to clarify the relationship between these dimensions. The sample selection method using adverts and posters may have led to participants with a higher level of motivation and a higher functional level being chosen. This may have resulted in a lack of obvious differences in the cognitive domains, such as memory or verbal fluency, reported in other studies. In the statistical analysis no explicit correction was made by multiple comparisons although this may not have been essential since a multivariate step-wise regression model was used.
ConclusionAn extensive cognitive study revealed that the subjects of the general population with greater frequency of SPS are also characterised by higher scores in depression and poor performance in the motor speed (i.e. a slower motor speed). This association would support the theory of continuum of psychosis, which divides the population into those with less serious psychotic symptoms, with no need for clinical care, and into those with the most severe symptoms, present in schizophrenia.37
Ethical responsibilitiesProtection of people and animalsThe authors declare that the procedures followed are in keeping with the ethical norms of the committee of responsible human experimentation and in accordance with the WMA International Code of Medical Ethics and the Helsinki Declaration.
Data confidentialityThe authors declare they have adhered to the protocols of their centre of work regarding the publication of patient data.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in the article. This document is in the possession of the author of correspondence.
FundingThis study was financed by the Fondo de Investigaciones Sanitarias (Instituto de Salud Carlos III) (VM, concession number FIS PI080017, FIS PI1102303) and the Gerencia Regional de Salud de Castilla y León (VM, concession number GRS 249/A/08, GRS 613/A/11); a pre doctoral grant from the Universidad de Salamanca and Banco Santander (VS); a Marie Curie Intra European grant, 7th European Commission Framework Programme (AD, 330 156-CODIP); Fondos de Investigación en Salud del Gobierno Español (SR and CV, concession number FIS: PS09/02002; PI10/01430; PI10/01746; PI11/01977; PI11/02708; PI12/02077, EC10-333, EC10-220 Cd08/00269; CM08/00213; CIBER CB07/09/0024 CIBER Red), European Regional Development Funds and local subsidies, including the Gobierno del País Vasco and Universidad del País Vasco (SR and CV, concession number 20009111047, 2011111113, 2011111170, 20111064, Kronik11/010; CANNABIS-SAIO10-PC10BF01, 2010111170, IT679-13, SAIO11-PE11BF006, SAIO11-PE11BF007) and Stanley (SR and CV, concession number 03-CR-003).
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Martín-Santiago O, Suazo V, Rodríguez-Lorenzana A, Ruiz de Azúa S, Valcárcel C, Díez Á, et al. Relaciones entre síntomas psicóticos subclínicos y rendimiento cognitivo en la población general. Rev Psiquiatr Salud Ment (Barc). 2016;9:78–86.