The objective of this analysis was to evaluate the clinical and economic value of the use of 50mg-desvenlafaxine compared to the usual care (mix of duloxetine and venlafaxine) in the outpatient treatment of major depressive disorder after first line treatment failure (relapse) in Spain.
Materials and methodsA Markov model was used to follow up a cohort of major depressive disorder patients for 1 year after failure of first-line treatment with a serotonin-specific reuptake inhibitor and estimate outcome measures (percentage remission and depression-free days) and accrued and direct costs incurred during outpatient treatment of major depressive disorder. In order to obtain the efficacy data related to the treatment alternatives, a literature review of clinical trials was performed. A panel of clinical experts validated the use of clinical resources employed in the estimation of economic outcomes together with model assumptions. The analysis was performed in 2014 from the perspective of the National Health System.
ResultsDue to fewer discontinuations, initiating second line treatment with desvenlafaxine was associated with more depression-free days and a higher percentage of patients in remission versus usual care: 1.7 days and 0.5%, respectively. This was translated into lower drug and events management costs, and an overall cost reduction of €108 for the National Health System.
ConclusionsIn patients who have not responded to a first-line serotonin-specific reuptake inhibitor therapy, desvenlafaxine-50mg was clinically similar in effectiveness, but a less costly option, compared with a weighted average of duloxetine and venlafaxine for the second-line treatment of major depressive disorder patients from a payer (National Health System) perspective in Spain.
El objetivo del análisis fue evaluar el valor clínico y económico del uso de desvenlafaxina-50mg comparado con la práctica médica (pool de pacientes tratados con duloxetina o venlafaxina) tras el fracaso del tratamiento de primera línea de la depresión mayor en España.
Materiales y métodosModelo Markov que sigue una cohorte de pacientes diagnosticados con depresión mayor, tras el fracaso del tratamiento de primera línea con inhibidores selectivos de la recaptación de serotonina y estima la respuesta al tratamiento (porcentaje de remisión y días libres de depresión) y los costes directos incurridos durante el tratamiento. Los datos de eficacia considerados en el análisis fueron obtenidos de ensayos clínicos a partir de una revisión de la literatura. Los principales supuestos del modelo, así como el uso de recursos, fueron validados por expertos clínicos. El análisis de realizó en el año 2014 desde la perspectiva del Sistema Nacional de Salud.
ResultadosDebido al menor número de discontinuaciones, iniciar el tratamiento de segunda línea con desvenlafaxina se asoció a un mayor número de días libres de depresión (+1,7) y un mayor porcentaje de pacientes en remisión (+0,5%). Esto se tradujo en un menor coste farmacológico y del manejo de los eventos y en un ahorro total para el Sistema Nacional de Salud de 108€.
ConclusionesEn pacientes no respondedores al tratamiento con inhibidores selectivos de la recaptación de serotonina en primera línea de la depresión mayor, desvenlafaxina-50mg mostró una efectividad clínicamente similar a los otros tratamientos usados en la práctica médica, pero con un menor coste para el Sistema Nacional de Salud.
Depression affects some 350million people in the world according to the World Health Organization,1 and it is foreseen that in 2020 it will become the second most common cause of disability, after cardiovascular diseases.2 An epidemiological study carried out on a representative sample of the Spanish population revealed a prevalence-life of mental disorders of 19% and a prevalence-year of 8.4%. It also revealed that major depressive disorder (MDD) is the most frequent mental disorder, with a prevalence-life of 10.6% (6.4% in men and 14.5% in women) and a prevalence-year of 4.0%.3,4 The variables that increase the risk of depression include personal, cognitive, social, family and genetic factors.5 Of the patients that attend primary care visits for any reason, 14.7% present depression; of these, 72% are diagnosed and 34% receive treatment with antidepressants.6 However, both the detection rates and treatment rates are higher in the more severe forms of depression.7
Depression is one of the main causes of disability and of costs for the healthcare system and for society as a whole. Its high cost is the result of a combination of factors, such as its high prevalence, the increased incidence in the developed nations, its high level of consumption of healthcare resources, its effects on general functioning, the associated loss of productivity and employment, and because its impact on disability is proportionally greater than that derived from other chronic illnesses.8 An estimate of the total costs of mental disorders in Spain showed that affective disorders (depression and bipolar disorder) represented costs of 2332million euros in 2002. Of these, the direct healthcare costs represented 648million euros (27.8% of the total cost) and the indirect costs, 1685million euros (72.2% of the total cost). The direct healthcare costs of affective disorders represented 1.8% of the total public healthcare expenses in Spain in 2002.9 There are several international estimates as to the burden and cost of depression. In 2004, the total costs in Europe of depression were said to be 117,851million euros. Of this amount, 35.4% corresponded to direct costs and 6.5% a indirect costs,10 loss of productivity attributable to morbidity or premature death from suicide and other causes. Another study indicated that the costs of depression in Sweden doubled between 1997 and 2005, finally reaching 3500million euros.11 Annual direct costs, including the cost of healthcare and pharmaceutical care, were higher than those borne by the standard patient in the healthcare system. In addition, these costs notably increase in relation to the severity of the clinical picture and the lack of therapeutic response.12
Most of the Clinical Practice Guidelines published since 2008 recommend the selective serotonin reuptake inhibitors (SSRIs) as the drugs of first choice in the treatment of moderate–severe MDD. The National Institute for Health and Care Excellence recommends continuing antidepressant treatment for at least 6 months after the remission of the episode, at which point the possibility of prolonging the treatment should be evaluated taking into consideration the number of previous episodes.13 Approximately 38% of the patients with MDD do not respond to treatment with SSRIs for 6–12 weeks and 54% do not achieve remission.14 Consequently, second-line treatment should be initiated with another group of drugs, among which are the serotonin–noradrenaline (or -norepinephrine) reuptake inhibitors (SNRIs). There are at present 3 SNRIs marketed in Spain: venlafaxine, duloxetine and desvenlafaxine. The main pharmacokinetic differences between desvenlafaxine compared to venlafaxine and duloxetine are: it presents a high bioavailability, it is not a substrate nor does it have any activity on glucoprotein P, and it has a metabolism that practically does not depend of the cytochrome P450 system, with the fact that it has minimum activity on the isoenzyme 2D6 and does not behave as a substrate of that isoenzyme being especially important. The enzyme CYP2D6 metabolises approximately 25–30% of the current drugs, with the consequence being the appearance of variations in clinical response reported with venlafaxine or other antidepressants or the need to adjust the dosage depending on the genotype.15 Given that the patient with depression frequently takes other drugs, comedication with a strong CYP2D6 inhibitor can turn a patient that metabolises rapidly or super-rapidly into one that metabolises slowly.16 In this sense, using drugs such as desvenlafaxine (in whose metabolism the CYP2D6 metabolic route barely intervenes) not only favours similar pharmacokinetics in individuals having both slow and quick metabolising phenotypes, it also does not interfere in the action of other concomitant drugs with metabolisation through CYP2D6.17
The STAR*D study was designed to assess the effect of 4 lines of sequential treatment in patients that had not responded to the initial treatment with citalopram, with a design that attempted to imitate standard clinical practice as much as possible.18 The probabilities of remission of 37% and 31% for first-line and second-line treatment, respectively, dropped substantially to 14% and 13% in third- and four-lines.19 This indicated the importance of establishing correctly, and in time, the first- and second-line treatments. Given the lack of clinical and economic evidence between different SNRI treatments recommended for second line in conditions of normal medical practice, the objective of this study was to assess the clinical and economic value of using desvenlafaxine instead of the normal clinical practice represented by using a mixture of venlafaxine and duloxetine in the treatment of MDD in Spain.
Material and methodsWe studied a hypothetical cohort of patients treated with MDD after the failure (relapse) of first-line treatment with SSRIs. The characteristics of this cohort reflect the design of the STAR*D clinical trial.18 All the data used to model the management of this cohort consequently reflect results of studies published (obtained using a literature review), in which the patients included had characteristics that were similar to the patients included in the STAR*D trial.
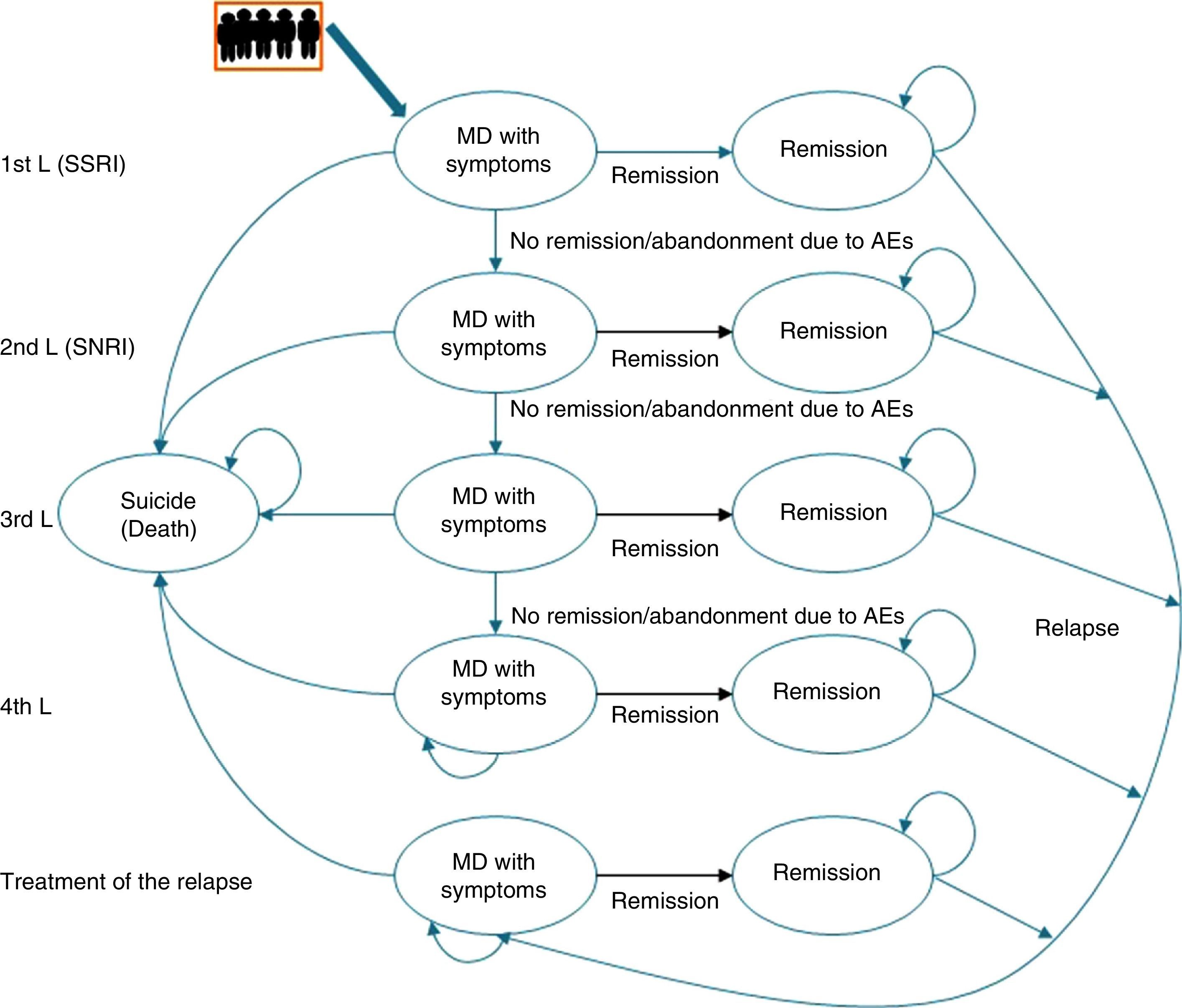
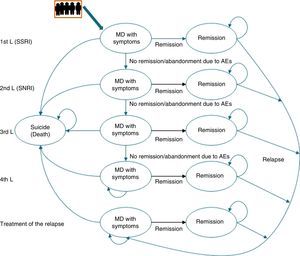
Description of the pharmaco-economic modelThe economic analysis was carried out using the Markov model defined by 2 health states: remission, MDD with symptoms, and death (suicide); these states replicated the evolution of the illness and the patient response in different lines of treatment (Fig. 1).20
The Markov model for studying the evolution of the illness and patient response to the various treatments for major depressive disorder in Spain. AE, adverse event; MDD, major depressive disorder; SSRIs, selective serotonin reuptake inhibitors; SNRIs, serotonin–norepinephrine reuptake inhibitors.
Markov Models are used to represent the natural history of diseases that evolve with states of health that change over time and that present events due to exposure to risks. In these models, the individuals can only remain in a specific state until the end of a defined period [sic]; the patient can “pass” between states.
Likewise, to simulate patient flow through the different lines of treatment of MDD, the patients entered the model in the first line of treatment in the health state “MDD with symptoms” and, depending on the efficacy of the treatment, they could cross (“remission”) or not. If there was no remission, the patients changed treatment to the next therapeutic line and their response to it was assessed again. Treatment efficacy was assessed every 8 weeks. A change in treatment was produced by the lack of remission, due to adverse events (AEs), and even in spite of having achieved remission.
This process was repeated in all the lines of treatment until a time cut-off of a year was reached or until the patients dropped out of treatment. Due to the time cut-off of the study, no type of discount was carried out.
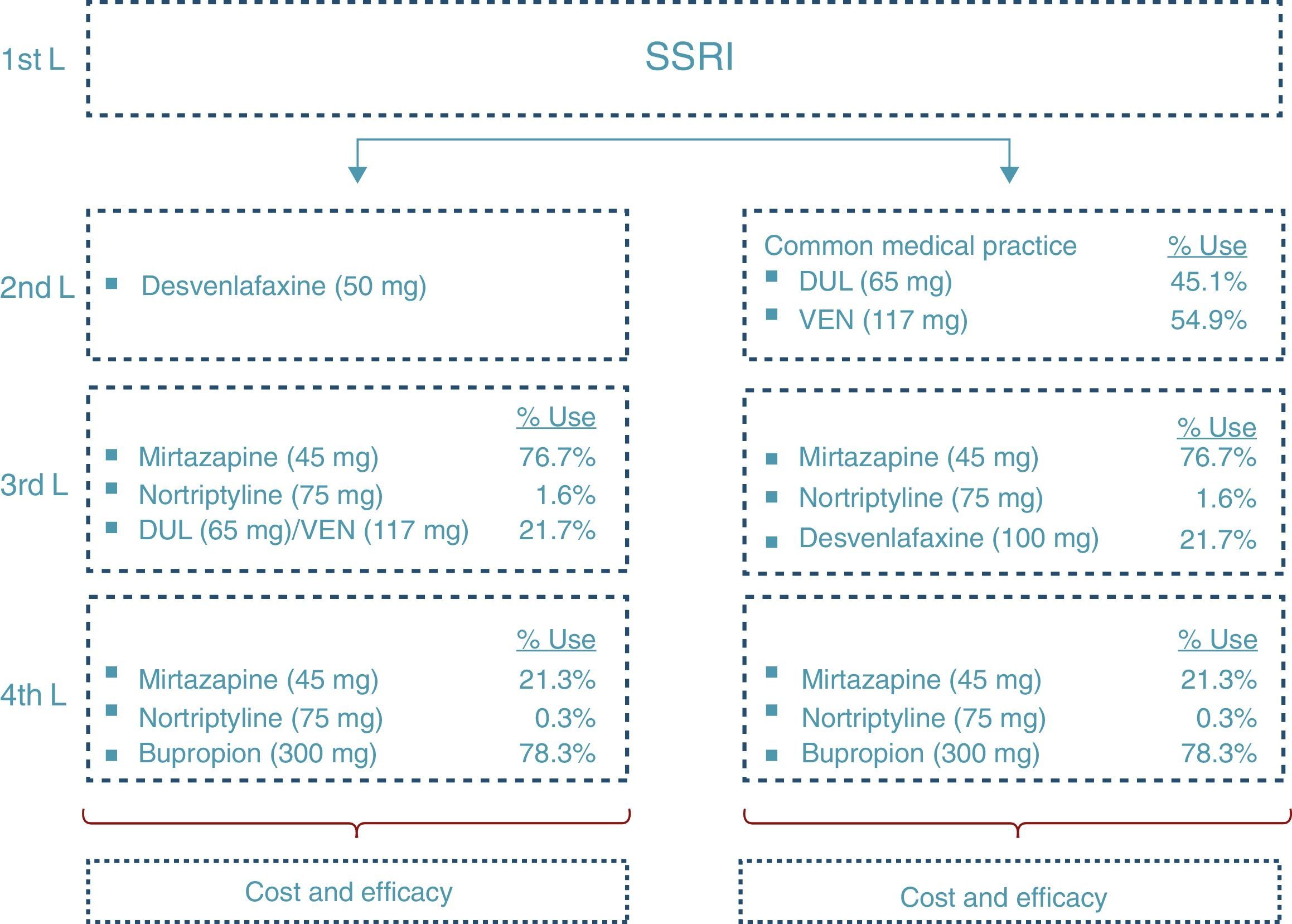
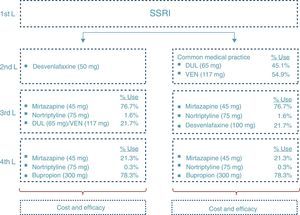
At the end of the time period analysed, we compared the economic costs (pharmacological cost, disease management, AEs, etc.) and clinical costs (remission, depression-free days) generated during the cycles considered for the 2 treatment alternatives: desvenlafaxine or treatment following the standard medical practice (represented by a mixture of venlafaxine and duloxetine according to their market quota as second-line alternatives after failure with an SSRI) (Fig. 2).
To assess the consistency of the results against the uncertainty as to the inputs, various analyses of sensitivity were carried out, varying the parameters identified as essential in the pharmaco-economic result analysis.
Disease managementManagement of MDD in Spain has been profiled based on the study of the Clinical Practice Guidelines and on the opinion of 2 representative clinical experts on the illness. To do so, a consultation was carried out by means of a structured questionnaire with which the standard clinical practice for each of them was sought, as well as the validation of the main inputs of the model. After that, the assumptions were validated and the analysis results were established by consensus.
Fig. 2 shows the ratio of patients treated with each drug and dosage for line of treatment in Spain according to the data obtained from IMS Health. The treatment alternative of choice after the second-line failure of the SNRIs was treatment with tricyclic antidepressants (TADs). In addition, to reflect clinical practice, third-line treatment with SNRIs was considered. Likewise, in the fourth line, the patients were treated both with psychoactive drugs (after failure of the TADs) and with TAD (after failure of the SNRIs).
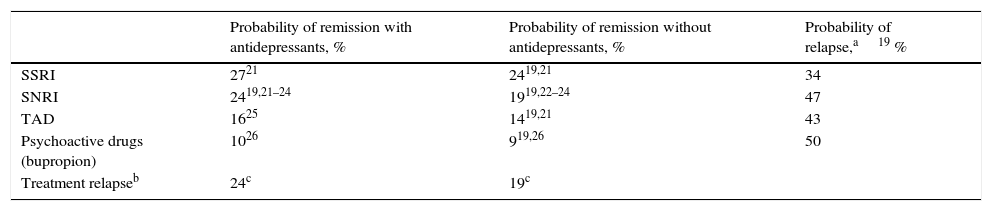
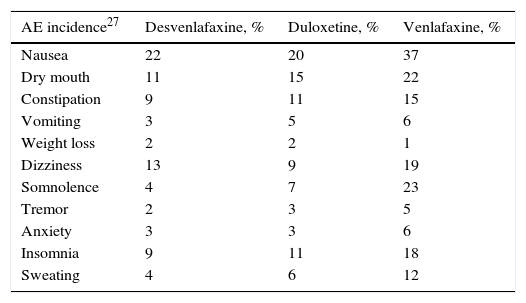
Clinical parametersTo model patient evolution and assess patient response to the drugs used in the treatment of MDD, we considered the probabilities of remission after receiving treatment and the probabilities of relapse after having previously achieved remission. The clinical evidence indicated that the efficacy of the drugs included in the groups of antidepressant treatments (SSRI, SNRI, TAD and psychoactive drugs) were not significantly different within each drug group. Consequently, the probabilities of remission and relapse were considered in the study to be equal within each group (Table 1). A patient diagnosed with MDD was considered as having achieved remission if he or she obtained a score of <7 on the Hamilton Rating Scale for Depression.13 The patients that did not achieve remission with their treatment changed it, with results of 67% with SSRI, 61% with SNRI and 48% with TAD and psychoactive drugs. In addition, 23% of the patients were considered to have changed treatment in spite of having achieved remission.19Table 2 presents the main AEs that occurred with the SNRIs. The analysis considered the probability of dropping out of the treatment as the result of a severe AE and it was assumed that all these patients continued in treatment in a later line. The analysis also contemplated the probability that the patients with MDD with symptoms would commit suicide, independently of whether they were taking antidepressant drugs or not (Table 2).
Probabilities of remission and relapses (evaluated at 8 weeks).
| Probability of remission with antidepressants, % | Probability of remission without antidepressants, % | Probability of relapse,a19 % | |
|---|---|---|---|
| SSRI | 2721 | 2419,21 | 34 |
| SNRI | 2419,21–24 | 1919,22–24 | 47 |
| TAD | 1625 | 1419,21 | 43 |
| Psychoactive drugs (bupropion) | 1026 | 919,26 | 50 |
| Treatment relapseb | 24c | 19c |
SNRIs, serotonin–noradrenaline reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TAD, tricyclic antidepressant.
Incidence of the most relevant adverse events occurring with use of the serotonin–noradrenaline reuptake inhibitors, treatment drop-outs due to these events and suicide rates.
| AE incidence27 | Desvenlafaxine, % | Duloxetine, % | Venlafaxine, % |
|---|---|---|---|
| Nausea | 22 | 20 | 37 |
| Dry mouth | 11 | 15 | 22 |
| Constipation | 9 | 11 | 15 |
| Vomiting | 3 | 5 | 6 |
| Weight loss | 2 | 2 | 1 |
| Dizziness | 13 | 9 | 19 |
| Somnolence | 4 | 7 | 23 |
| Tremor | 2 | 3 | 5 |
| Anxiety | 3 | 3 | 6 |
| Insomnia | 9 | 11 | 18 |
| Sweating | 4 | 6 | 12 |
| Treatment drop-out due to AE | Percentage of patients |
|---|---|
| SSRI | 9%21 |
| SNRI | 5% (DES)22–24; 9.1% (CMP) (9.2% DUL/9% VEN)28–37 |
| TAD | 32%19 |
| Psychoactive drugs (bupropion) | 34%19 |
| Suicide attempts | Suicide rate |
|---|---|
| Suicide attempts that result in demise (semester rate) | |
| Patients taking an antidepressant | 40/100,00038 |
| Patients without antidepressant treatment | 27/100,00039 |
| Suicide attempts not resulting in demise (semester rate) | |
| Patients with antidepressant treatment | 93/100,00038 |
| Patients without antidepressant treatment | 62/100,00038,39 |
AE, adverse advent; CMP, common medical practice; DES, desvenlafaxine; DUL, duloxetine; SNRIs, serotonin–norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TAD, tricyclic antidepressant; VEN, venlafaxine.
The efficacy data considered in the analysis were obtained from clinical trials based on a review of the literature. The main suppositions of the model and the efficacy data used, as well as the use of resources, were validated by clinical experts in the treatment of depression in Spain.
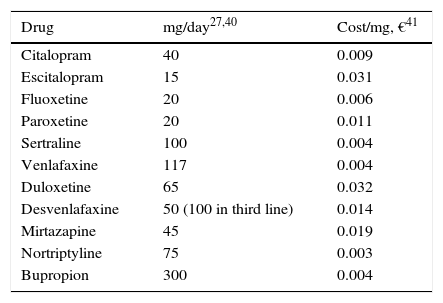
Use of resources and costsFor this analysis, the use of resources and the unit costs of MDD management presented in Table 3 were borne in mind. The majority of the AEs were considered to have occurred in the first 2 weeks after treatment commencement, to avoid redundancy in assigning costs. To estimate the cost of the AEs, the amount of a visit to the specialist physician was assumed for 10% of the patients (Table 3). A successful suicide attempt (death) was considered not to incur any additional direct cost. The direct cost of suicide in Spain was calculated as 10 days of hospitalisation as a result of a failed suicide attempt. This figure was obtained from the mean number of days of hospitalisation of patients admitted to hospital for events possibly related to suicide: poisoning or toxic drug effect (GRD 449, GRD 450), neurotic depression (GRD 426) and psychosis (GRD 430) 43, and it was multiplied by the mean cost per day of hospitalisation estimated from the same database, yielding €7835.90. The model was developed from the perspective of the Spanish National Health System (SNS in Spanish); only direct medical costs were included: pharmacological cost and direct cost del treatment of the disease (Table 3).
Use of resources and unit costs for the management of major depressive disorder.
| Drug | mg/day27,40 | Cost/mg, €41 |
|---|---|---|
| Citalopram | 40 | 0.009 |
| Escitalopram | 15 | 0.031 |
| Fluoxetine | 20 | 0.006 |
| Paroxetine | 20 | 0.011 |
| Sertraline | 100 | 0.004 |
| Venlafaxine | 117 | 0.004 |
| Duloxetine | 65 | 0.032 |
| Desvenlafaxine | 50 (100 in third line) | 0.014 |
| Mirtazapine | 45 | 0.019 |
| Nortriptyline | 75 | 0.003 |
| Bupropion | 300 | 0.004 |
| Resource | Use of resources | Unit cost, €42 |
|---|---|---|
| At the commencement of treatment | ||
| Initial visit to the specialist physician (diagnosis) | 1a | 129.55 |
| Basic metabolic panel | 1a | 78.27 |
| Thyroid function test | 1a | 16.37 |
| Complete blood count | 1a | 4.2 |
| MDD with symptoms | ||
| Successive visits to the specialist physician | 16.8/year12 | 65.56 |
| Visit to the doctor if a dosage adjustment was required | 1a | 65.56 |
First of all, 2 additional analyses were carried out, comparing the use of desvenlafaxine with venlafaxine and duloxetine, respectively, separately. To highlight the economic impact of varying the dosages used in clinical practice, different analyses of sensitivity were performed: an analysis considering the dosage of 150mg of venlafaxine (this being the dosage used to the greatest extent by the specialists); varying the dosage of duloxetine and venlafaxine according to the indications in the Summary of Product Characteristics (60mg duloxetine and 75mg venlafaxine) and, finally, considering an average dosage of 67mg of desvenlafaxine (assuming that 2/3 of the patients receive the indicated dosage of 50mg, while 1/3 receive a dosage of 100mg).
In addition, the effect on the results from the percentage of patients using SNRIs in third-line treatment (in 10.9% and in 32.6%) and from the percentage of patients in remission after treatment with some of the therapeutic alternatives (varying ±20%) were assessed. In turn, as the drop-out rate due to AEs was the only differentiating parameter as to efficacy of the treatments in second line, we assessed the results of the analysis equalling the drop-out rate of duloxetine and venlafaxine to that of desvenlafaxine.
In addition, given that the model considers the existence of up to 23% of patients that change their treatment in spite of being in remission, an analysis in which this percentage was assumed to be 0% was carried out.
Finally, an analysis was performed considering a cost 40% lower than the treatment with duloxetine, which corresponds to its expected reference price, once it loses its patent protection.
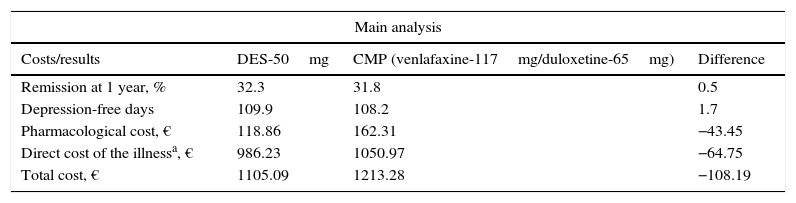
ResultsInitiating second-line treatment with desvenlafaxine as compared to the common medical practice was associated with a slightly higher percentage of patients in remission at 1 year: 32.2% against 31.8%, respectively. This meant a greater number of depression-free days per patient and year: 109.9 with desvenlafaxine and 108.2 with venlafaxine or duloxetine, which was linked with lower direct costs of the treatment of the illness (Table 4). In this sense, initiating second-line treatment with desvenlafaxine instead of duloxetine or venlafaxine, according to the common medical practice, would save the SNS an average €108 per patient and year, derived from a lower cost for pharmacological treatment and for disease management (AE, suicide and medical follow-up of the patients; Table 4).
Costs and results in health of the therapeutic pattern of the model for major depressive disorder.
| Main analysis | |||
|---|---|---|---|
| Costs/results | DES-50mg | CMP (venlafaxine-117mg/duloxetine-65mg) | Difference |
| Remission at 1 year, % | 32.3 | 31.8 | 0.5 |
| Depression-free days | 109.9 | 108.2 | 1.7 |
| Pharmacological cost, € | 118.86 | 162.31 | −43.45 |
| Direct cost of the illnessa, € | 986.23 | 1050.97 | −64.75 |
| Total cost, € | 1105.09 | 1213.28 | −108.19 |
| Analysis of sensitivity | |
|---|---|
| Scenario | Total cost difference, € |
| DES-50mg vs venlafaxine-117mg | −49 |
| DES-50mg vs duloxetine-65mg | −180 |
| DES-50mg vs CMP (venlafaxine-75mg/duloxetine-60mgb) | −96 |
| DES-50mg vs CMP (venlafaxine-150mg/duloxetine-65mg) | −114 |
| DES-67mg vs CMP | −89 |
| DES-50mg vs CMP (% SNRI in 3L=32.6%) | −110 |
| DES-50mg vs CMP (% SNRI in 3L=10.9%) | −107 |
| DES-50mg vs CMP (remission rate +20%) | −113 |
| DES-50mg vs CMP (remission rate −20%) | −104 |
| DES-50mg vs CMP (drop-out rate due to AEs equal among the SNRIs) | −114 |
| DES-50mg vs CMP (% change of treatment given remission 0%) | −2 |
| DES-50mg vs CMP (duloxetine cost −40%) | −81 |
DES, desvenlafaxine; AE, adverse event; SNRIs, serotonin–norepinephrine reuptake inhibitors; CMP, common medical practice.
As the analysis was performed separately (desvenlafaxine against duloxetine, and desvenlafaxine against venlafaxine), the use of desvenlafaxine (50mg) would save the SNS, per patient and year, €180 compared to the use of duloxetine (65mg) and €49 compared to the use of venlafaxine (117mg).
In the analysis using the dosages recommended in the Summary of Product Characteristics for duloxetine and venlafaxine, we found a savings of €96 for desvenlafaxine compared to the common medical practice. Using a dosage of 150mg of venlafaxine, the savings were €114 and, assuming a dosage of 67mg of desvenlafaxine (which corresponds to a mixture of 2/3 of 50mg and 1/3 of 100mg), they were €89.
Increasing the percentage of patients treated with SNRIs in third line (32.6%) would result in a saving of desvenlafaxine against common clinical practice of €110 and decreasing it (10.9%) would lead to a saving of €107.
Likewise, the savings of desvenlafaxine against common medical practice would range between €104 and €113, depending on whether the remission rate was 20% lower or higher than those used in the main analysis.
Equalling the drop-out rate due to AEs showed that, even having the same efficacy, initiating treatment with desvenlafaxine would be the most economical alternative for the SNS (−€114) against common clinical practice. Lowering the percentage of patients that change treatment, even having obtained remission at 0%, would lower this savings to €2. Finally, against a reduction of 40% of the cost of duloxetine, desvenlafaxine would continue to be the most economical alternative (−€81).
DiscussionOur study shows how desvenlafaxine in second-line treatment against the treatments used in common medical practice (a mixture of venlafaxine and duloxetine) slightly improves the clinical parameters: remission at 1 year and depression-free days. This improvement was mainly due to the lower drop-out rate due to AEs with desvenlafaxine. Bearing in mind this small clinical improvement in the face of the other alternatives studied, and that the direct costs were a bit lower in the group treated in second line with desvenlafaxine compared to the rest of the SNRIs, and given the current Spanish of MDD. Considering a mean saving of €108 annually per patient with MDD treated with desvenlafaxine instead of the other SNRIs, and bearing in mind the adult population in Spain with MDD44 as well as the current prevalence per year of MDD,4 this could mean a potential high saving for the SNS.
In general, the variability in the management of depression is high. In studies carried out n Primary Care, the rate of response (frequency of partial remission) in the patients that received antidepressants was around 60%, with a marked placebo effect being observed.45 As was commented earlier, clinical evidence shows how the efficacy of the various drugs included in each group was not significantly different. The differences in cost consequently become a key factor when it is time to decide on one drug or another within each group. There is little published evidence on economic assessment of SNRIs. To date, there is no publication on desvenlafaxine and only 2 publications involving economic assessments comparing venlafaxine to duloxetine. Both studies showed the similarities (both clinical and economic) that there are between the 2 SNRIs, which corroborate the results of this study.46,47
This study presents the limitations inherent in modelling and using assumptions that make it possible to simulate the clinical and economic consequences of a specific illness, using the best evidence available. One of these limitations is that the model analyses a hypothetical cohort of patients and that the data used for modelling its evolution reflect results of studies obtained through a review of the literature. Likewise, these studies have been chosen in such a way that the characteristics of the patients are similar to those of patients included in the STAR*D clinical trial, and that their results are comparable.
Another limitation is that, given that when the study was carried out there was no publication available on direct comparisons between the various antidepressants analyses in this study, the data for efficacy of tolerability of the comparative studies with placebo had to be used for each of the treatments. To attempt to minimise the uncertainty as to the inputs and, consequently, in the results and conclusions of the pharmo-economic analysis, clinical experts validated all the data (both clinical and economic) together with the assumptions made. Some patients with MDD might require a combination of antidepressants. However, the indications, the choice and the way of using these combinations are debatable.48
Due to the difficulty in modelling the response to treatment using combination of drugs (in part due to the little clinical evidence and in part due to the great variability in clinical management of this illness), this analysis assessed only the clinical and economic consequences of the drugs used in monotherapy.
Depression is one of the factors most often related to suicidal behaviour,49 which is the result of the confluence of a great number of situations and factors combining among themselves to generate a variety of situations that go from simple fleeting suicidal ideation up to consummated suicide. The variables that intervene in the production of suicidal behaviour are numerous and include biological factors and sociodemographic, psychiatric and psychosocial variables.50 That is why it was necessary to calculate the cost of failed suicides for this study; however, given that there is no information about this matter, an extrapolation was performed based on the mean number of days of hospitalisation of the patients admitted for poisoning or toxic drug effects, neurotic depression and psychosis.43
The results of the analyses of sensitivity carried out on the main model parameters confirm that desvenlafaxine is superior to common clinical practice and highlighted that, even having the same efficacy as other second-line therapeutic alternatives, desvenlafaxine is still the treatment of choice. There are 2 main reasons for this: its better safety profile and the fact that its pharmacokinetic profile needs less follow-up to adjust the dosage.22,23,51 Because of the high prevalence of depression, the cost its treatment involves, its role as one of the primary factors of risk for suicide and its impact on the productivity of people, depression has an enormous economic role not only in the healthcare system, but also in society. For that reason, it is vitally important to establish healthcare policies aimed at treating this condition with efficacy to reduce the social burden that it represents.
In conclusion, by putting into context the small differences in cost between the second-line SNRIs after the failure of the first-line SSRI antidepressants with the total of patients with MDD in Spain, a significant saving is obtained for the SNS with desvenlafaxine. In moments of tight budgets, this saving could be relevant.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe economic model was developed by RTI (USA) and was funded by Pfizer, Inc. The adaptation of the model, whose results are presented in this study, was carried out by Oblikue Consulting, S.A. (Barcelona, Spain) and was funded by Pfizer, Inc. Milagrosa Blanca Tamayo and Josep Gascón Barrachina received fees for their participation as clinical experts in the Spanish adaptation of the model, but they have not received any remuneration as authors of the document. Javier Rejas Gutiérrez and Beatriz Armada Peláez are employees at Pfizer S.L.U., the Spanish subsidiary of Pfizer, Inc.
Please cite this article as: Rejas Gutiérrez J, Blanca Tamayo M, Gascón Barrachina J, Armada Peláez B. Evaluación económica de la desvenlafaxina en el tratamiento de la depresión mayor en España. Rev Psiquiatr Salud Ment (Barc). 2016;9:87–96.